Mench M.M. Fuel Cell Engines
Подождите немного. Документ загружается.

c02 JWPR067-Mench December 19, 2007 17:26 Char Count=
30 Basic Electrochemical Principles
where T
L
and T
H
are the heat rejection and heat addition reservoir temperatures, respec-
tively. As a result, when the difference between the ambient and operating temperature is
low, heat engines are inefficient. Although an electrochemical reaction engine is not subject
to Carnot limitations, it is important to understand that this does not mean the following:
(a) An electrochemical reaction has no limits on efficiency (we will discuss this in
Chapter 3).
(b) An electrochemical reaction always has greater thermodynamic efficiency than
its chemical analog. Indeed, depending on the operating conditions, a chemically
based cycle can be more efficient than an electrochemically based cycle.
Many electrochemical reactions do have the potential to be much more efficient and operate
at lower temperatures compared to a chemical reaction. There are many examples where
electrochemical processes are more common than mechanical or chemical alternatives,
including the following:
1. Chemical or Material Production From the early 1800s to around 1900, aluminum
was typically produced through a chemical reduction of aluminum chloride. Alu-
minum was a rare and expensive material produced this way and was treated as
a precious metal. A 2.8-kg pyramid, one of the largest casts of aluminum at the
time, was used to cap the Washington Monument in 1884 [1]. The electrochemical
route, known as the Hall–Heroult process of 1886, greatly reduced cost and ease
of production. The process of recovering aluminum by high-temperature electrol-
ysis of alumina dissolved in a molten salt bath was named after C. M. Hall and
P. L. Heroult, who nearly simultaneously patented the process in the United States
and France, respectively. Considering the importance of aluminum in aviation, it is
doubtful commercial airline travel would be feasible without this electrochemical
process for aluminum production. Other important examples of electrochemical
production include chlorine, hydrogen, oxygen, and other gas-phase species.
2. Batteries According to a 2005 study, over 70 billion batteries are produced a
year with a value of over $38 billion and growing [2]. Although actually small in
comparison to aluminum production, this value should continually increase as the
need for portable and wireless power grows.
3. Electroplating This important electrolytic process includes not only jewelry and
other aesthetic applications but also electrical contacts and coatings for protection
from corrosion (Figure 2.1). Electroplating alone was already a $10 billion market
in 1991.
4. Sensors and Measurement Devices There are many sensors based on electrochem-
ical reactions. A common example is the thermocouple, which exploits the thermo-
dynamically governed relationship between temperature and voltage between the
junction of two dissimilar metals. Other electrochemical-based sensors for physical
parameters, species detection, or other uses are common.
New developments in electrochemical processes continue to occur at a fast pace, and the
field is always expanding. The Electrochemical Society (ECS) is one of the oldest technical
societies in America, dating back to 1902. Members of the ECS have included H. H. Dow

c02 JWPR067-Mench December 19, 2007 17:26 Char Count=
2.2 Electrochemical Reaction 31
Figure 2.1 The purification of copper sheets by electrolysis. Pure copper sheets act as the cathode
and are spaced between impure copper sheets in an electrolyte bath. The process results in growth
of the pure copper sheet. (Image from D. D. Ebbing, General Chemistry, Third Edition, Houghton
Mifflin Company, Boston, 1990.)
(Founder of Dow Chemicals), C. M. Hall, and Thomas Edison. The need for electrochemical
expertise and development, including fuel cells, will continue for the foreseeable future.
