Mench M.M. Fuel Cell Engines
Подождите немного. Документ загружается.


c02 JWPR067-Mench December 19, 2007 17:26 Char Count=
40 Basic Electrochemical Principles
e
-
Voltmeter
H
2(g)
Switch
Zn anode
Cathode
compartment
Anode
compartment
-
NO
3
Na
+
-
NO
3
Zn
2+
-
NO
3
e
-
H
+
Zn(s)
2e
-
Zn
2+
(aq)
+
2H
+
(aq)
+
2e
-
H
2(g)
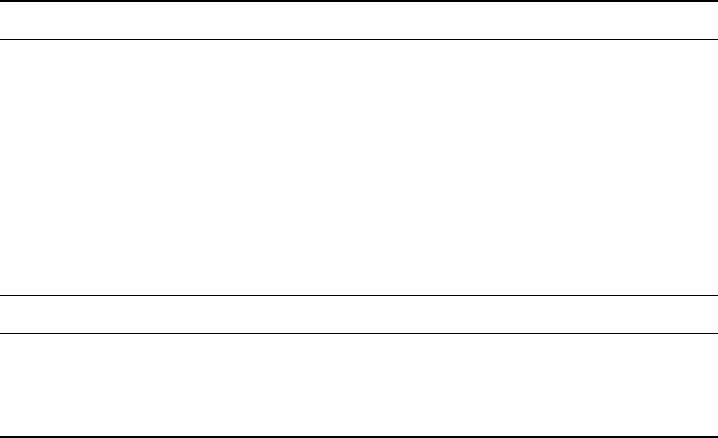
Figure 2.7 The SHE used to reference a zinc oxidation reaction. (Adapted from Ref. [5].)
In a galvanic reaction, the potential difference between the cathode and the anode is
positive, and the anode is at a lower potential compared to the cathode. As discussed, this
voltage difference is a measure of the thermodynamic potential for reaction and is related
to the chemical energy difference between products and reactants. The majority of fuel
cells operate with oxygen in air at the cathode and hydrogen fuel at the anode. Thus, at
open-circuit conditions the anode is nearly at SHE conditions and is therefore at around
0 V. The cathode potential is analogous to the top of the waterfall in Figure 2.6.
Using Table 2.1, the overall cell voltage is simply the difference in the reduction
potential between the cathode and anode:
E
cell
= E
cathode,red
− E
anode,red
(2.19)
There is a minus sign in Eq. (2.19) since both half-cell reactions are taken as reduction
reactions whereas Eq. (2.18) uses one reduction and one oxidation reaction. At standard
conditions (pure oxygen and hydrogen, 1 atm pressure, and 298 K), we expect an open-
circuit cell voltage of 1.23 V.
Resistance and Conductance Electrical resistance measured in ohms (), is a measure
of the potential loss associated with moving a rate of charge. Put another way, in order
to move a given rate of charge through a conductor, some voltage potential is lost. It is
important to realize the energy is not lost. Indeed, conservation of energy still applies.
Instead, the potential to do electrical work is lost and is dissipated into heat. The greater
voltage potential lost per rate of charge passed, the greater the resistance. Resistance can
be defined as
R = V/A = (J/C)/(C/s) = Js/C
2
(2.20)
One joule-second per coulomb squared is defined as one ohm, and its inverse (i.e., 1/)is
defined, quite directly, as a mho. A mho is the unit of conductance, also known as a siemen

c02 JWPR067-Mench December 19, 2007 17:26 Char Count=
2.3 Scientific Units, Constants, and Basic Laws 41
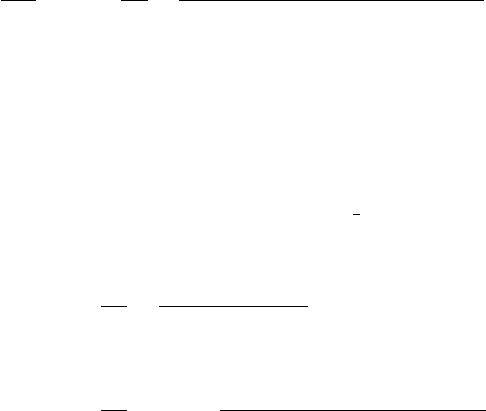
A = Cross-sectional area
ρ=
RA
l
l
=Ω-m
= Linear path of ion travel
Figure 2.8 Illustration of current through a wire.
