Mench M.M. Fuel Cell Engines
Подождите немного. Документ загружается.


c01 JWPR067-Mench December 19, 2007 19:42 Char Count=
Table 1.1 Fuel Cell Types, Descriptions, and Basic Data
Fuel Cell
Type
Electrolyte
Material
Operating
Temperature
(
◦
C) Major Poison Advantages Disadvantages
Most Promising
Applications
Alkaline
fuel cell
Solution of
potassium
hydroxide in
water
60–250
a
CO
2
High efficiency, low
oxygen readuction
reaction losses
Must run on pure oxygen
without CO
2
contaminant
Space applications
with pure O
2
/H
2
available
Phosphoric
acid fuel
cell
Solution of
phosphoric acid
in porous silicon
carbide matrix
160–220 Sulfur, high levels
of CO
1–2% CO tolerant,
good-quality waste heat,
demonstrated durability
Low power density,
expensive, platinum
catalyst used, slow
start-up, loss of
electrolyte
Premium stationary
power
Solid oxide
fuel cell
Yttria (Y
2
O
2
)
stabilized
zirconia (ZrO
2
)
600–1000 Sulfur CO tolerant, fuel flexible,
high-quality waste heat,
inexpensive catalyst
Long start-up time,
durability under thermal
cycling, inactivity of
electrolyte below
∼600
◦
C
Stationary power
with
cogeneration,
continuous-power
applications
Molten
carbonate
fuel cell
Molten alkali metal
(Li/K or Li/Na)
carbonates in
porous matrix
600–800 Sulfur CO tolerant, fuel flexible,
high-quality waste heat,
inexpensive catalyst
Electrolyte dissolves
cathode catalyst,
extremely long start-up
time, carbon dioxide
must be injected to
cathode, electrolyte
maintenance
Stationary power
with
cogeneration,
continuous-power
applications
Polymer
electrolyte
fuel cell
b
Flexible solid per-
fluorosulfonic
acid polymer
30–100 CO, Sulfur, metal
ions, peroxide
Low-temperature
operation, high
efficiency, high H
2
power density, relatively
rapid start-up
Expensive catalyst,
durability of components
not yet sufficient,
poor-quality waste heat,
Intolerance to CO,
thermal and water
manangement
Portable,
automotive, and
stationary
applications
a
Modern AFCs < 100
◦
C.
b
Includes direct methanol fuel cell and direct alcohol fuel cells.
10
c01 JWPR067-Mench December 19, 2007 19:42 Char Count=
1.4 Classification of Fuel Cells 11
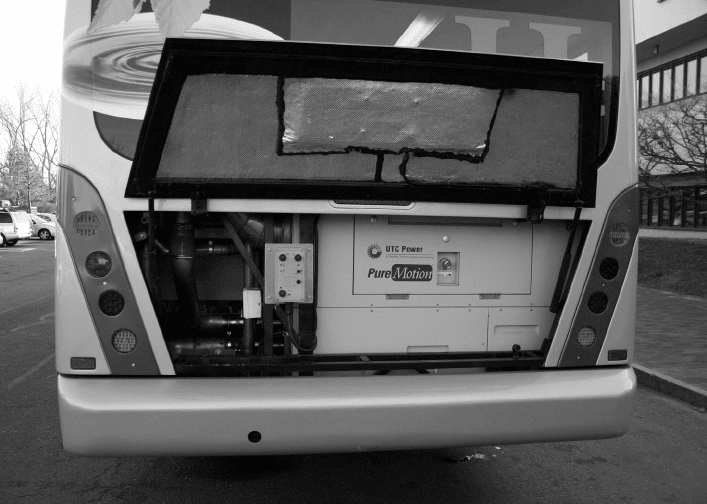
Figure 1.9 UTC Power supplies fuel cell bus powerplants for transit programs in the United States
and Europe. The fuel economy of transit buses powered by a UTC Power PureMotion
TM
fuel cell
system is two times better than a diesel-powered bus. Fuel cell-powered buses also emit no harmful
tailpipe emissions and operate quietly. (Image Courtesy of UTC Power Corporation.)
is fueled either by pure hydrogen or from a diluted hydrogen mixture generated from a
fuel reformation process. A stack power density of greater than 1.3 kW/L is typical. Since
the operating temperature is from room temperature to ∼80
◦
C, a noble metal platinum
catalyst is typically used on the anode and cathode. Figure 1.9 is a picture of a PEFC engine
developed by UTC Power for city bus applications. The H
2
PEFC has many technical issues
that complicate performance and control. Besides issues of manufacturing, ancillary system
components, cost, and market acceptance, the main remaining technical challenges for the
fuel cell itself include (1) water and heat management, (2) durability, and (3) freeze–thaw
cycling and frozen-start capability.