2.2 ELECTROCHEMICAL REACTION
As discussed in Chapter 1, when an electrochemical reaction occurs, the overall global
reaction and thus the chemical energy difference between the beginning and end states of
the reactants and products are identical to the analogous chemical reaction. However, an
electrochemical reaction circulates current through a continuous circuit to complete the
reaction, while a purely chemical reaction does not. Current is strictly defined as motion
of a charged specie and can be in the form of anions (negatively charged species such as
O
2−
), cations (positively charged species such as H
+
), or negatively charged electrons. An
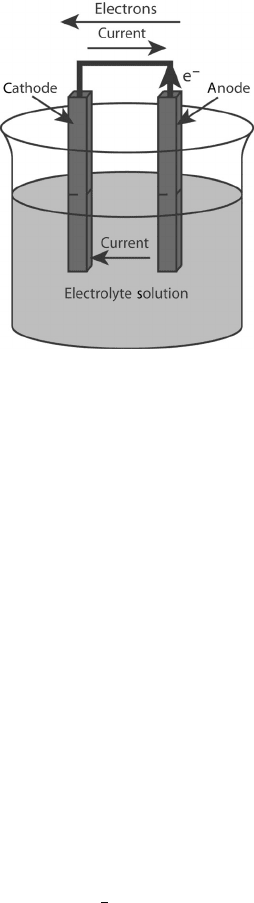
electrochemical reaction also has more requirements than a purely chemical one. Shown in
Figure 2.2 is a basic electrochemical reaction cell. For an electrochemical reaction to take
place, there are several necessary components:
1. Anode and Cathode Electrode The electrochemical reactions occur on the electrode
surfaces. Oxidation occurs at the anode and reduction at the cathode. The reduction
reaction is accompanied by the oxidation reaction, and the pair is often referred to
as a redox reaction. Electrochemical reduction occurs in a reaction that consumes
electrons, reducing the valence state. Electrochemical oxidation results in the loss
of electrons and an increase in the valence state.
2
2
The valence state of an element is a measure of the electrons required to reach a filled outer electron shell.
c02 JWPR067-Mench December 19, 2007 17:26 Char Count=
32 Basic Electrochemical Principles
Figure 2.2 Basic reaction circuit.
2. Electrolyte The main function of the electrolyte is to conduct ions from one
electrode to the other. The electrolyte can be a liquid or a solid, and also serves to
physically separate the fuel and the oxidizer and prevent electron short-circuiting
between the electrodes. This is fundamentally different from a chemical reaction
where the fuel and oxidizer react together.
3. External Connection between Electrodes for Current Flow If this connection is
broken, the continuous circulation of current cannot flow and the circuit is open.
When all components are in place, a complete circuit is formed, and continuous flowing
current of ions and electrons can be maintained under the proper conditions. If any of
these components are not present, the circuit is open, and no flow of current will occur.
An example of this is a battery sitting on the shelf at a store. Since the system is missing
an external connection, there is no continuous reaction. It is important to emphasize that
current is not only the flow of electrons in the external connection between electrodes but
also the flow of ions from one electrode to the other through the electrolyte and overall, the
sum of the charges is conserved in the reaction.
Global versus Elementary Reaction An important distinction between reaction steps,
which is needed to understand the material presented in the rest of the book, is the concept
of a global and elementary reaction. Consider the overall fuel cell reaction:
H
2
+
1
2
O
2
→ H
2
O (2.2)
We know that in a fuel cell the hydrogen and oxygen are separated by the electrolyte, so this
reaction cannot possibly occur in one step as shown. This is the global hydrogen–oxygen
redox reaction. Next, if we focus on just one electrode, the anode, we also have a global
anodic reaction. For example, consider hydrogen oxidation for an acid electrolyte (PAFC
and PEFC):
H
2
→ 2H
+
+ 2e
−
(2.3)
This looks very simple, but in reality it is still very unlikely to occur in a single step as
shown. Equation (2.3) is the global hydrogen electrochemical oxidation mechanism. The

c02 JWPR067-Mench December 19, 2007 17:26 Char Count=
2.2 Electrochemical Reaction 33
following elementary steps are believed to be mainly responsible for this overall oxidation
mechanism in an acid electrolyte:
H
2
⇔ 2(H − M)
ad
(2.4)
(
H − M
)
ad
⇔ H
+
+ e
−
(2.5)
In these equations, M represents the nonreacting catalyst surface. The first step is the
hydrogen dissociative chemisorption step, referred to as the Tafel reaction. In this step, the
hydrogen bond is broken and a layer of atomic hydrogen is adsorbed on the catalyst surface.