(S). The siemen is named after Werner von Siemens (1816–1892), a German electrical
engineer and businessman [7]. Electrical resistance is analogous to flow potential losses in
a pipe. A small-diameter pipe has greater resistance to flow than a large-diameter pipe and
suffers greater pressure loss per length for a given flow rate. Similarly, a material with high
electrical resistance will have greater voltage potential loss per length for a given current.
From experience and common sense, we know that fluid flow pressure losses through
a pipe are a function of the geometry of the pipe, and the pressure loss is not an intrinsic
property of all pipes. Similarly, electrical resistance is not an intrinsic property of a wire.
Consider Figure 2.8, which shows a cylindrical wire axially conducting current. The resis-
tance to current (which is physically a measure of the resistance to the flow of electrons) is
proportional to the cross-sectional area A and inversely proportional to the length of travel.
Obviously, the longer the wire, the greater the loss of voltage potential. An exceptionally
thin wire has more electron interaction with the wire surface, leading to increased resistance
to flow compared to a larger diameter wire. Resistivity ρ (m) is an intrinsic property of
a material because it normalizes the resistance of the material for cross-sectional area (A)
and length (l), removing geometric factors:
ρ =
RA
l
R = ρ
l
A
=
l
σ A
(2.21)
Some resistivities of common materials are given in Table 2.2. The inverse of resistivity,
1/ρ,istheconductivity σ [(m)
−1
]. Generally, the conductivity of a material is used if the
purpose is to conduct charged species, and its resistivity is used if it is an insulating material,
although either choice is appropriate. Metals are generally good electron conductors. Gas-
phase conductivity is negligible except at extreme temperatures where significant gas
ionization can occur. Electrolytes are designed to be excellent ionic conductors and poor
electron conductors.
Dependence of Conductivity on Operational Parameters The conductivity of various
substances, and in particular fuel cell materials, can depend strongly on operating pa-
rameters. For example, the electron conductivity of solid materials such as graphite and
steel decreases with increasing temperature due to increased molecular collision frequency.
However, the ionic conductivity of the SOFC and PEFC electrolyte is a positively related
function of temperature. In the PEFC, water saturation in the electrolyte also plays a major
role. These functional dependencies are highly material specific and are discussed in later
chapters.
c02 JWPR067-Mench December 19, 2007 17:26 Char Count=
42 Basic Electrochemical Principles
Table 2.2 Electrical and Ionic Resistivities of Selected Materials
Electron conductors Electron Resistivity at 293 K (m)
Gold 2.44 × 10
−8
Aluminum 2.28 × 10
−8
Copper 1.7 × 10
−8
Silver 1.6 × 10
−8
Stainless steel 7.2 × 10
−7
Platinum 1.1 × 10
−7
Ruthenium 7.1 × 10
−8
Palladium 10 × 10
−8
Carbon 3.5 × 10
−5a
Water (deionized) 2.5 × 10
5
Polytetrafluoroethylene (Teflon) 10
16
–10
17
Ionic Conductors Ionic Resistivity (m)
Nafion PEFC electrolyte by DuPont, fully humidified ∼10 at 353 K
SOFC electrolyte 0.1–1 at 600–1000 K
Liquid electrolytes Highly concentration, temperature,
and ion dependent
a
Dependent on direction and molecular structure.
2.3.2 Ohm’s Law
Ohm’s law is named after the work of Georg Simon Ohm (1789–1854), a Bavarian mathe-
matician and teacher. The basic form of Ohm’s law can be shown as
V = IR (2.22)
It is important to note that in liquid solutions Ohm’s law is true only for electrolytes without
ionic concentration gradients, which may not always be strictly accurate [8, 9], but can be
considered to be true for most fuel cell studies.
Often, students have difficulty following the units involved in this simple relationship
since they are often familiar with only volts, ohms, and amperes. Unit matching and
conversion are critical components of any engineering analysis, and electrochemistry is no
exception. If one breaks down the units of this relationship into units for energy, power,
and so forth, we see that Ohm’s law is dimensionally consistent:
V = IR
V = A ·
J/C = (C/s)(Js/C
2
) = J/COk!
Electrical Power Power is the rate of work, whether in mechanical, electrical, thermal,
or other form. Electrical power (P
e
) can be expressed as
P
e
= IV
(2.23)
W = (C/s)(J/C) = J/s = WOk!

