Mench M.M. Fuel Cell Engines
Подождите немного. Документ загружается.

c04 JWPR067-Mench January 28, 2008 17:28 Char Count=
180 Performance Characterization of Fuel Cell Systems
On a semiempirical basis, the crossover current density i
x
can be modeled as a base
current density that occurs even under open-circuit conditions. In this approach the current
density at the cathode is modeled as i + i
x
. At OCV, the external current i = 0, but i
x
is still
active. For instance, if a Tafel kinetics approach is used on the cathode, we would model
the cathode kinetic overpotential in the absence of concentration affects as
η
a,c
=−
R
u
T
α
c
F
ln
i + i
x
i
o,c
(4.101)
This has the desired effect of decreasing the OCV to observed levels but is really not an
accurate representation of the true mixed potential reaction, in part because the exchange
current density on the cathode for the ORR is different than for the HOR on the cathode.
If a full analytical model of the surface is desired, the mixed potential must be properly
accounted for and include separate terms to account for the kinetic losses associated with the
individual reactions present on the particular electrode. Except in the cases where detailed
analysis of the OCV effects is desired, this is often not necessary, since the OCV departure
affects the starting potential at zero current but has little effect beyond the OCV loss.
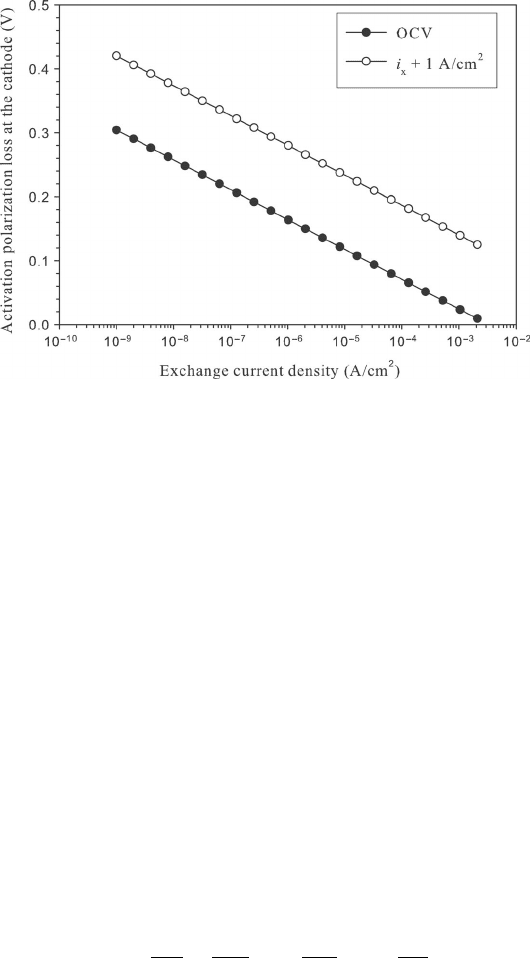
Example 4.12 Calculating Crossover Losses In ref. [9], the authors noted a hydrogen
crossover loss of 3.3 mA/cm
2
for their automotive H
2
PEFC applications. Calculate the mass
crossover rate of hydrogen through the membrane. Also, calculate and plot the cathode
activation overpotential loss at open circuit and 1 A/cm
2
as a function of cathodic exchange
current density. Assume the cathodic charge transfer coefficient at the cathode is 1.5 at a
temperature of 353 K, and the fuel cell has a 50 cm
2
geometric area.