The second reaction step is responsible for the actual charge transfer and current generation
and is referred to as the Volmer reaction. As a rule, the intermediate reactions must sum to
the overall global mechanism. So even for the simple hydrogen oxidation reaction, there
are multiple reaction steps. For the oxygen reduction reaction, the reaction is much more
complex, can involve dozens of potential reaction steps, and is still a subject of research.
We can, however, summarize the global oxidation and reduction reactions that occur at the
anode and cathode of fuel cells with acid or alkaline electrolytes:
For acid aqueous electrolytes that transport positive ions through the electrolyte (e.g.
PEFC, PAFC):
Anode global hydrogen oxidation reaction (HOR): H
2
→ 2H
+
+ 2e
−
(2.6)
Cathode global oxygen reduction reaction (ORR): O
2
+ 4H
+
+ 4e
−
→ 2H
2
O (2.7)
For alkaline aqueous electrolytes that transport negative ions through the electrolyte
(e.g. AFC, MCFC, SOFC):
Anode HOR: H
2
+ 2OH
−
→ 2H
2
O + 2e
−
(2.8)
Cathode ORR: O
2
+ 2H
2
O + 4e
−
→ 4OH
−
(2.9)
It should be noted that many alternative pathways also exist to describe the oxygen reduction
reaction, and more advanced publications should be consulted for current understanding in
this evolving area.
Conservation of Charge An excess of charge cannot be maintained in equilibrium. Con-
servation of charge is perhaps more difficult to grasp than conservation of energy or mass,
but upon careful consideration, it is just as obvious a physical law. Since electrons and ions
that carry current are discrete physical entities, the units of charge carried are also discrete.
Consider the independent anodic and cathodic reactions of the fuel cell shown in Eq. (2.10):
Anode: H
2
→ 2H
+
+ 2e
−
Cathode: 2H
+
+ 2e
−
+
1
2
O
2
→ H
2
O
Overall: H
2
+
1
2
O
2
→ H
2
O
(2.10)
Although the anode and cathode reactions are independent, they are clearly coupled to
each other by the necessity to balance the overall reaction, so that the electrons produced
in the HOR are consumed in the ORR. Note that the overall balanced chemical equation
has no stray charged species and is identical to the chemical combustion of hydrogen in
air. However, in the electrochemical reaction, the anode oxidation and cathode reduction
reactions are separate and produce or consume the charged species that make up the current.

c02 JWPR067-Mench December 19, 2007 17:26 Char Count=
34 Basic Electrochemical Principles
When the products of an electrochemical reaction are at a lower chemical energy
state than the reactants, the reaction is thermodynamically favorable, and the reaction
will generate current, a flow of electrons or ions. Such a reaction is termed galvanic.
Thermodynamics, discussed in detail in Chapter 3, can be used to determine if a given
electrochemical reaction is thermodynamically favorable but cannot determine the rate
of reaction. In fact, even a highly thermodynamically favorable reaction may proceed
so slowly that no appreciable current can be detected. Discussion of the determination
of rates of reaction described by electrochemical kinetics is given in Chapter 4. A fuel
cell, a battery, and corrosion are examples of galvanic electrochemical reactions. Galvanic
reactions occur without external input when the proper conditions are met, including all
components necessary for the basic circuit shown in Figure 2.2.