Direct Methanol Fuel Cell The liquid-fed direct methanol fuel cell (DMFC) is generally
seen as the most viable alternative to lithium ion batteries in portable applications because
DMFC systems require less ancillary equipment and can therefore potentially be more
simplified compared to an H
2
PEFC. Additionally, the usc of a liquid fuel simplifies
storage. The DMFCs can potentially compete favorably with advanced Li ion batteries
(which currently power many wireless portable applications) in terms of gravimetric energy
density of ∼120–160 Wh/kg and volumetric energy density of ∼230–270 Wh/L. While both
H
2
PEFCs and DMFCs are strictly PEFCs (both use the same flexible polymer electrolyte),
the DMFC feeds a liquid solution of methanol and water to the anode as fuel. The additional
complexities of the low-temperature methanol oxidation reaction prevent the DMFC from

c01 JWPR067-Mench December 19, 2007 19:42 Char Count=
12 Introduction to Fuel Cells
(a)
(b)
Figure 1.10 Photograph of (a) PDA/smart phone concept model and (b) a handheld entertainment
system concept model. Both are powered by Mobion
ő
fuel cell technology, which uses a direct
methanol fuel cell for power. (Image Courtesy of Mechanical Technology, Inc. (MTI).)
c01 JWPR067-Mench December 19, 2007 19:42 Char Count=
1.4 Classification of Fuel Cells 13
obtaining the same level of fuel cell power density as the H
2
PEFC. Figure 1.10 is a picture
of a portable DMFC developed by MTI Micro for hand-held power application.
Four main technical issues affecting performance remain: (1) two-phase flow man-
agement in the anode and cathode, (2) methanol crossover, (3) poor catalyst activity, and
(4) high catalyst loading. While significant progress has been made by various groups
to develop optimized catalysts, total noble metal catalyst loading is still on the order of
10 mg/cm
2
. Typically a platinum–ruthenium catalyst is utilized on the anode for methanol
oxidation, and a platinum catalyst is utilized on the cathode as in the H
2
PEFC [3]. The
DMFC is discussed in greater detail in Chapter 6.
Solid Oxide Fuel Cell The SOFC represents a high-temperature fuel cell system with a
solid ceramic electrolyte. The historical operating temperature of SOFC systems is around
800–1000
◦
C, although developing technology has demonstrated 500
◦
C operation [4], where
simplified system sealing and materials solutions are feasible. Due to the elevated operating
temperature, the catalysts used are non–noble metal and other inexpensive raw materials.
High electrolyte temperature is required to ensure adequate ionic conductivity (of O
2−
)
in the solid-phase ceramic electrolyte. Operating efficiencies as high as 60% have been
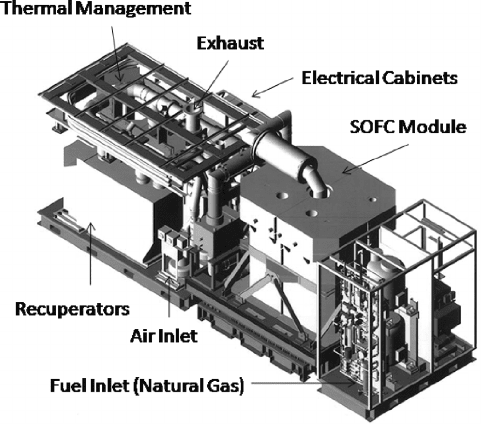
attained for a 220-kW cogeneration system [5]. Figure 1.11 is a schematic of the Siemens
Westinghouse 100-kW tubular SOFC system. It is interesting to realize that most of the
volume of larger fuel cell systems is not in the fuel cell itself but in ancillary components,
including fuel processing and power conditioning systems.