SOLUTION The mass flux of hydrogen can be calculated from Faraday’s law and a unit
conversion using the molecular weight of hydrogen. Note the need to keep consistent units
of current density in the expression:
n
x
=
i
x
A
nF
=
3.3/1000 × 50
2 × 96,485
= 8.55 × 10
−7
mol/s
To convert to mass crossover rate, we use the MW:
˙
m
x
= n
x
· MW
H
2
= (8.55 × 10
−7
mol/s)(2 g/mol = 1.71 × 10
−6
g/s
We can assume Tafel kinetics at the cathode; then
η
a,c
=−
R
u
T
α
c
F
ln
i + i
x
i
o,c
=−0.0203 ln
i + 0.0033
i
o,c
At OCV this becomes
η
a,c
=−
R
u
T
α
c
F
ln
i
x
i
o,c
=−0.0203 ln
0.0033
i
o,c
c04 JWPR067-Mench January 28, 2008 17:28 Char Count=
4.6 Polarization Curve Model Summary 181
Plotting the activation losses as a function of cathodic exchange current density, we find:
COMMENTS: Even a very small crossover results in an extremely significant polarization
loss near an open circuit. In the PEFC at low temperatures, 0.1–0.2 V is typically sacrificed
to crossover. At high current density, the added effect of the crossover is minimal, though,
as i >> i
x
.
4.6 POLARIZATION CURVE MODEL SUMMARY
Returning again to our overall model, we now have a complete representation of the
polarization curve. If we know several key paramaters relating to the kinetic, ohmic, and
mass transfer processes, we can predict the overall polarization curve of the fuel cell.
Much more complex models exist in the literature to cover multidimensional, multiphase,
and transient aspects as well as approach the problem from various length scales from
molecular to full-size stack simulation. However, the approach taken here does include the
most important physicochemical phenomena that affect fuel cell performance:
E
cell
= E
◦
(T , P) − η
a,a
−
η
a,c
− η
r
− η
m,a
−
η
m,c
− η
x
(4.102)
Extension of these relations into multi-dimensional is a matter of additional mathematics,
not fundamental understanding.
From Chapter 3
E
◦
(T , P) =−
G
nF
+
R
u
T
nF
ln
P
fuel
P
∗
fuel
υ
fuel
P
O
2
P
∗
O
2
υ
oxidizer
(4.103)
The anode and cathode activation polarization losses (η
a,a
and η
a,c
) for most fuel cell
reactions can be determined from Eq. (4.35), the BV equation or a simplified form [i.e.,
Eqs. (4.52)–(4.55)].

c04 JWPR067-Mench January 28, 2008 17:28 Char Count=
182 Performance Characterization of Fuel Cell Systems
The ohmic polarization, η
r
can be determined from Eq. (4.71), including contact, ionic,
and electronic resistances.
The concentration polarization values, η
m,a
and η
m,c
from Eq. (4.86), and the crossover
losses can be included by adding either the fuel crossover current density to the cathode
current (mass transfer) or an internal short resistor for the case of a mixed conductivity in
the electrolyte.
We still cannot fully investigate the concentration polarization without the tools of
Chapter 5, which will allow us to predict the mass transfer limiting current density.
Alternative Simplified Empirical Model The complexity of the model shown requires
calculation or estimation of many parameters that are themselves functions of operating
conditions. Additionally, due to the many complexities and material, geometry, fluid mani-
folding, and so on, not included in our bulk model, the calculated results often have a large
deviation from experimental results. As stated, much more complex computational models
exist, but even these have significant deviations from measured results because not every
phenomenon can be accurately modeled and many of the transport parameters needed still
have significant uncertainty in their values. It must be emphasized that the basic model
presented in this chapter is a mere starting point and is not intended as a quantitative or
exact solution for all operating fuel cells. Instead, it serves to promote understanding of the
underlying physicochemical phenomena that control performance, so that an understanding
of the engineering trade-offs in design optimization can be achieved based on the qualitative
trends predicted.
Given the error and experimental effort associated with estimation of all the parameters
in the analytical model and the complexity and time required for computational simulation,
which, ultimately, still relies on the accuracy of the input parameters, there is a need for
an entirely empirical approach to quickly characterize performance of fuel cells and stack
designs. The following semiempirical model can be used to quickly glean some comparative
information:
E
cell
= E
OCV
− A ln
(
i
)
− iR− B ln
1 −
i
i
l
(4.104)
or, using Eq. (4.90) [20],
E
cell
= E
OCV
− A ln
(
i
)
− iR− m exp
(
ni
)
(4.105)
where A, R, B, i
l
, m, and n, are parameters taken from curve-fits of experimental data.