In comparison, some reactions require energy input to occur, and the products are at a
higher chemical energy state than the reactants. Electrical-energy-consuming electrochemi-
cal reactions are termed electrolytic. The generation of hydrogen and oxygen by electrolysis
of water is an example of an electrolytic process. Many industrial processes are also elec-
trolytic, such as gold plating and production of certain chemicals such as aluminum. As an
example, compare the galvanic HOR of a common fuel cell (Eq. 2.2) and the electrolytic
water electrolysis reaction:
Anode: OH
−
→
1
2
O
2
+ H
2
O + 2e
−
Cathode: H
2
O + e
−
→
1
2
H
2
+ OH
−
Overall: H
2
O → H
2
+ 1/2O
2
(2.11)
The fact that the galvanic HOR of Eq. (2.2) can be reversed is remarkable but is a typical
feature of electrochemical reactions. Consider being able to reverse chemical combustion
and produce gasoline from the tailpipe exhaust of an automobile. Of course, the energy
required to electrolytically return the product water to its reactant state of hydrogen and
oxygen is greater than the chemical energy released in the galvanic process or an unlimited
supply of energy would be possible. However, the fact that the products of reaction can
be returned to the initial chemical state is utilized in some fuel cell applications. In a
reversible fuel cell system, the galvanic reactions of Eq. (2.10) provide power until the fuel
and oxidizer are expended. Then, external power is required for the electrolysis of water to
generate oxygen and hydrogen through the mechanism shown in Eq. (2.11). Thus, the fuel
and oxidizer compartment can be sealed, and no refueling is needed. The reversible fuel
cell system is ideal for space applications, where the cost of delivering weight into orbit can
reach $5000/kg. During orbit, for example, the reversible fuel cell provides power when
solar energy is unavailable, and solar panels provide power to electrolyze water when solar
energy is available. A commercially available portable reversible fuel cell demonstration,
unit is illustrated in Figure 2.3.
A few general conventions are useful to remember considering electrochemical reac-
tions.
1. Current is the flow of charged species through the electrolyte (ions) and through
the external circuit (electrons).
2. Current is defined as the flow of positive charge and is thus movement in a direction
opposite to the electron flow (although this convention is not universal).
3. For both galvanic and electrolytic reactions, electrons are conducted from the anode,
through the external circuit, and to cathode.

c02 JWPR067-Mench December 19, 2007 17:26 Char Count=
2.3 Scientific Units, Constants, and Basic Laws 35
Figure 2.3 Reversible fuel cell demonstration kit from Eco Soul of Tustin, California.
4. For both galvanic and electrolytic reactions, oxidation occurs at the anode and
reduction occurs at the cathode. Thus, in a hydrogen fuel cell, the hydrogen is being
oxidized while the oxygen is being reduced.
5. The sign of the electrode depends on type of cell. For a galvanic reaction, reduction
occurs at a higher voltage potential than oxidation and thus the cathode is designated
as the positive electrode. For an electrolytic cell, the opposite is true and the anode
is the positive electrode. In an automotive battery, the cathode is the positive (+),
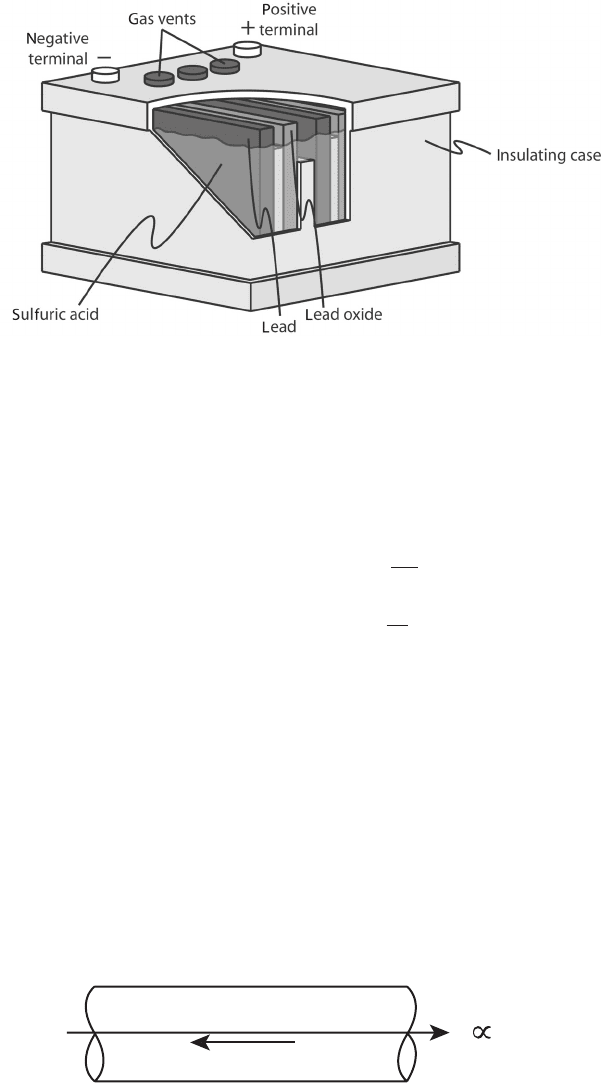
red-labeled electrode (Figure 2.4).