Figure 1.11 Siemens 100 kW tubular solid oxide fuel cell and cogeneration system. The system
has a peak power of ∼140 kW, typically feeding 109 kW into the local grid and 64 kW of hot water
into the local district heating system. at an electrical efficiency of 46% [6]. (Image courtesy Siemens
Power Generation.)
c01 JWPR067-Mench December 19, 2007 19:42 Char Count=
14 Introduction to Fuel Cells
There has been much recent development in the United States on SOFC systems,
incubated by the Department of Energy Solid State Energy Conversion Alliance (SECA)
program. The 10-year goal of the SECA program (started in the fall of 1999) is to develop
3–10-kw SOFC units at <$400/kW with rated performance achievable over the lifetime of
the application with less than 0.1% loss per 500 h operation by 2021 [7].
Besides manufacturing and economic issues, the main technical limitations of the
SOFC include achieving a reduced operating temperature, controling start-up time, dura-
bility, and proper cell-sealing. The SOFC is discussed in depth in Chapter 7.
Molten Carbonate Fuel Cell Molten carbonate fuel cells are commercially available from
several companies, including a 250-kW unit from FuelCell Energy in the United States and
several other companies in Japan. Some megawatt-sized demonstration units are installed
worldwide based on natural gas or coal-based fuel sources which can be internally reformed
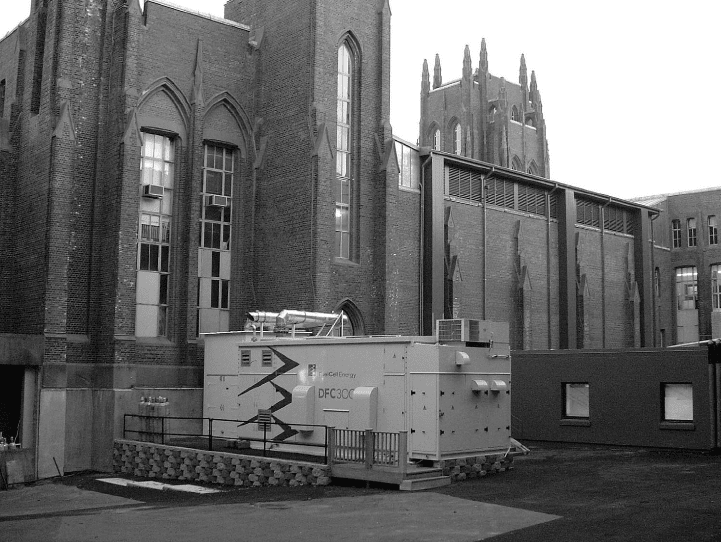
within the anode of the MCFC. Figure 1.12 shows a picture of a 250-kWe net MCFC
developed by FuelCell Energy and installed at Yale University. MCFCs operate at high-
temperature (600–700
◦
C) with a molten mixture of alkali metal carbonates (e.g., lithium
and potassium) or lithium and sodium carbonates retained in a porous ceramic matrix
through a delicate balance of gas-phase and capillary pressure forces. Technical details of
the MCFC are discussed in detail in Chapter 7. A major advantage of the MCFC is the lack
of precious metal catalysts, which greatly reduce the system raw material costs. Original
development on the MCFC was mainly funded by the U.S. Army in the 1950s and 1960s.
Figure 1.12 FuelCell Energy’s DFC
ő
300A 250 kWe net molten carbonate fuel cell system
installed at Yale University in Connecticut. (Image courtesy of FuelCell Energy, Inc.)

c01 JWPR067-Mench December 19, 2007 19:42 Char Count=
1.4 Classification of Fuel Cells 15
The U.S. Army desired operation of power sources from logistic fuel already available,
thus requiring high temperatures with internal fuel reformation that can be provided by
the MCFC. During this period, significant advances of this liquid electrolyte alternative
to the SOFC were made [8]. Development waned somewhat after this early development,
but advances have continued and MCFC commercialization has been achieved. The main
disadvantages of MCFCs include (1) complex electrolyte management and loss through
finite vapor pressure, (2) extremely long start-up time (the MCFC is generally suitable only
for continuous power operation), (3) durability, and (4) carbon dioxide injection into the
anode is needed to maintain electrolyte stability.
Phosphoric Acid Fuel Cell The PAFC was originally developed for commercial appli-
cation in the 1960s. The PAFC has an acidic, mobile (liquid) electrolyte of high con-
centration phosphoric acid contained by a porous silicon carbide ceramic matrix and
operates at around 160–220
◦
C. A 200-kW PAFC array that powers the Verizon call
routing center in New York is shown in Figure 1.13. Like the MCFC, the electrolyte
is bound by capillary and gas pressure forces between porous electrode structures. The
PAFC is in many ways similar to the PEFC, except the acid-based electrolyte is in liq-
uid form and the operating temperature is slightly higher. Over two-hundred 200-kW
commercial PAFC units were developed and sold by United Technologies Corporation
through several different divisions and subsidiaries, and many are still in operation.
