Mench M.M. Fuel Cell Engines
Подождите немного. Документ загружается.


c04 JWPR067-Mench January 28, 2008 17:28 Char Count=
170 Performance Characterization of Fuel Cell Systems
Porous diffusion layer
Catalyst layer electrode
Channel
Land Land
iA
nF
Suction of
into electrode
iA
nF
Figure 4.34 Schematic of channel flow suction into catalyst layer of fuel cell.
Equations (4.81) and (4.83) can be used for any fuel and oxidizer reaction with different
reaction orders.
Considering flow through a fuel cell channel, there is a flux of products away from
the catalyst, a suction of reactants to the catalyst surface due to reaction, and inert species
not participating in the reaction, as shown in Figure 4.34. At higher current densities,
the mass transport limitations can reduce the concentration of the reactants at the catalyst
surface to well below the flow field channel concentration and cause a sharp decline in the
output voltage. This concentration loss can be precipitous and corresponds to (region III in
Figure 4.1). This region of the polarization curve is really a combined thermodynamic- and
kinetic-related phenomenon that occurs independently at both electrodes. To determine an
expression for η
m,a
and η
m,c,
the mass concentration polarizations at each electrode, consider
Eqs. (4.81) and (4.83). From these, a general form of the voltage change at an electrode for
concentration changes in reactant R from some state 2 to state 1 can be written as
V
C
2
−C
1
= η
c
= V
C
2
− V
C
1
=
R
u
T
(
n + γ
)
F
ln
C
R,s,2
C
R,s,1
(4.84)
Example 4.10 Kinetic and Thermodynamic Effect of Change in Oxygen Concentration
Determine the change in voltage expected for an increase in oxygen mole fraction from a
fully humidified state at 90
◦
C, 1.5 atm, to a fully humidified state at 70
◦
C, 1.5 atm pressure.
Assume an ORR order γ of 0.75. For this example, ignore changes in exchange current
density with temperature.
SOLUTION In this example, we are considering increasing the operating temperature
of the fuel cell at constant relative humidity. This will have a positive effect in terms of
the exchange current density (ignored here) but will also decrease the available oxygen
mole fraction since the saturation pressure is strongly related to temperature, as we have
discussed in Chapter 3. First we need to calculate the mole fraction of oxygen in both cases.
From Chapter 3
P
sat
(T )(Pa) =−2846.4 + 411.24 T (
◦
C) − 10.554 T (
◦
C)
2
+ 0.16636 T (
◦
C)
3
Evaluation gives P
sat
(70) and P
sat
(90) = 0.31 and 0.69 atm, respectively.
From
RH =
P
v
P
sat
(
T
)
=
y
v
P
total
P
sat
(
T
)
we find that
y
v
(
70
)
=
1 × 0.31
1.5
= 0.21 y
v
(
90
)
=
1 × 0.69
1.5
= 0.46

c04 JWPR067-Mench January 28, 2008 17:28 Char Count=
4.4 Region III: Concentration Polarization 171
Next we need to covert the vapor mole fraction to the oxygen mole fraction. The ratio of
oxygen to nitrogen in dry air is constant in humidified air at y
O
2
,dry
= 0.21. For the moist
air at 70
◦
C
y
air,70
= 1 − y
v,70
= 0.79 y
O
2
= 0.21y
air
= 0.166
For the moist air at 90
◦
C
y
air,90
= 1 − y
v,90
= 0.54 y
O
2
= 0.21y
air
= 0.113
From simplification of Eq. (4.84) at 70 and 90
◦
C, with γ = 0.75, we have
V
70−90
=
R
u
T
F
ln
C
o
2
,70
C
o
2
,90
Finally:
V
70
− V
90
=
R
u
T
F
ln
(
0.166
)
ln
(
0.113
)
=
R
u
F
[
343 ln
(
0.166
)
− 363 ln
(
0.113
)
]
= 15 mV
So decreasing the temperature from 90 to 70
◦
C actually increases the expected voltage by
15 mV for a fully humidified system because the oxygen mole fraction will increase. There
will also be an expected decrease in the exchange current density which can partially or
completely offset this trend. However, this example illustrates another issue with operating
PEFCs at elevated temperatures where the oxygen mole fraction in a moist system is
decreased.