2.3 SCIENTIFIC UNITS, CONSTANTS, AND BASIC LAWS
Although the reader is assumed to have at least a cursory knowledge of basic chemistry and
electrical engineering concepts, a summary of some of the most basic relations, constants,
and units common to electrochemistry is included to provide an understanding of the
physical meaning of the commonly used parameters and allow us to make some basic
calculations.
2.3.1 Electrical Charge, Current, Voltage, and Resistance
Current and Charge The electricity that powers electric motors, radios, and so on, is
really a flow of current. The flow of electrical current through a circuit, powering an
electrical motor or other device, is analogous to the flow of water in a pipe, powering a
c02 JWPR067-Mench December 19, 2007 17:26 Char Count=
36 Basic Electrochemical Principles
Figure 2.4 Cut-away illustration of car battery. (Adapted from http://www.tiscali.co.uk/reference/
encyclopaedia/hutchinson/m0016566.html.)
water wheel and providing mechanical energy, as depicted in Figure 2.5. The electrical
current (I) is the rate of the flow of charged species and is analogous to the mass flow rate
of water in the pipe. The total charge passed is analogous to the total mass of fluid passed
over a given time:
Pipe: Total mass passed = m =
dm
dt
dt =
˙
mdt
Wire: Total charge passed = q =
dc
dt
dt =
Idt
(2.12)
The International System (SI) unit of current (I)istheampere, named after the French
mathematician and physicist Andr
´
e-Marie Amp
`
ere (1775–1836) [3]. Note from Figure 2.5
that the electron flow is shown moving in the direction opposite to the current because the
direction of current is defined as the flow of positive charge and thus moves in a direction
opposite to the negatively charged electron flow (although this convention is not universal).
The total electrical charge passed (C) is designated with the SI unit of the coulomb,
after the French physicist Charles Augustin Coulomb (1736–1806). A coulomb is the
conventional unit of charge passed through the circuit and is equivalent to the total charge
passed by the flow of an ampere of electrons in one second:
1C= 6.28 × 10
18
electrons passed = 1 As (2.13)
e
I
˙
m
Current conductor or water pipe
Figure 2.5 Flow through a pipe or a wire is analogous.
c02 JWPR067-Mench December 19, 2007 17:26 Char Count=
2.3 Scientific Units, Constants, and Basic Laws 37
Thus, the charge on a single electron is only −1.6 × 10
−19
C. Since a coulomb is approxi-
mately equal to 6.28 × 10
18
elementary charges, one ampere is equivalent to 6.28 × 10
18
elementary charges moving through a surface in one second, or 1 C/s.
Sometimes, it is more convenient to describe total charge passed in units of ampere-
hour (Ah). While this can seem confusing at first, examining the units reveals it is simply
a unit of total charge passed:
1Ah= (C/s) · h × 3600 s/h = 3600 C (2.14)
Total energy is often also expressed in a similar fashion. A look at an electric bill or a laptop
computer battery will show the measure often used to define total energy as a watt-hour,
even though it is really equivalent to a joule.
1Wh= (1 Jh/s)(3600 s/h) = 3600 J (2.15)
Voltage A volt (V) is a measure of the potential to do electrical work. Mechanical work is
done when a weight is moved through a distance, and electrical work is done when current
flows through a resistance. The volt (V) is named after Italian physicist Alessandro Volta
(1745–1827), who demonstrated the first electrochemical battery in 1800 [4]. The higher the
voltage, the greater the potential there is to do electrical work. Science students are familiar
with the joule as the standard SI unit of energy. Voltage potential is derived from the same
thermodynamic origin of energy difference between the chemical bonds of the reactants
and products. In this context, we can convert to units appropriate for electrochemical work.