c02 JWPR067-Mench December 19, 2007 17:26 Char Count=
2.4 Faraday’s Laws: Consumption and Production of Species 43
If the voltage is the fuel cell voltage, the output is the electrical power from the fuel
cell. If instead the voltage in Eq. (2.23) is the voltage potential lost due to resistance, the
power generated is thermal dissipation. The delineation between electrical power and heat
dissipation rate will become clear in future chapters if it is not now. Thus, electrical power
has units of watts (J/s), as expected. Ohm’s law can also be substituted into Eq. (2.17) to
obtain alternate expressions for electrical power:
P
e
= IV = I · IR = I
2
R
(2.24)
P
e
= IV =
V
R
· V =
V
2
R
Since it is often the case that mechanical and electrical systems operate in conjunction
with one another, it is imperative to be able to convert among the units used for electrical
systems and thermo-mechanical systems.
Example 2.1 Simple Electrical Calculations A given circuit has a continuous 5 A DC
(direct current) and an overall resistance of 10 . Calculate
(a) the potential loss, in volts, to maintain steady state;
(b) the electrical power dissipated as heat during operation, in watts; and
(c) the total heat dissipated in 2 h.
SOLUTION
(a) Potential loss is found via Ohm’s law for this circuit: V = IR = 50 V.
(b) Electrical power dissipated as heat is P
h
= IV = 250 W.
(c) The total heat dissipated is given as
Total heat Q =
t
0
P
h
dt = (250 W)(2 h)[1 (J/s)/W](3600 s/h) = 1800 kJ
COMMENT: The resistance shown represents the resistance in a generic electric circuit
not a fuel cell.
2.4 FARADAY’S LAWS: CONSUMPTION AND
PRODUCTION OF SPECIES
In this section we consider a fundamentally important question: How much mass of a given
reactant is required to produce a given amount of current? Conversely, how much current
is required to produce a certain amount of product? Clearly, the fundamental relationships
should be based on conservation of mass and charge. We cannot produce mass from an
electrochemical reaction, only rearrange it, and a given amount of reactant can produce a
fixed number of charged species, based on the balanced chemical reaction. Consider 1 mol

c02 JWPR067-Mench December 19, 2007 17:26 Char Count=
44 Basic Electrochemical Principles
of reactant in a generic electrochemical reaction:
ν
A
A + ν
B
B → ν
C
C + ν
D
D (2.25)
where ν
i
is the molar (stoichiometric) coefficient required to balance each species i in the
reaction equation. Consider the number of electron charges moved through the circuit to
react with 1 mol of species A in a one-electron process (e.g., for every mole of A consumed,
one electron is moved through the circuit). An example is given for the anodic portion of
the silver redox reaction:
Ag
s
→ Ag
+
aq
+ e
−
(2.26)
We know from common experience that the overall silver redox reaction will oxidize
(tarnish) silver over time. In this oxidation process, 1 mol of electrons and 1 mol of
silver cation is produced per mole of silver reacted. However, not every reaction is a 1
mol–one electron process. Faraday’s constant F represents the charge per mole of equivalent
electrons, that is,
F =
6.023 × 10
23
electrons/mole equivalent
6.242 × 10
18
electrons/C
= 96,485 C/eq (2.27)
Faraday’s constant can also be written in terms of ampere-hour:
F = 96,485 C/eq
3600 C/Ah
= 26.8Ah/eq (2.28)
The equivalent electrons (eq) is very important but is commonly omitted. Many electro-
chemical reactions do not exchange 1 mol of electrons for 1 mol of reactant, as in Eq.
(2.26). For example, consider the ORR that occurs at the cathode of many fuel cells:
4e
−
+ 4H
+
+ O
2
→ 2H
2
O (2.29)
Here, 4 mol of electrons react per mole of oxygen. In this case, the charge carried per mole
of oxygen reacted would be 4F. The scaling factor n is defined as the number of electrons
transferred per mole of species of interest.
n =
number of electrons
mole of species of interest
= eq/mol (2.30)
Thus, the combination of nF is the charge passed per mole of species of interest, and the
units of nF are coulombs per mole. Note that the unit “mole” is specific to the chosen
species. That is, it is really a mole of the species of interest. The choice of the species of
interest makes a difference, and n simply permits determination of the relationship between
charge passed and reactant consumption (or product generation) of any species chosen. For
example, in Eq. (2.29), n is 4 eq electrons/mol O
2
for oxygen consumption. Alternatively,
considering water produced as the species of interest, the value of n is 2, and there are 2F
coulombs passed per mole of H
2
O produced.