Figure 1.13 The Verizon call routing center in Garden City, New York, is home to the largest U.S.
commercial fuel cell installation of its kind. The fuel cells from UTC Power generate 200 kilowatts
each, providing a total of 1.4 megawatts of clean power to the center. (Image Courtesy of UTC Power
Corporation.)
c01 JWPR067-Mench December 19, 2007 19:42 Char Count=
16 Introduction to Fuel Cells
However, ubiquitous commercial application has not been achieved, primarily due to the
high cost of approximately $4500/kW, about five times greater than cost targets for conven-
tional stationary applications [9]. The main advantages of the PAFC include (1) the high
operating temperature allows operation with 1–2% CO in fuel stream, (2) the highly con-
centrated acid electrolyte does not need water for conductivity, making water management
very simple compared to the PEFC, and (3) the demonstrated long life and commercial
success for premium stationary power of the PAFC. Besides the high system cost, the main
technical disadvantages of the PAFC include (1) a bulky, heavy system compared to PEFC
with area-specific power less than half of the PEFC (0.2–0.3 W/cm
2
[10], (2) continued use
of platinum catalyst with nearly the same loading as PEFCs, (3) the relatively long warm-up
time until the electrolyte is conductive at ∼160
◦
C (although warm-up time is much less
than the MCFC or SOFC), and (4) the liquid electrolyte has finite vapor pressure, resulting
in continual loss of electrolyte in the vapor phase. Modern PAFC design includes cooling
and condensation zones to mitigate this loss. The PAFC is discussed in greater detail in
Chapter 7.
Alkaline Fuel Cell Alkaline fuel cells utilize a solution of potassium hydroxide in wa-
ter as an alkaline, mobile (liquid) electrolyte. Alkaline fuel cells were originally devel-
oped as an auxillary power unit APU for space applications by the Soviet Union and the
United States in the 1950s and served on the Apollo program as well as the Space Shut-
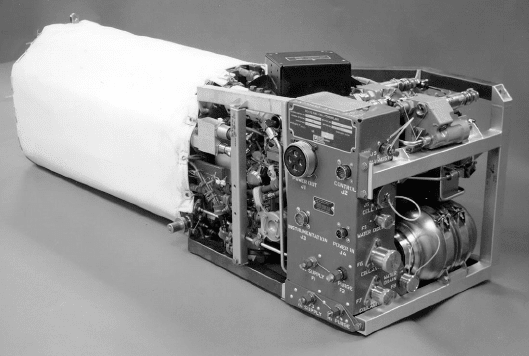
tle orbiter. A 12-kW AFC used to provide power and potable water for astronauts in the
Space Shuttle orbiter is shown in Figure 1.14. The AFCs were chosen for space applica-
tions for their high efficiency and robust operation. The AFC operates around 60–250
◦
C
with greatly varied design and operating conditions. Modern designs tend to operate at
the lower range of temperature and pressure near ambient conditions. The primary ad-
vantages of the AFC are the lower cost of materials and electrolyte and high operating
efficiency (60% demonstrated for space applications) due to use of an alkaline electrolyte.
Figure 1.14 12-kW fuel cell power plant for the Space Shuttle Orbiter. Three units power the
orbiter while in space as well as provide the drinking water for the astronauts. (Image Courtesy of
UTC Power Corporation.)
c01 JWPR067-Mench December 19, 2007 19:42 Char Count=
1.5 Potential Fuel Cell Applications and Markets 17
For alkaline electrolytes, the oxidizer reduction reaction (ORR) kinetics are more effi-
cient than acid-based electrolytes (e.g., PEFC, PAFC). Many space applications utilize
pure oxygen and hydrogen for chemical propulsion, so the AFC was well suited as an
APU. However, the alkaline electrolyte suffers an intolerance to even small fractions of
carbon dioxide (CO
2
) found in air which react to form potassium carbonate (K
2
CO
3
)in
the electrolyte, gravely reducing performance over time. For terrestrial applications, CO
2
poisoning has limited lifetime of AFC systems to well below that required for commercial
application, and filtration of CO
2
has proven too expensive for practical use. Due to this
limitation, relatively little commercial development of the AFC beyond space applications
has been realized. Some recent development of alkaline-based solid polymer electrolytes
is underway, however. The AFC is discussed in greater detail in Chapter 7.