COMMENTS: High-temperature PEFC operation has several disadvantages, includ-
ing: (a) electrolyte material limitations (conventional electrolyte materials are limited to
<120
◦
C), (b) reduced oxygen mole fraction for humidified conditions, and (c) need for
larger humidification system. The ideal operating temperature depends on the trade-offs
between enhanced kinetics and decreased reactant concentration, humidification require-
ments, and longevity. For high-temperature fuel cell operation, an elevated pressure some-
times is preferred since it reduces the oxygen mole fraction decrease with temperature for
humidified flow.
Mass-Limiting Current Density We previously examined ohmic limiting current density.
Now we consider the case of mass transfer limiting current density i
l
. At the mass transport
limiting current density, the rate of mass transport to the reactant surface is insufficient to
promote the rate of consumption required for reaction. In this case, the local concentration
of reactant will be reduced to zero, which, from Eq. (4.84), must also reduce the cell
voltage to zero.
3
Assuming the surface concentration (C
R
) is zero at the limiting state (i
l
)
3
Small localized areas of fuel or oxidizer starvation in the fuel cell can exist, but if a significant area is depleted
of fuel or oxidizer, the local voltage potential is reduced to zero, and since the catalyst layers are conductive, the
voltage of the entire cell will be reduced to zero. Thus, when we operate a fuel cell, the flow stoichiometry of the
anode and cathode must always be greater than unity to enable operation at sufficient voltage.
c04 JWPR067-Mench January 28, 2008 17:28 Char Count=
172 Performance Characterization of Fuel Cell Systems
and decreases linearly from state 1 to state 2, we can show that
C
R,s,2
= C
R,s,1
− C
R,s,1
i
i
l
= C
R,s,1
1 −
i
i
l
(4.85)
which we can plug into Eq. (4.84) and show that
V
C
2
−C
1
= η
m
=−
R
u
T
(
n + γ
)
F
ln
1 −
i
i
l
(4.86)
If we assume the reaction occurs only at the catalyst layer interface, which is true for
high-current-density mass-transport-limited reactions, and we neglect kinetic effects, we
are left with the Nernst equation at each electrode:
η
m
=−
R
u
T
nF
ln
1 −
i
i
l
(4.87)
In practice, the concentration loss is more gradually observed than predicted by Eqs.
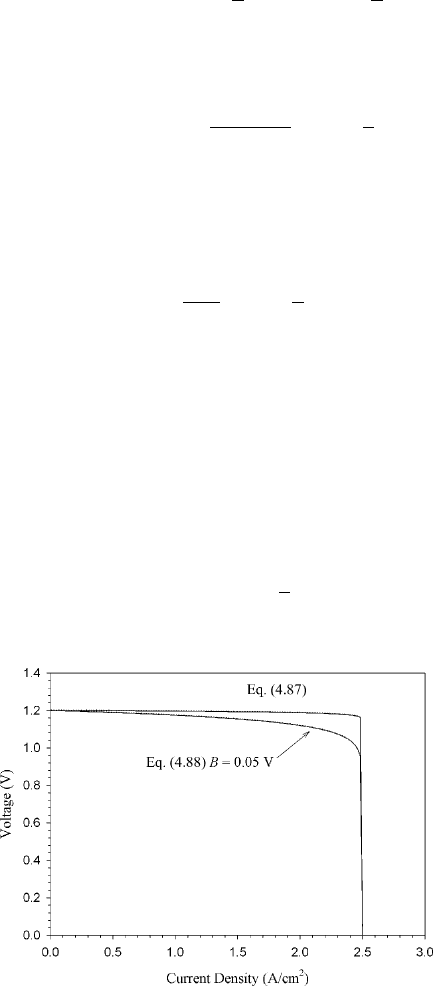
(4.87) or (4.86), but the qualitative result of the equation holds (see Figure 4.35). The
deviation from predicted values is a result of the dependence of the kinetics on reactant
concentration, the accumulation of liquid water or the accumulation of inert species such as
nitrogen, which cannot be easily predicted without inclusion of fuel cell geometry, material,
and other parameters. To account for this deviation, a semiempirical approach is often used
where Eq. (4.87) is changed slightly to include a constant (B) to better fit with experimental
data:
η
m
=−B ln
1 −
i
i
l
(4.88)
Figure 4.35 Comparison of concentration polarization predicted with Nernst equation and that
predicted with semiempirical modification with B factor of 0.05 V at 353 K and i
l
= 2.5 A/cm
2
.