Now, consider the HOR that occurs at the anode of many fuel cells:
H
2
→ 2e
−
+ 2H
+
(2.31)

c02 JWPR067-Mench December 19, 2007 17:26 Char Count=
2.4 Faraday’s Laws: Consumption and Production of Species 45
Here, there are 2F coulombs passed per mole of H
2
(n = 2 eq electrons/mol H
2
). Note
that it is possible that some purely chemical reactions can also occur in parallel with
electrochemical reactions. In this case, the purely chemical component of the reaction must
also be known to determine the relationship between charge passed and consumption or
generation in the overall multi-reaction process.
Faraday’s Laws In the early 1830s, Michael Faraday reported that the quantity of species
electrolytically separated was proportional to the total charge passed, establishing a link
between the flow of charge and mass [9]. This became the basis for Faraday’s first law of
electrolysis:
1. For a specific charge passed, the mass of the products formed are proportional to
the electrochemical equivalent weight of the products.
Although Faraday’s results were purely experimental, the important implication from this
proportional relationship was that electrical current was a result of discrete particles we
now understand to be ions and electrons that are conserved as part of an overall balanced
reaction equation. This understanding enabled quantification of many electrical phenomena
and helped reveal the nature of protons and electrons.
In his work, Faraday also discovered that the mass of the product species was directly
proportional to the charge passed. This law provides a connection between the charge passed
and the mass generated or consumed in a reaction through the balanced electrochemical
reaction equations. Faraday’s second law of electrolysis is stated as follows:
2. The amount of product formed or reactant consumed is directly proportional to the
charge passed.
The second law is the most important for fuel cell study. This result is what we could
expect from modern common sense and conservation of mass; the current generated is
proportional to the mass reacted or produced:
m ∝ I (2.32)
Considering a purely electrochemical reaction, with an understanding of the discrete nature
of charged particles that carry current, it seems obvious that there is a proportional rela-
tionship between charge passed and consumption or production of the involved species.
The proportionality is based on the balanced electrochemical reaction equation and can be
written as
˙
n
x
=
iA
nF
=
I
nF
(2.33)
Let us examine the parameters and units involved:
˙
n
x
= rate of molar consumption or production of species x (mol of x/s)
I = current (A)
A = superficial electrode area (cm
2
)
i = current density, = I /A (A/cm
2
)
n = equivalent electrons per mole of reactant x (eq/mol)
F = charge carried on one equivalent mole (C/eq)

c02 JWPR067-Mench December 19, 2007 17:26 Char Count=
46 Basic Electrochemical Principles
The superficial (or geometric) electrode area A is the active area of the electrode as viewed
from above. For a given electrochemical reaction, Eq. [2.33] can be used to determine the
amount of reactant consumed to produce a given current and the amount of product formed
for a known current. The actual amount of reactant delivered to an electrode can be greater
than determined by Faraday’s law but can never be less. The actual amount of product
produced in a given reaction will never be greater than predicted.
Example 2.2 Faraday’s Law Calculations Consider a single hydrogen fuel cell at 4 A
current output:
Anode oxidation:
H
2
→ 2e
−
+ 2H
+
Cathode reduction:
4e
−
+ 4H
+
+ O
2
→ 2H
2
O
Global reaction:
H
2
+
1
2
O
2
→ H
2
O
(a) What is the molar rate of H
2
consumed for the electrochemical reaction?
(b) What is the molar rate of O
2
consumed for the electrochemical reaction?
(c) What is the minimum molar flow rate of air required for the electrochemical
reaction? Assume air is a mixture of 21% oxygen and 79% nitrogen by volume.
(d) What is the maximum molar flow rate of air delivered for the electrochemical
reaction?
(e) What is the rate of water generation at the cathode in grams per hour? The molecular
weight of water is 18 g/mol.
(f) Can the generation rate of water be greater or less than the value predicted in part
(e)?
SOLUTION (a) Consider the hydrogen as the “reactant of interest,” x in Faraday’s law.