Other Fuel Cells Many other fuel cell systems exist, and new versions are constantly
being developed. Many of these are simply existing fuel cell systems with a different fuel.
For example, PEFCs based on a direct alcohol solution offer alternatives to DMFCs for
portable power and include those based on formic acid [11], dimethyl ether [12], ethylene
glycol, dimethyl oxalate, and other so-called direct alcohol fuel cells (DAFCs) [13, 14].
A completely different concept is the biologically based fuel cell. Biologically based
fuel cells use biocatalysts for conversion of chemical to electrical energy and can be
classified into two basic categories: (1) microbial fuel cells (MFCs) and (2) enzyme-
based fuel cells. In the MFC, electricity is generated by anerobic oxidation of organic
material by bacteria. The catalytic activity and transport of protons are accomplished using
biological enzymes or exogenous mediators [15–17]. Although performance is relatively
quite low, on the order of 0.1–1 mA/cm
2
, the potential for generating some power, or
simply power-neutral decomposition and treatment of domestic waste matter, currently a
multibillion-dollar cost to society, is potentially quite significant.
The enzyme-based biological fuel cell has significantly greater power density
(1–10 mA/cm
2
) than the microbial fuel cell, although power produced is still orders of
magnitude lower than a conventional precious metal catalyzed H
2
PEFC [17]. However,
enzymatic fuel cells have distinct advantages in terms of potential cost and operation at
ambient temperature in near-neutral-pH environments. Enzymatic fuel cells are envisioned
as implantable power devices in humans or as using environmentally derived fuel from tree
saps for long-term remote sensor applications [18]. While biologically based fuel cells are
probably the least-developed fuel cell power source, the unique aspects of the catalytic pro-
cess and potential for natural sugar-based power are intriging. Another potentially interest-
ing application on which this author has pondered is a weight loss fuel cell where blood sugar
would be used to power an external fuel cell device, effectively burning calories with no
physical exercise required. The feasibility of this concept is further explored in Problem 22.
1.5 POTENTIAL FUEL CELL APPLICATIONS
AND MARKETS
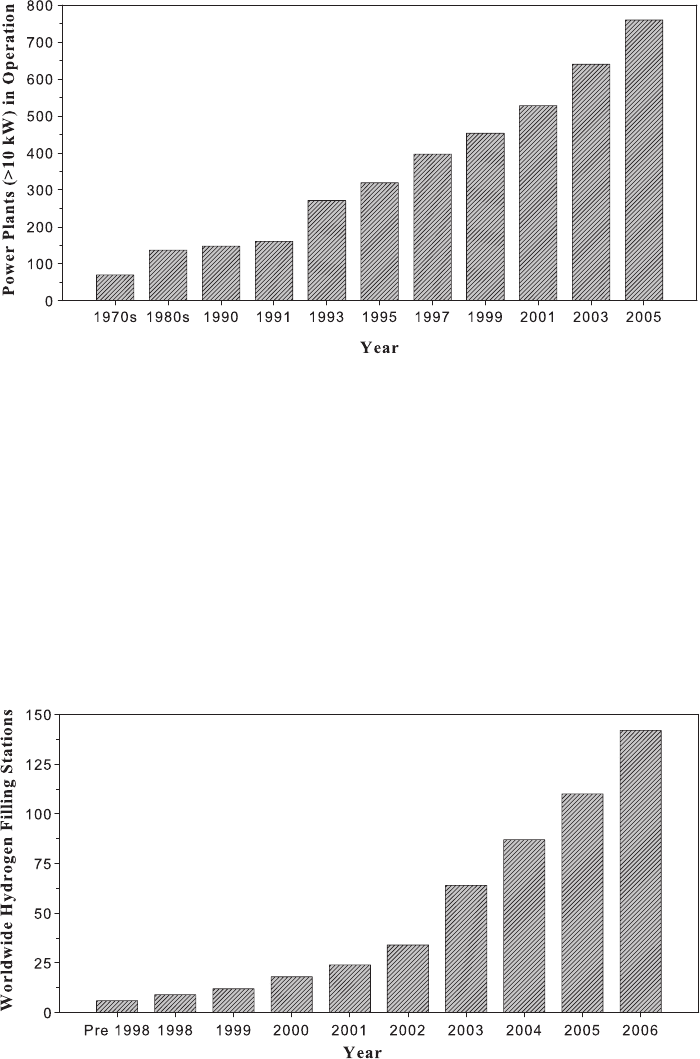
Fuel cells have the potential to replace existing power sources for many applications. Figures
1.15 and 1.16 show the exponential progression of commercial stationary power fuel cell
installations and hydrogen refueling stations worldwide. Although the trends look promis-
ing, they currently represent only an infinitesimal fraction of the fueling infrastructure
c01 JWPR067-Mench December 19, 2007 19:42 Char Count=
18 Introduction to Fuel Cells
Figure 1.15 Commercial large (>10-kW) stationary power fuel cell installations under operation
versus year. (Data adapted from Ref. [19].)