The volt is defined as the measure of potential to do electrical work:
1V= 1J/C (2.16)
A volt is thus a measure of the work required to conduct one coulomb of charge. The higher
the voltage, the higher the potential is available to move this charge. The words potential
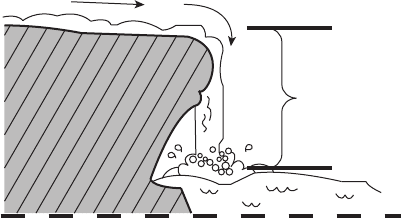
and volt in fact are often synonymous with one another. Figure 2.6 illustrates a waterfall
analogy to help understanding. The potential for the water flowing over the waterfall to
do work is proportional to the difference in height between the top and the bottom of the
waterfall and the flow rate of the water. The greater the difference in height between the
top and the bottom, the more work a hydraulic turbine could extract from the same flow.
Similarly, the electrodes in an electrochemical reaction are like the top and the bottom
of the waterfall. The potential to convert chemical bond energy into electrical power is
proportional to the difference in the potential (voltage) between the electrodes, not the
absolute value of the electrodes (imagine if the bottom of the waterfall was only 1 m below
the top—not much of a waterfall). The current is of course analogous to the mass flow rate
of the water going down the waterfall (a trickle of water is not going to generate much
power), and the total charge is analogous to the integration of the mass flow rate over time,
or the total mass passed through the waterfall. Just as the mechanical power generated by
the turbine scales directly with water flow rate and height of the falls, the electrical power
scales directly with current and voltage:
P
e
= IV = IE
cell
(2.17)
c02 JWPR067-Mench December 19, 2007 17:26 Char Count=
38 Basic Electrochemical Principles
E
cathode
E
anode
Flow rate over the falls—current
Standard reference: standard hydrogen electrode (SHE = 0.0 V)
E
cell
= E
cathode
– E
anode
Figure 2.6 Waterfall analogy to voltage potential. E represents the voltage potential.
Since the voltage of a given reaction is a measure of the potential energy for the
reaction, it must be referenced to some standard datum, selected as an arbitrary zero-voltage
point. The standard commonly used in electrochemistry is the so-called standard hydrogen
electrode (SHE). The SHE is the voltage of a platinum sheet electrode immersed in an
aqueous electrolyte solution, with unit H
+
ion activity i.e., 1 M concentration in contact
with hydrogen gas at 1 atm hydrogen pressure [6]. The SHE is arbitrarily assigned to 0 V.
Thus, other electrodes have an oxidation (negative) or reduction (positive) potential relative
to the SHE. Since most fuel cells operate on hydrogen, the result is that the anode potential
is around 0 V. In practice, the SHE is not always convenient, and many other reference
electrodes with easily reproducible potentials have been devised and can be used instead
of the SHE. The theoretical electrode voltage can be determined based on thermodynamic
considerations, discussed in Chapter 3. The standard electrochemical reduction half-cell
reaction series is shown in Table 2.1 and shows the reduction potential of the given half
reaction relative to the SHE for the reactions shown. The oxidation potential of the reverse