Each form has fit parameters for activation, ohmic, and concentration polarizations. For
the OCV, an empirical equation or constant can be used based on experimental data. This
approach is useful to quickly model real fuel cell performance, and by comparison with
other similar curve its, the relative impact of the ohmic, concentration, and activation
polarizations between different designs can be compared.
Example 4.13 Predicting Change in Polarization Curve Based on our understanding of
fuel cell polarizations, we can now diagnose and predict basic polarization curve behavior
as a function of the relevant parameters. We should be careful not to generalize too much,
as many other minor effects and differently combined variables can lead to similar bulk
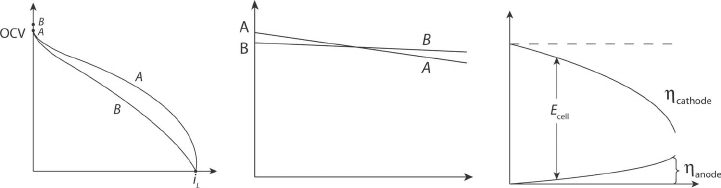
c04 JWPR067-Mench January 28, 2008 17:28 Char Count=
4.7 Summary 183
polarization curve results. Nevertheless, at this stage basic conclusions and predictions can
be made based on the understanding of this chapter:
(a) Sketch a typical hydrogen PEFC polarization curve with electrolyte A. Then,
sketch a polarization curve with the same operating conditions, but with a thicker
electrolyte B. Be sure to think about all of the effects of this change.
(b) Sketch a typical high-temperature fuel cell (SOFC, MCFC) polarization curve
operating at temperature A. Then, sketch a polarization curve B with elevated
temperature conditions. Be sure to think about all of the effects of this change.
(Ignore the effects on mass transport region until Chapter 5.)
(c) Sketch the shape of the anode and cathode electrode polarizations versus current
for the hydrogen PEFC.
SOLUTION
COMMENTS: When thinking about changes in operating conditions, the effects on all the
regions of the polarization must be considered. Some of these we cannot yet fully understand
(without Chapter 5, that is). Note that in many cases what seems like a completely positive
effect has other limitations that require an engineering optimization.
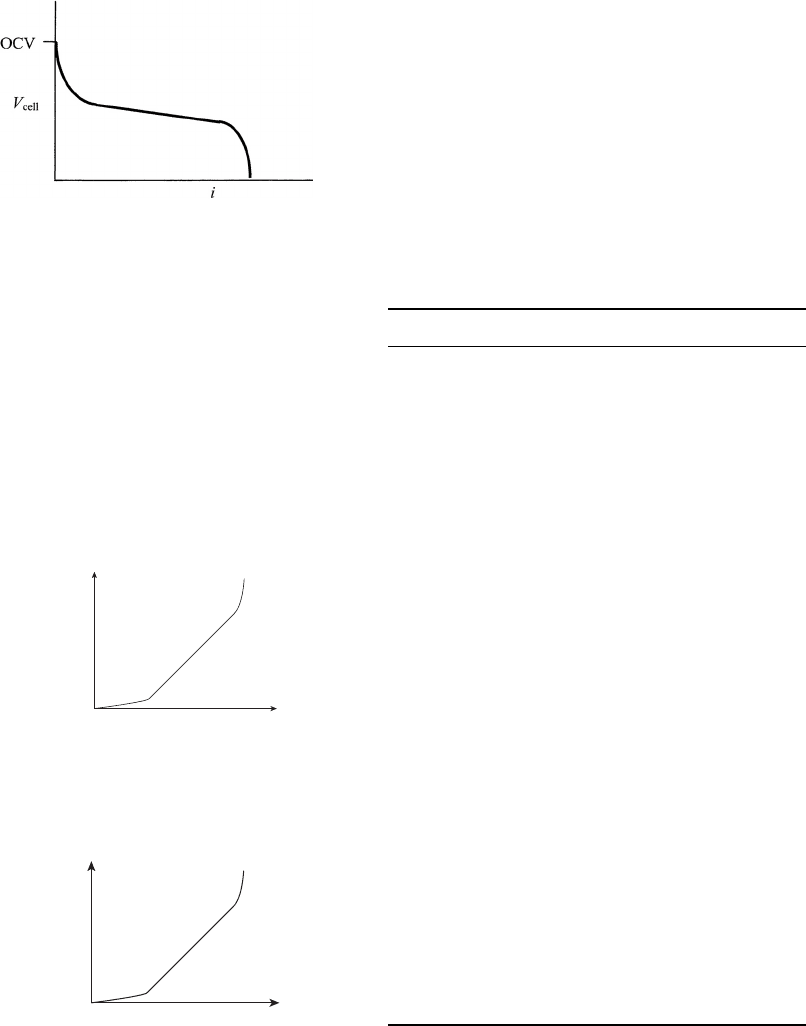
4.7 SUMMARY
This chapter presents a fundamental background in the various polarization losses on a fuel
cell system. The main figure of merit for a fuel cell system is the polarization curve, shown in
Figure 4.1. Starting at the maximum (unobtainable) thermal voltage E
◦◦
, the potential is re-
duced to the predicted Nernst open circuit potential due to entropy and concentration effects.