c04 JWPR067-Mench January 28, 2008 17:28 Char Count=
4.4 Region III: Concentration Polarization 173
Therefore, the total concentration polarization of the fuel cell can be written as
η
m,a
+ η
m,c
=−B
a
ln
1 −
i
i
l,a
− B
c
ln
1 −
i
i
l,c
(4.89)
Each electrode will have this loss (and a separate limiting current density), although the
anode loss is typically negligible for a hydrogen feed due to the high concentrations
and mass diffusivity, so that a single expression is often used to represent concentration
polarization. If the anode contribution to the concentration and activation polarization is
ignored, little error would result for air–H
2
systems. This is not the case for fuel cells
without neat hydrogen as fuel or with significant dilution or impurities in the anode side,
such as when the hydrogen gas from a fuel reformer is used. It is important to understand
that each electrode has a different mass transport limiting current density, which can be
approximated with the concepts discussed in Chapter 5. However, the minimum value of
limiting current density between the electrodes will be the determining value on the fuel
cell polarization curve.
Example 4.11 Determine Concentration Polarization Given the anodic mass-transport-
limited current density is 15 A/cm
2
and the cathode mass-transport-limited current density
is 2.5 A/cm
2
, determine the anode and cathode concentration polarization at 0.1 and 1.0
A/cm
2
. Assume the B factor is 0.045 V on both electrodes and Eq. (4.88) is appropriate and
is determined from curve-fit of several polarization curves.
SOLUTION On the anode
η
m,a
=−0.045 ln
1 −
i
15
which is 0.3 and 3.1 mV for 0.1 and 1.0 A/cm
2
, respectively. On the cathode
η
m,c
=−0.045 ln
1 −
i
2.5
which is 1.8 and 23.0 mV for 0.1 and 1.0 A/cm
2
, respectively.
COMMENTS: The fuel cell maximum current density would be limited by the cathode
i
l
in this case. It would also be possible that the maximum current density is limited by
kinetic or ohmic effects as well.
Alternate Empirical Approach Another completely empirical approach to describe the
overall fuel cell concentration polarization has been proposed [18–20]:
η
m
= m exp
(
ni
)
(4.90)
If this equation is used, the constants m and n are typically fit from several polarization
curves, and the total (anode +cathode) concentration polarization is included in this single
expression. According to [20], typical values of the m constant are around 3 × 10
−5
V,
and the n constant is around 8 × 10
−3
cm
2
/mA for a PEFC. Although this expression
completely loses physical meaning, it can be used to simply model the complex fuel cell
stack mass transport limitations if plentiful polarization curve data are available.
c04 JWPR067-Mench January 28, 2008 17:28 Char Count=
174 Performance Characterization of Fuel Cell Systems
Functionally, the mass-limiting current density at each electrode depends on:
1. Fuel cell design (discussed in other chapters for various fuel cells)
2. Operating conditions and stoichiometry
3. Mass transport to the catalyst layer (discussed in Chapter 5)
(a) Thickness and porosity of the diffusion media (PEFC and AFC) and catalyst
layer
(b) Transport mode to electrode (diffusion, forced convection)
(c) free-stream concentration, temperature, pressure
4. Impurities/blockage/water accumulation/flooding or liquid electrolyte can cover the
catalyst, hindering access to the catalyst
Flow Stoichiometry Until now, we have discussed the flow into a fuel cell and not
explored the fact that the concentration of reactant is depleted inside the fuel cell from con-
sumption of reactant. Flow comes into a fuel cell with a molar flow rate of reactant shown in
Chapter 2:
˙
n
in
= λ
iA
nF
(4.91)
The reactant consumed can be determined from Faraday’s law:
˙
n
consumed
=
iA
nF
(4.92)
Therefore the amount of reactant out of a fuel cell is
˙
n
in
−
˙
n
consumed
=
(
λ − 1
)
iA
nF
(4.93)
We can see now why the flow stoichiometry must always be greater than unity in a fuel
cell, since in a unity condition, the flow leaving the fuel cell would contain no reactant,
automatically assuring that the catalyst layer in this region would have no reactant and,
according to the Nernst equation, zero voltage. It is important to understand that the entire
fuel cell need not suffer reactant depletion. Since the fuel cell catalyst layer is electrically
conductive, any significant region devoid of reactant can induce zero fuel cell voltage for
a single cell. Even smaller, locally starved regions can reduce performance and accelerate
degradation. If the cell is in a stack, then the individual fuel cell in series with severe reactant
depletion can suffer voltage reversal. Voltage reversal can result in rapid degradation of
the catalyst and support, and therefore local reactant depletion should be avoided.