required for practical widespread use. Fuel generation, storage, and delivery infrastructure
are still major barriers that must be overcome. Hydrogen generation, storage, and delivery
are discussed in Chapter 8. Hydrogen infrastructure is a vast and speculative subject, and
various summaries can be found [20, 21]. The most likely consumer applications for fuel
cells include portable (0–100-W), stationary (0–25-kW), and transportation (∼100-kW)
applications. Each market has unique demands that tend to be more suited to a particular
type of fuel cell. For portable applications, high system power density and simplicity are de-
sired over efficiency and cost. For stationary applications, durability and high efficiency are
higher priorities. For transportation applications, compact size, rapid start-up, robustness,
and high efficiency are the primary technical goals.
Figure 1.16 Cumulative worldwide hydrogen fueling stations. Upper bars in 2005 and 2006 are
anticipated station installations. (Data adapted from Ref. [22].)
c01 JWPR067-Mench December 19, 2007 19:42 Char Count=
1.5 Potential Fuel Cell Applications and Markets 19
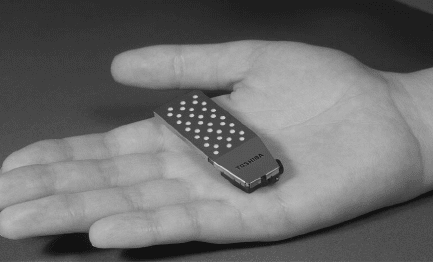
Figure 1.17 Toshiba 100 mWe micro direct methanol fuel cell which weighing 8.5 g (0.3 oz) [23].
(Image Courtesy Toshiba Corporation.)
Portable Applications Perhaps where fuel cells show the most promise for ubiquitous
near-term implementation is in portable power (0–100-W) applications, such as cell phones
and laptop computers. Current battery technology has not yet provided the energy density re-
quired for long-term operation, and recharging is time consuming. Additionally, the cost of
existing premium power battery systems is already on the same order as contemporary fuel
cells, with additional development anticipated. With replaceable fuel cartridges, portable
fuel cell systems have the additional advantage of instant and remote rechargeability that can
never be matched with secondary battery systems. A hand-held DMFC for portable power
developed by Toshiba is planned for production. The 8.5-g DMFC shown in Figure 1.17
is rated at 100 mW continuous power (up to 20 h) and measures 22 mm × 56 mm × 4.5
mm, including a maximum of 9.1 mm for the concentrated methanol fuel tank [24]. As the
wireless economy progresses, demand for higher power, smaller, and instantly recharge-
able technologies will undoubtedly continue to push forward development of portable
fuel cells.
Stationary and Distributed Power Applications Stationary (1–500-kW) applications in-
clude power units for homes or auxiliary and backup power generation units. Stationary
applications are designed for nearly continuous use and therefore must have far greater
lifetime than automotive units, although operation at a near-continuous steady state is
advantageous for durability. Stationary devices typically range from 1 kW temporary or
auxiliary power generator units, examples of which are the Ballard fuel cell generator units
shown in Figure 1.18, to 100-kW systems for regular power of buildings. Unlike the portable
fuel cell, where ancillary components are reduced as much as possible, the stationary fuel
cell system is not similarly constrained, and typically has an array of components to achieve
high-efficiency, durable operation.
A plot showing the estimated number of demonstration and commercial units in the
stationary power category from 1985 to 2002 is given in Figure 1.19. Not surprisingly,
the exponential growth in the number of online units follows a similar qualitative trend
to the available patents granted for various fuel cell technologies shown in Figure 1.1.
The early rise in stationary units in 1997 was primarily a result of PAFC systems sold
by United Technologies Corporation, although recently most additional units have been
PEFCs from various manufacturers. Data are estimated from the best available compilation