From this initial maximum possible starting point, four main modes of polarization occur:
1. Activation polarization dominates at low current density and occurs at each elec-
trode.
2. Ohmic polarization occurs throughout the polarization curve as a result of ionic
and electronic bulk and contact resistance through all components of the fuel cell,
but typically is dominated by the electrolyte ionic transfer resistance.
3. Concentration polarization dominates at high current density and occurs at each
electrode.
4. Crossover or mixed-conductivity polarization is mainly responsible for the depar-
ture from the expected Nernst potential at open-circuit conditions.
c04 JWPR067-Mench January 28, 2008 17:28 Char Count=
184 Performance Characterization of Fuel Cell Systems
The activation polarization is commonly modeled with the BV expression, which
assumes the electrochemical reaction is rate limited by a charge transfer process:
i
cell
= i
o
exp
α
a
F
R
u
T
η
− exp
−α
c
F
R
u
T
η
Several simplifications that allow explicit calculation of the activation overpotential are
possible from this expression, including:
1. Tafel kinetics for high polarization (branch ratio 10 : 1):
η =±
R
u
T
α
j
F
ln
i
i
o
2. Linerarized kinetics for low polarization (α
i
Fη/R
u
T < 0.15):
η =
i
i
o
R
u
T
(
α
a
+ α
c
)
F
=
i
i
o
R
u
T
nF
3. A sinh simplification for the case where the anodic and cathodic charge transfer
coefficients are equal:
η =
R
u
T
αF
sinh
−1
i
cell
2i
o
In electrochemical reactions, the dominating kinetic parameter is the exchange current
density i
o
, which is a strong function of temperature and reactant concentration. The
exchange current density is not an intrinsic parameter of a catalyst and is also a strong
function of the morphology of the electrode structure:
i
o
(T , C
R
) = i
o,ref
exp
−
E
a
R
u
T
C
R
C
R,ref
γ
The ohmic polarization loss can be represented symbolically as
n
r
= iA
n
k=1
r
k
where the summation is performed over the contacts and bulk ionic and electronic resis-
tances of all components. The losses are typically dominated by ionic resistance in the
electrolyte, which is discussed in Chapter 5.
The concentration polarization is a result of decreasing surface concentration of reac-
tant. The restriction can be a result of gas-phase transport limits, impurity absorption at the
catalyst surface, liquid blockage in low-temperature fuel cells, or other reasons. The ther-
modynamic (Nernst) and kinetic concentration polarization at an electrode can be written
as
η
m
=−B ln
1 −
i
i
l
c04 JWPR067-Mench January 28, 2008 17:28 Char Count=
Application Study: Polarization Curve Losses 185
where B is an empirical constant and i
l
is the mass transfer limiting current density at that
electrode. Another approach is to use an empirically derived expression:
η
m
= m exp
(
ni
)
The departure from Nernst OCV is a result of reactant crossover through the electrode
and mixed reaction potential, or electrical shorts from mixed electrolyte conductivity. For
most fuel cell systems, the major concern is reactant crossover, although high-temperature
SOFC systems suffer from some electrical conductivity in the electrolyte. For the mass
crossover case, the effect can be modeled by inclusion of the crossover current density
with the fuel cell current density activation overpotential at the electrode with the mixed
potential reaction.