Also recall that 1 A = 1 C/s:
H
2
→ 2H
+
+ 2e
−
n = 2 electron eq/mol H
2
˙
n
H
2
=
iA
nF
=
4C/s
(2 e
−
eq/mol H
2
)(96,485 C/eq)
= 2.072 × 10
−5
mol H
2
/s
(b) Here, we are looking for the molar oxygen flow rate of oxygen consumed, which
is determined from the oxidation–reduction reaction:
2H
+
+ 2e
−
+
1
2
O
2
→ H
2
O
n = 4 electron eq/mol O
2
˙
n
O
2
=
iA
nF
=
4C/s
(4 e
−
eq/mol O
2
)(96,485 C/eq)
= 1.036 × 10
−5
mol O
2
/s
c02 JWPR067-Mench December 19, 2007 17:26 Char Count=
2.4 Faraday’s Laws: Consumption and Production of Species 47
(c) Now we are concerned with determination of the minimum required molar flow
rate of air, which contains 79% nitrogen. The only required reactant here is oxygen, and
the molar flow rate of oxygen required was solved in part (b). To achieve the same flow
rate of oxygen in air, we simply must divide by the mole fraction of reactant (oxygen) in
the air mixture. Note that nitrogen flow is inert here and simply “goes along for the ride”:
˙
n
O
2
0.21
=
˙
n
air
=
iA
nF
=
4C/s
(0.21 × 4e
−
eq/mol O
2
)(96,485 C/eq
= 4.94 × 10
−5
mol O
2
/s
(d) There is no maximum of reactant supplied. The amount of reactant supplied is
governed by, for example, the pumps and blowers that make up the system. Faraday’s law
only imposes a minimum amount of mass required to generate a given current.
(e) Here, we are to determine the rate of water generation in grams per hours, which
will require some minor unit conversion. The first step is to solve for the molar rate of water
generation, which occurs at the cathode:
2H
+
+ 2e
−
+
1
2
O
2
→ H
2
O
n = 2 electron eq/mol H
2
O
˙
n
H
2
O
=
iA
nF
=
4C/s
(2 e
−
eq/mol H
2
O)
(96,485 C/eq) = 2.072 × 10
−5
mol H
2
O/s
Now simply convert to the appropriate units using the molecular weight:
˙
m
H
2
O
=
iA
nF
× MW =
4C/s
(2 e
−
eq/mol H
2
O)(96,485 C/eq)
(18 g/mol)(3600 s/h)
= 1.34 g
H
2
O
/h
(f) The value for water produced cannot be greater for a given current, since this would
violate conservation of mass. Normally, the products cannot be less than that predicted
either, unless another electrochemical reaction (called a side reaction) takes place that
consumes some of the charge passed.
COMMENTS: Notice the small numbers when units of moles per second are used. Be
sure to keep enough significant digits. Also note from part (e) that a hydrogen fuel cell is
also a water generator. The water produced must be removed from the fuel cell to allow
reactant to reach the catalyst layer. In high-temperature systems, the water product is vapor,
while lower temperature fuel cell systems can produce liquid effluent, which complicates
design.
Example 2.3 Fuel Cell Stack Calculations Consider a 20-cell stack operating steadily
in series with 100 cm
2
active area per electrode, with a current density of 0.8 A/cm
2
.The
fuel cell nominal voltage is 0.6 V per plate.
(a) Determine the water production in grams per hour for this stack.
(b) Determine the stack voltage and electrical power output.
SOLUTION (a) This example is quite similar to the previous one, but with an additional
complication that this is now a stack of fuel cells. Since they are in series, each fuel cell

c02 JWPR067-Mench December 19, 2007 17:26 Char Count=
48 Basic Electrochemical Principles
produces the same total current (0.8 A/cm
2
× 100 cm
2
= 80 A), and thus the total water
produced is simply 20 times the rate of water generation of an individual fuel cell in the
stack. Consider the ORR at the cathode:
4e
−
+ 4H
+
+ O
2
→ 2H
2
O
For water production,
n = 2 electron eq/mol H
2
O
˙
n
H
2
O
=
iA
nF
=
(0.8C/s · cm
2
100 cm
2
)
(2 e
−
eq/mol H
2
O)(96,485 C/eq)
= 4.15 × 10
−4
mol H
2
O/s per fuel cell
Now simply scale for the entire stack and to the appropriate units using the molecular
weight and number of plates in series:
˙
m
H
2
O,stack
= 20
iA
nF
· MW
= (20 cells)(4.15 ×10
−4
mol H
2
O/s per fuel cell)(18 g/mol)(3600 s/h)
= 538 g H
2
O/ h per stack
(b) Since the plates are in series, the stack voltage is
20 cells × 0.6 V per fuel cell = 12 V per stack
The cells are all in series and therefore all carry the same current of 80 A. The stack electric
power is simply P
e
= IV, or 960 W.