To avoid depletion, the minimum requirement is an inlet anode and cathode stoichiom-
etry greater than unity. In a combustion engine, this would be like having a requirement
that the engine have unburned gasoline and air in the exhaust. For both an internal com-
bustion engine and a fuel cell this is obviously wasteful. If not recycled, the fuel cell loses
the potential for the energy released by the reaction of the unreacted fuel and oxidizer, in
addition to being a possible safety risk if released to the environment. In fuel cell stacks,
the hydrogen or other fuel used is typically recycled back into the anode, which carries a
parasitic energy penalty. In the cathode, since air is usually used, recycling is not neccesary.

c04 JWPR067-Mench January 28, 2008 17:28 Char Count=
4.5 Region IV: Other Polarization Losses 175
Some fuel cell designs have attempted to boost fuel utilization and reduce system
parasitic losses with a dead-ended hydrogen fuel compartment. That is, the hydrogen
flow channels have no exit, and fuel is either continuously or sporadically supplied at the
consumption rate required for suitable performance. A major drawback of this approach
is that inerts and poisons in the flow stream build up in the dead end over time, and at
least periodic purging is needed. In low-temperature systems, liquid accumulation is also
a common problem, and some flow in the channels is beneficial to remove liquid droplet
accumulations [20].
Returning to our ultimate goal of being able to analytically describe the fundamental
physics of the polarization curve, we now have an expression that includes the starting
equilibrium voltage, the departure from this voltage resulting from activation overpotential
at each electrode, the ohmic polarization, and concentration polarization:
E
cell
= E
◦
(T , P) − η
a,a
−
η
a,c
− η
r
− η
m,a
−
η
m,c
Can now solve
−η
x
(4.94)
Only the crossover and shorting polarization losses need to be modeled, which are covered
in the next section.
4.5 REGION IV: OTHER POLARIZATION LOSSES
The final piece of the polarization curve to be modeled is the departure from the expected
OCR given by the Nernst equation. For low-temperature PEFCs, the OCV is predicted to be
around 1.2 V, but in practice, only about 1.0 V is observed. For a high-temperature SOFC,
however, the actual OCV can be very close to the theoretical OCV. For the PEFC, the 0.2 V
represents an incredibly significant efficiency loss before any useful current is even drawn.
The departure from the theoretical OCV is typically a result of two phenomena:
1. Electrical short circuits in the fuel cell
2. Crossover of reactants through the electrolyte and subsequent mixed-potential re-
action at the opposite electrode
Electrical Shorts Electrical short circuits can happen if the cell is poorly designed or
assembled or, more commonly, the electrolyte is not completely insulating for electrons. For
low-temperature fuel cells, this is usually not a major problem, but for higher temperature
fuel cells, especially SOFCs, the electrolyte phase can have a mixed conductivity for
electrons that is dependent on material properties, temperature, and oxygen partial pressure.
The transference number (t
i
) is the ratio of electrolyte ionic conductivity to the total
conductivity (ionic plus electronic), defined as [22]
t
i
=
σ
i
σ
i
+ σ
e
(4.95)
Obviously, a transference number close to unity is desired, and a value greater than 0.9
is found in conventional SOFCs. When a significant electrical conductivity exists in the

c04 JWPR067-Mench January 28, 2008 17:28 Char Count=
176 Performance Characterization of Fuel Cell Systems
electrolyte, current is short circuited through the electrolyte, causing a departure from the
Nernst potential at open-circuit conditions, as well as additional losses throughout the entire
polarization curve. At an open circuit, the current passed as a result of the electronic leakage
equals the ionic exchange, and the following equation can be developed:
E
OCV
= E
◦
(
T, P
)
Nernst
×
σ
i
σ
i
+ σ
e
= E
◦
(
T, P
)
× t
i
(4.96)
One goal of SOFC research is the development of electrolyte structures with high ionic
conductivity at low operating temperatures [22–26]. A problem with achieving this goal
has been the mixed conductivity of the electrolyte. Ironically, a long-term goal of PEFC
operation in to increase the operating temperature to provide tolerance to impurities and
better heat rejection, while a long-term goal of SOFCs is to reduce operating temperature
so that less expensive materials can be used. Phosphoric acid fuel cells do operate at an
intermediate temperature but have power density and cost limitations.
In fuel cells with coolant flow, ionic impurities which accumulate in the coolant over
time can also cause short currents through the coolant to develop.
Species Crossover In a high-temperature SOFC, the Nernst potential is reduced compared
to a low-temperature PEFC, as discussed in Chapter 3. However, the normally observed
OCV of a SOFC and a PEFC are similar, due to crossover losses in the PEFC. Crossover of
reactants through the electrolyte can be a major issue degrading the performance of a PEFC.