The various polarization losses are summarized in the following equation for fuel cell
voltage as a function of current density:
E
cell
= E
◦
(T , P) − η
a,a
−
η
a,c
− η
r
− η
m,a
−
η
m,c
− η
x
where E
◦
(T, P) is solved from the Nernst equation.
APPLICATION STUDY: POLARIZATION CURVE LOSSES
Now that this chapter is completed, we have some real analytical tools to work with to
describe fuel cells in operation. The methods shown in the chapter can be applied to
any fuel cell system, given appropriate transport and electrochemical parameters. First,
the empirical method can be used to curve-fit any experimental polarization curve and to
understand a little about the general performance of a fuel cell. The basic analytical model
we described can be applied to more fundamentally understand the roles of particular types
of materials and geometry on the performance, provided all of the unknown quantities can
be properly fit or estimated from existing resources. Fortunately, there are a lot of existing
data available that are continuously updated in technical journals, which can be used as a
starting point for evaluation of polarization behavior. For this assignment:
(a) Find a recent fuel cell technical journal article or publication with some experi-
mental polarization curves in it at different experimental conditions for either a
fuel cell or stack. Perform an empirical curve fit of the form of Eq. (4.105). Find a
best-fit kinetic, ohmic, and mass transfer parameter fit.
(b) Try to estimate (or use vales provided) for a more complete analytical model from
this chapter. Use given parameters where available and reasonable assumptions or
approximations where there is not enough information.
(c) Use the results of parts A and B to estimate fuel cell performance at different
operating conditions. Discuss how reasonable the results are and what would be
needed to have a more accurate predictive tool.
Some good journals to refere to are the Journal of Power Sources, Journal of the Electro-
chemical Society, Electrochimica Acta, and others your professor can provide. Your school

c04 JWPR067-Mench January 28, 2008 17:28 Char Count=
186 Performance Characterization of Fuel Cell Systems
library likely has online access to some of these resources or can get the relevant texts
for you. The websites of these journals provide free topical searches and abstracts to get
you started. In lieu of these data, you can also use the data provided in the table below
for a PEFC operating at 65
◦
C, 1 atm back pressure, 100% RH inlets, anode and cathode
stoichiometry of 1.5 and 2.0, respectively.
Current Density (A/cm
2
) Voltage (V)
0.0000 0.9317
0.0539 0.8493
0.1817 0.7995
0.4129 0.7490
0.6944 0.6992
0.9132 0.6493
1.0850 0.5989
1.2894 0.4993
1.4452 0.3988
1.5612 0.2990
1.6509 0.1987
1.7300 0.0000
PROBLEMS
Calculation/Short Answer Problems
4.1 Draw a typical polarization curve and label the regions
dominated by activation polarization (A), ohmic polariza-
tion (B), and concentration polarization (C). Also label the
specific locations of the OCV (D), Mass-limiting current
density i
l
(E), heat-generating region (F), and useful elec-
tric region (G). Discuss the factors that result in the various
polarizations (e.g., the catalyst choice has a strong effect on
the activation region, as do many other factors).
4.2
(a) Consider a fuel cell stack with poor overall com-
pression. What region of the polarization curve
would be most affected?
(b) Consider a poor catalyst layer. What region of the
polarization curve would be most affected?
(c) Consider the electrolyte becomes very dry in a
PEFC and the ionic conductivity decreases. What
region of the polarization curve would be most
affected?
4.3 Describe what is happening when a low-temperature
H
2
–O
2
PEM fuel cell is “flooded” and what region of the
polarization curve in Problem 4.1 (A, B,orC) would be
most affected?
4.4 Explain why the operating stoichiometry of a fuel cell
reactant can never be equal to or less than 1 (without recy-
cling effluent gas).