COMMENTS: This works out to be about 538 cm
3
of water per hour. Larger fuel cell
engines have a significant amount of water production to manage. Consider that an auto-
motive fuel cell engine should be on the order of 100 kW, and about 932 kg (around 2 lb)
of water per minute would be produced! Much of this leaves the system as vapor, however.
2.5 MEASURES OF REACTANT UTILIZATION
EFFICIENCY
There are various metrics utilized to quantify the efficiency of different aspects of an
electrochemical reaction. One type of efficiency for a purely electrochemical reaction is
based on species consumption. For a galvanic process, there will be a minimum amount
of reactant required for a given reaction, as calculated by Faraday’s law, Eq. [2.33]. In
practice, we are not constrained to provide exactly the minimum amount of reactant. For a
given current, there is a calculated minimum amount of reactant, but there is no maximum.
The actual flow rate of reactants is a function of the pumps and blowers that are used
for reactant delivery. Obviously, the more flow delivered, the higher the parasitic power
required, so we generally seek to deliver something close to the minimum requirement. The
Faradic efficiency is a measure of the percent utilization of reactant in a galvanic process:
ε
f
=
theoretical required rate of reactant supplied
actual rate of reactant supplied
(2.34)
c02 JWPR067-Mench December 19, 2007 17:26 Char Count=
2.5 Measures of Reactant Utilization Efficiency 49
Faradic efficiency is often called the fuel utilization efficiency (µ
f
) when applied to the fuel
in a galvanic redox reaction:
µ
f
=
theoretical required rate of fuel supplied
actual rate of fuel supplied
(2.35)
For an electrolytic process, some side reactions and inefficiencies may occur and result in
less than complete conversion. The current efficiency is defined as:
ε
c
=
actual rate of species reacted or produced
theoretical rate of species reacted or produced
(2.36)
Stoichiometric Ratio In fuel cell parlance, the term stoichiometry is defined as the in-
verse of the Faradic efficiency. Students may be confused with this terminology, since the
stoichiometric condition typically describes a balanced chemical reaction equation with no
excess oxidizer. Here, the term stoichiometry is used slightly differently, and its meaning is
similar to the definition of equivalence ratio used in combustion. Unlike chemical reactions,
the reduction and oxidation reactions are separated by electrolyte, so each electrode can
have a discrete stoichiometry:
The cathodic stoichiometry is defined as:
λ
c
=
1
ε
f,c
=
actual rate of oxidizer delivered to cathode
theoretical rate of oxidizer required
(2.37)
The anodic stoichiometry is defined as:
λ
a
=
1
ε
f,a
=
actual rate of fuel delivered to anode
theoretical rate of fuel required
(2.38)
To avoid confusion the reader should be aware that other symbols for stoichiometry, besides
λ, are commonly used in the literature, including ζ and ξ . The theoretical rate of reactant
required is calculated by Faraday’s law, and the actual rate of reactant delivered is a function
of the fuel or oxidizer delivery system. One important point is worth mentioning: Fuel cells
must always have an anode and cathode stoichiometry greater than 1. For a value less than
unity, the current specified could not be produced. For reasons explained in Chapter 4, a
stoichiometry of exactly 1 is not possible either, so that a Faradic efficiency of 100% is not
possible on the anode or cathode for a single pass of reactant.
3
Example 2.4 Stoichiometry and Utilization Consider a portable 20 cm
2
active area fuel
cell operating steadily at 0.75 V, 0.6 A/cm
2
. The fuel utilization efficiency is 50%, and the
cathode stoichiometry is 2.3. The fuel cell is expected to run for three days before being
recharged. The cathode operates on ambient air, and the anode runs off of compressed
hydrogen gas.
(a) Determine the volume of the hydrogen fuel tank required if it is stored as a
compressed gas at 200 atm (20.26 MPa), 298 K.
(b) How large would a pure oxygen container be if it was used to provide the oxi-
dizer? Consider 200 atm (20.26 MPa) storage pressure and 298 K average ambient
temperature.
3
Fuel recirculators can be used to increase the effective faradic efficiency to 100%, but we are talking about a
single pass of reactant through the fuel cell here.