The 0.2-V loss typically suffered at the open circuit in a PEFC represents ∼20% efficiency
loss without a useful electron being drawn. In a DMFC, the effect is even more extreme,
since the liquid solution used as a fuel has a higher molecular density, thus crossing over
more readily. For the DMFC, an actual OCV of 0.7 V (∼1.2 V is predicted) is normally
observed due to this loss. Intensive research and development has been invested to lessen
the crossover effect in DMFCs while not sacrificing operating performance.
Physically, a large concentration gradient in the reactant between the anode and cath-
ode sides acts as a driving force for diffusion of reactants across the electrolyte that
compete with the opposing electrode reactions (Figure 4.36). Recall that with the Nernst
equation we establish the thermodynamic equilibrium potential between the two elec-
trodes. With crossover, the Nernst potential will be altered by slightly changing the surface
Anode Cathode
H
2
O
2
Thin-film
electrolyte
Figure 4.36 Reactant crossover in PEFC.

c04 JWPR067-Mench January 28, 2008 17:28 Char Count=
4.5 Region IV: Other Polarization Losses 177
concentrations at the catalyst surface, but this effect is negligible. The major effect of
crossover on OCV is due to kinetics. At the hydrogen electrode, the exchange current
density is typically orders of magnitude higher for the hydrogen electro-oxidation than for
any reaction with crossover oxygen, despite the high reduction potential (see Table 4.1).
Therefore, there is little interference from oxygen crossover at the anode, and a nearly true
equilibrium can be established. However, at the cathode, the hydrogen crossover typically
has a much higher exchange current density for oxidation than the ORR, which is very slow.
At the cathode, a true thermodynamic equilibrium cannot be established due to hydrogen
crossover oxidation reaction and relatively slower oxygen reduction kinetics, resulting in a
mixed reaction and lowered OCV. It should be noted that, although the oxygen crossover
to the anode has a small impact on the OCV departure, it does have an important impact
on PEFC durability, discussed in Chapter 6. Because of these reasons, we seek to eliminate
crossover of reactants in PEFCs and other fuel cells in general.
From concepts discussed in the next chapter, the crossover can be related to the
diffusion coefficient, thickness, and concentration gradient across the electrolyte through
the one-dimensional version of Fick’s law [27]:
n
− D
∂C
∂x
(4.97)
where D is a mass diffusivity coefficient, C is the molar concentration, and x is the length
scale in the direction of transport. Even a very small amount of reactant crossover can have
a large impact on OCV.
Several approaches have been used to limit reactant crossover:
Ĺ Change in Material Properties of Electrolyte If the PEFC electrolyte is made less
permeable to the reactants [e.g., D in Eq. (4.97) is reduced], crossover losses will be
reduced. However, this approach has the severe limitation of reducing the reactant
transport to the catalyst, where it is needed, resulting in severe mass transport losses.
From the concept of the reaction surface of Chapter 1, some diffusion of reactants
through the electrolyte covering catalyst particles is needed for adequate perfor-
mance. Other alterations of the electrolyte material porosity or use of composite
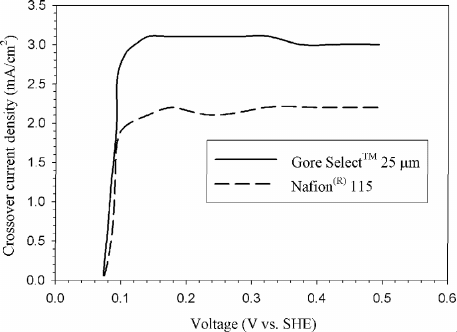
structures have been tried with some success. Figure 4.37 shows the measured hydro-
gen crossover current density for several PEFC membranes. Notice that the thicker
membrane limits crossover, as expected. Experimentally, the hydrogen crossover is
oxidized until a mass transfer limiting condition is reached, which is proportional
to the rate of hydrogen crossover. The equivalent current density produced by the
crossover hydrogen can be solved from Faraday’s law, that is, i
x
=
˙
n
x
2F/A
Ĺ Use of Thicker Electrolyte From Eq. (4.97), the longer the distance of diffusion,
the lower the flux. This approach has been used extensively in the DMFC and
other alcohol-based liquid solution fuel cells. Although this is effective to reduce
crossover, the ohmic losses are directly proportional to electrolyte thickness, which
limits the use of this technique in high-power applications.