4.5 Consider a hydrogen fuel cell with 80% H
2
and 20%
water vapor in the anode at 2 atm total pressure. If the hy-
drogen mole fraction is reduced to 70% because the water
content has increased to 30%, what must your anode total
pressure be to offset the effect of the increased water con-
tent on expected OCV? Consider Nernst and kinetic effects
with a hydrogen reaction order of 1.
4.6 Fill out the plot for a typical PEFC. The plot is of fuel
cell voltage at a given steady-state current versus compres-
sion pressure:
c04 JWPR067-Mench January 28, 2008 17:28 Char Count=
Problems 187
4.7 Consider the following typical polarization curve for
a fuel cell. Be careful to consider all the effects of what the
question poses!
(a) Consider the case where we have half the thickness
of the electrolyte. Draw the expected polarization
curve. Label the curve A.
(b) Consider the case where we increase the exchange
current density of both electrodes. Draw the ex-
pected polarization curve. Label the curve B.
(c) Consider the case when we increase the tempera-
ture of operation in reference to the baseline case
already drawn. Label the curve C.
4.8 Consider the polarization curve of an electrode below.
If we decrease the activity of this electrode by using a worse
catalyst, what will the same curve look like? Redraw the
curve below with a worse catalyst.
log i
η
activation
4.9 On the single-electrode polarization plot below indi-
cate the regions where (a) Butler–Volmer kinetics, (b) Tafel
kinetics, (c) the sinh solution, and (d) facile kinetics (lin-
earized Butler–Volmer) apply.
log i
η
activation
4.10 Consider a hydrogen fuel cell with 75% N
2
, 19%
oxygen, and 6% H
2
O in the cathode at 1 atm total pressure.
If the oxygen mole fraction is reduced because water vapor
has been added to the cathode (new balance 15% O
2
, 25%
H
2
O, and 60% N
2
), the expected OCV will fall. To counter
this loss, you suggest changing the cathode pressure. To
what value must your cathode total pressure be changed to
in order to maintain the OCV? Conditions on the anode are
not to be changed.
4.11 Given the table below of parameters for a 50-cm
2
-
active-area fuel cell, solve the following:
(a) Using the appropriate kinetics, calculate the ca-
thodic activation overpotential η
a,c
at 0.8 A/cm
2
.
(b) Calculate the total ohmic overpotential η
R
at 0.5
A/cm
2
, including contact resistance.
Parameter Value Unit
T
cell
340 K
Ionomer fraction, anode 0.4 —
Ionomer fraction, cathode 0.3 —
Anode thickness 30 µm
Cathode thickness 20 µm
Average electrolyte
conductivity
7.0 S/m
Electrolyte thickness 50 µm
Anode GDL thickness 250 µm
Cathode GDL thickness 300 µm
α
a
(for anode) 0.6 —
α
c
(for anode) Solve —
α
a
(for cathode) Solve —
α
c
(for cathode) 0.4 —
E(T,P) 1.15 V
Anode GDL porosity 0.3 —
Cathode GDL porosity 0.4 —
Anode i
o
1.5 A/cm
2
Cathode i
o
5.0 × 10
−4
A/cm
2
Anode pressure 101,325 Pa
Cathode pressure 300,000 Pa
B (replaced RT/nF in
concentration loss)
0.05 V
n anode elementary reaction 1 —
n cathode elementary reaction 2 —
R contact, total for cell 35 m·cm
2
Landing to channel width ratio 1:1 Unitless
I
n
(crossover) 3.0 mA/cm
2
g
O
2
average mole fraction 0.15 Unitless
y
H
2
average mole fraction 0.75 Unitless

c04 JWPR067-Mench January 28, 2008 17:28 Char Count=
188 Performance Characterization of Fuel Cell Systems
4.12 Do you expect the voltage of a hydrogen–air fuel
cell to increase, decrease, or stay the same if the anode and
cathode pressures are both doubled.