reaction is simply the same value with opposite sign. For a complete cell redox reaction,
the standard cell voltage E
cell
is simply the sum of the oxidation and reduction potentials:
E
cell
= E
anode
+ E
cathode
(2.18)
If positive, the reaction is galvanic. If the cell voltage (E
cell
) is negative, this is the minimum
applied voltage required to initiate the electrolytic reaction. As an example, consider a
redox couple of the oxidation of zinc and the reduction of hydrogen:
Anode (oxidation):
Zn → 2e
−
+ Zn
2+
Cathode (reduction):
2e
−
+ 2H
+
→ H
2
Overall:
Zn + 2H
+
→ Zn
2+
+ H
2
From Table 2.1, the reduction potential of the zinc reaction is −0.76 V, so that oxidation
of zinc relative to the SHE at standard conditions is 0.76 V. The cathode is the SHE, so the

c02 JWPR067-Mench December 19, 2007 17:26 Char Count=
2.3 Scientific Units, Constants, and Basic Laws 39
Table 2.1 Partial Electrochemical Reduction Potential Series at 298
◦
C
Half Reaction Voltage E
◦
(V)
Ag
+
aq
+ e
−
→ Ag
s
+0.799
AgBr
s
+ e
−
→ Ag
s
+ Br
−
aq
+0.095
AgCl
s
+ e
−
→ Ag
s
+ Cl
−
aq
+0.222
HClO
aq
+ H
+
+ e
−
→
1
2
Cl
2,g
+ H
2
O
l
+1.63
Cu
2+
aq
+ 2e
−
→ Cu
s
+0.337
Fe
2+
aq
+ 2e
−
→ Fe
s
−0.440
Fe
3+
aq
+ 3e
−
→ Fe
s
+0.771
2H
+
aq
+ 2e
−
→ H
2,g
0.000
2H
2
O
l
+ 2e
−
→ H
2,g
+ 2OH
−
aq
−0.830
HO
−
2aq
+ H
2
O
l
+ 2e
−
→ 3OH
−
aq
+0.880
H
2
O
2,aq
+ 2H
+
aq
+ 2e
−
→ 2H
2
O
l
+1.776
K
+
aq
+ e
−
→ K
s
−2.925
Li
+
aq
+ e
−
→ Li
s
−3.05
Mg
2+
aq
+ 2e
−
→ Mg
s
−2.37
N
2,g
+ 4H
2
O
l
+ 4e
−
→ 4OH
−
aq
+ N
2
H
4,aq
−1.16
N
2,g
+ 5H
+
aq
+ 4e
−
→ N
2
H
−
5,aq
−0.23
NO
−
3aq
+ 4H
+
aq
+ 3e
−
→ NO
g
+ 2H
2
O
l
+0.96
Na
+
aq
+ e
−
→ Na
s
−2.71
Na
2+
aq
+ 2e
−
→ Ni
s
−0.28
Zn
2+
aq
+ 2e
−
→ Zn −0.76
O
2,g
+ 4H
+
aq
+ 4e
−
→ 2H
2
O
l
+1.23
O
2,g
+ 2H
+
aq
+ 2e
−
→ H
2
O
2,aq
+0.68
O
2,g
+ 2H
2
O
l
+ 4e
−
→ 4OH
−
aq
+0.40
O
3,g
+ 2H
+
aq
+ 2e
−
→ O
2,g
+ H
2
O
l
+2.07
S
s
+ 2H
+
aq
+ 2e
−
→ H
2
S
g
+0.141
H
2
SO
3,aq
+ 4H
+
aq
+ 4e
−
→ S(s) +3H
2
O
l
+0.450
HSO
−
4,aq
+ 4H
+
aq
+ 2e
−
→ H2SO
3,aq
+ H
2
O
l
+0.170
Source: From [5].
voltage is 0.0 V. Overall, then, the voltage of the cell illustrated in Figure 2.7 at standard
conditions is 0.76 V.
Individual Electrode Behavior In Figure 2.6, a waterfall is shown to illustrate the voltage
potential for the overall reaction. At each electrode, there is an independent half-cell global
reaction coupled with the other electrode reaction only through conservation of mass and
charge. Just as the potential for work from the waterfall is a result of the difference in
location between the top and the bottom, the overall electrochemical cell voltage is a result
of the difference in potential between the anodic and cathodic reactions, not the voltage of
the individual reactions themselves. Consider a hydrogen-filled cathode and anode from
Table 2.1. This electrochemical reaction circuit would have no overall potential for reaction,
since there would be no potential difference between the two electrodes. This also illustrates
the function of the electrode to separate fuel and oxidizer. If the electrolyte permits passage
of reactants through it, they will mix at the electrodes and reduce voltage potential.