Ĺ Alteration of Morphology or Structure of Porous Media and/or Catalyst Lay-
ers to Limit Diffusion Through Electrolyte This approach is favored in DMFC
applications because this approach allows the use of high-methanol-concentration
c04 JWPR067-Mench January 28, 2008 17:28 Char Count=
178 Performance Characterization of Fuel Cell Systems
Figure 4.37 Measured hydrogen crossover current density with different electrolyte structures [28].
The crossover current density is measured directly with a hydrogen anode and an inert humidified
cathode compartment. The limiting current density of hydrogen oxidation at the cathode is determined
to be the crossover current density.
solution. A very simple concept, a diffusion barrier is put between the catalyst layer
and the diffusion media, causing a sharp diffusion gradient across this boundary
which does not add to overall ionic impedance since it is not in the electrolyte. Since
the methanol reaction is kinetically limited, the goal of this approach is to tailor the
diffusion barrier to permit only the amount of methanol to reach the catalyst layer
as is needed for the electrochemical oxidation reaction, since additional methanol is
assumed to cross to the cathode:
n CH
3
OHcatalyst −n CH
3
OHreacted = n CH
3
OHcrossover (4.98)
Ĺ Alteration of Electrolyte Composition to Consume Crossover before Reaching Elec-
trode A novel approach by Wantanabe [29] was used where platinum particles were
embedded within the PEFC electrolyte (Figure 4.38). The concept was designed to
react the crossover hydrogen with crossover oxygen within the electrolyte to gen-
erate water and maintain high membrane humidity levels while reducing crossover
losses. This approach is more costly since it involves additional platinum, and it may
also lead to reduced durability.
Ĺ Recirculation of Liquid Electrolyte In fuel cells with a liquid electrolyte, such as
an alkaline fuel cell, recirculation of the electrolyte can be used to filter impuri-
ties and prevent crossover. This adds ancillary equipment and other system-related
difficulties.
Obviously, each approach has design limitations that result in a balance depending
on the application. For example, for reduced ohmic losses, the ideal electrolyte thickness
is infinitely thin. However, in this limit electrical shorts and crossover dominate. Some
thickness is required to provide a physical separation barrier. Ultimately, since a very small
amount (even milliamperes per cubic centimeter) of crossover will cause a large decrease

c04 JWPR067-Mench January 28, 2008 17:28 Char Count=
4.5 Region IV: Other Polarization Losses 179
Figure 4.38 Schematic of embedded catalyst for crossover elimination and internal water genera-
tion based on concept of Wantanabe [29].
in OCV, it is nearly impossible to completely eliminate this problem in polymer electrolyte
systems.
Modeling Crossover Losses There are several approaches used to empirically, semiem-
pirically, or analytically account for the noted OCV deviation caused by internal currents
or reactant crossover. For electrical conductivity effects, if the transference number (t
i
)is
known, it is a simple matter to add this effect to the performance deviation, since it can be
modeled as a short circuit in parallel with the external current, whose resistance is
R
short
=
δ
electrolyte
Aσ
e
(
)
(4.99)
where δ
electrolyte
is the electrolyte thickness and A is the geometric area of the electrolyte.
For mass crossover losses, several approaches have been taken, with different levels of
accuracy and physicochemical relevance. The first approach is purely empirical, based on
experimental data only. This is particularly useful for computational modeling that is not
overly concerned with conditions at OCV. The difficulty with this approach is it may not
be accurate and does not take into account the many related phenomena on OCV.
From the Nernst equation with unity reactant activity we can show the theoretical
equilibrium potential, which is close to accurate for the SOFC but assumes zero crossover
and only accounts for equilibrium effects. From experimental observation of a hydrogen
PEFC, Parthasarathy and co-workers [30] found that
OCV (cathode potential vs. RHE) = 0.0025T + 0.2329 (4.100)
where T is in Kelvin. From the Nernst equation, the OCV decreases with temperature, while
experimentally, the OCV is determined to increase slightly with the OCV for a PEFC. This
is due to crossover losses. As the temperature increases, the exchange current density on
the cathode increases dramatically, reducing the mixed potential effect of the crossover
hydrogen. The empirical trend is only valid over the low-temperature range of PEFCs (up
to ∼100
◦
C), however, and should also be a function of catalyst, electrolyte thickness and
material, age, and other factors not included in this simple fit. However, Eq. (4.100) has
been used conveniently in modeling studies to simplify calculation. Other empirical forms
can be derived based on experimental or theoretically determined data as needed.