4.13 Given the table of parameters included in problem 11
for a 100-cm
2
-active-area fuel cell, determine the follow-
ing:
(a) Using the appropriate kinetics, calculate the an-
odic activation overpotential η
a,a
at 0.5 A/cm
2
.
(b) Using the appropriate kinetics, calculate the ca-
thodic activation overpotential η
a,c
at 0.5 A/cm
2
.
(c) Calculate the total ohmic overpotential η
R
at 0.5
A/cm
2
.
4.14
(a) Discuss under what conditions the Butler–Volmer
kinetics model is valid.
(b) Discuss under what conditions the Tafel kinetics
model is valid.
(c) Discuss under what conditions the linearized
Butler–Volmer model is valid.
(d) Discuss under what conditions the sinh solution to
the Butler–Volmer model is valid.
(e) Define and discuss the meaning of the charge
transfer coefficient.
(f) Define the exchange current density and list what
factors affect it.
(g) Define and discuss the basic assumptions of the
Temkin and Langmuir kinetics model in contrast
to the Butler–Volmer model.
4.15 Consider you are designing a 50-cm
2
-active-area
hydrogen–oxygen PEM fuel cell system to operate at a
nominal current density of 1.2 A/cm
2
at 100
◦
C. The current
collector landing–channel width ratio is 1 : 2 on the anode
and cathode sides.
(a) In order to enhance power output, you would like
to increase operating pressure from 1 to 3 atm
on the cathode with 1 atm constant on the an-
ode. However, in doing so, the contact resistance
between the cathode and gas diffusion layer in-
creases by 30 m·cm
2
. What is the net effect of
changing the anode from 1 to 3 atm pressure on
voltage at 1.2 A/cm
2
?
(b) Determine the operating current density at which
the positive effect of the pressure increase is ex-
actly offset by the negative impact from the in-
creased contact resistance.
4.16 Consider a fuel cell with 100 cm
2
superficial active
area and a measured contact resistance of 20 m · cm
2
at
the anode and 10 m · cm
2
at the cathode. Consider the
Nafion 115 (0.005-in.thick) electrolyte as fully saturated
with moisture everywhere. Consider the anode and cathode
electrodes to be 20 and 30 µm thick, respectively, and 25
and 30% equivalent ionomer fraction. Find the fraction of
the total potential power lost at 1.2 A/cm
2
current density
from ohmic losses if E
◦
=1.25 V. Since the ionic resistance
is much greater than the electronic resistance, you may
neglect the electronic resistance. Assume the area ratio is
1 : 1.3 between the landing and channel.
4.17 In Equation (4.96), it was shown that
E
OCV
= E
(
T, P
)
Nernst
×
σ
i
σ
i
+ σ
i
= E
◦
(
T, P
)
× t
i
Derive this relationship. Recall it is for open-circuit con-
ditions where the ionic and electronic current exchange is
equivalent. That is, i
e
= i
i
.
4.18 Sketch a typical hydrogen PEFC polarization curve
with electrolyte A. Then, sketch a polarization curve with
the same operating conditions, but with a thicker electrolyte
B. Be sure to think about all of the effects of this change.
4.19 Sketch a typical high-temperature fuel cell (SOFC,
MCFC) polarization curve operating at temperature A.
Then, sketch a polarization curve B with reduced temper-
ature conditions. Be sure to think about all of the effects
of this change. (Ignore the effects on mass transport region
until Chapter 5.)
4.20 Sketch the shape of the anode and cathode electrode
polarizations versus current for the hydrogen PEFC on the
same plot as a DMFC.
4.21 Using total kinetics, plot the ORR over potential ver-
sus current density for a PEFC with a PT-C catalyst at 300 K,
1 atm for roughness factors of 1 (smooth electrode), 500,
and 2000.
Computer Problems
4.22 Using a spreadsheet or other computer program, put
together a basic fuel cell model program for a chosen fuel
cell system that includes the various terms and parameters
discussed in this chapter for the activation, ohmic, con-
centration, and crossover polarization losses. For now, use
reasonable parameters for the unknown quantities based
on the examples in this chapter. After Chapters 5–7, more
specific parameters for a given fuel cell can be added.
c04 JWPR067-Mench January 28, 2008 17:28 Char Count=
References 189
(a) Select appropriate anodic and cathodic kinetics
based on the fuel cell chosen and plot the acti-
vation polarization for each electrode as a func-
tion of current density for each electrode. Check
your code by a hand calculation at a given cur-
rent density. The act of checking the computed
values with hand calculations or exact analyti-
cal solutions is called model verification and is
a necessary step every time a computer model is
used.
(b) Plot the ohmic polarization as a function of current
density. Check your code by a hand calculation at
a given current density.
(c) Plot the concentration polarization as a function
of current density for both electrodes. Check your
code by a hand calculation at a given current den-
sity.
(d) Now add crossover to the calculation and show a
plot of the OCV as a function of crossover flux.
Check your code by a hand calculation at a given
current density.
(e) Plot the polarization curve for this fuel cell.
4.23 Using a spreadsheet or other computer program, find
a polarization curve for a given fuel cell from the litera-
ture and curve fit the results using the empirical fuel cell
model. Discuss the meaning of the mass transfer, kinetic,
and ohmic parameters. How could this model be expanded
to use for other test conditions? Hint: Your professor can
help you find some typical polarization curves to use.
REFERENCES
1. A. J. Bard and L. R. Falkner, Electrochemical Methods, Fundamentals and Applications, 2nd
ed., Wiley, New York, 2001.
2. T. E. Springer, T. A. Zawodzinski, M. S. Wilson, and S. Gottesfeld, “Characterization of Polymer
Electrolyte Fuel Cells Using AC Impedance Spectroscopy,” J. Electrochem. Soc., Vol. 143, No.
2, pp. 587–599, 1996.
3. P. M. Gomadam and J. W. Weidner, “Analysis of Electrochemical Impedance Spectroscopy in
Proton Exchange Membrane Fuel Cells,” Int. J. Energy Res., Vol. 29, pp. 1133–1151, 2005.
4. R. S. Berry, S. A. Rice, and J. Ross, Physical Chemistry, Oxford University Press, New York,
2000.
5. K. Eguchi, “Internal Reforming,” In Handbook of Fuel Cells—Fundamentals, Technology and
Applications, Vol. 4, W. Vielstich, A. Lamm, and H. A. Gasteiger, Eds., Wiley, New York, 2003,
pp. 1057–1069.
6. X. Li, Principles of Fuel Cells, Taylor and Francis Group, New York, 2006.
7. J. O’M. Bokris and S. Srinivasan, Fuel Cells: Their Electrochemistry, McGraw-Hill, New York,
1969.
8. S. H. Chan, K. A. Khor, and Z. T. Xia, J. Power Sources, Vol. 93, pp. 130–140, 2001.
9. H. A. Gasteiger, W. Gu, R. Makharia, M. F. Mathias, and B. Sompalli, “Beginning-of-Life
MEA Performance—Efficiency Loss Contributions,” In Handbook of Fuel Cells—Fundamentals,
Technology and Applications, Vol. 3, W. Vielstich, A. Lamm, and H. A. Gasteiger, Eds., Wiley,
New York, 2003, pp. 593–610.
10. J. Tafel, Z. Physik. Chem., Vol. 50A, p. 641, 1905.
11. T. E. Springer, T. Rockward, T.A. Zawodzinski, and S. Gottesfeld, “Model for Polymer Electrolyte
Fuel Cell Operation on Reformate Feed—Effects of CO, H2 Dilution, and High Fuel Utilization,”
J E lectrochem. Soc., Vol. 148, No. 1, pp. A11–A23, 2001.
12. E. Gileadi, Electrode Kinetics for Chemists, Chemical Engineers and Materials Scientists, Wiley-
VCH, New York, NY, 1993.
13. S. Park, R. J. Gorte and J. M. Vohs, “Applications of heterogeneous catalysis in the di-
rect oxidation of hydrocarbons in a solid-oxide fuel cell,” Appl. Catal., A, Vol. 200, p. 55,
2000.
