Mench M.M. Fuel Cell Engines
Подождите немного. Документ загружается.

c04 JWPR067-Mench January 28, 2008 17:28 Char Count=
150 Performance Characterization of Fuel Cell Systems
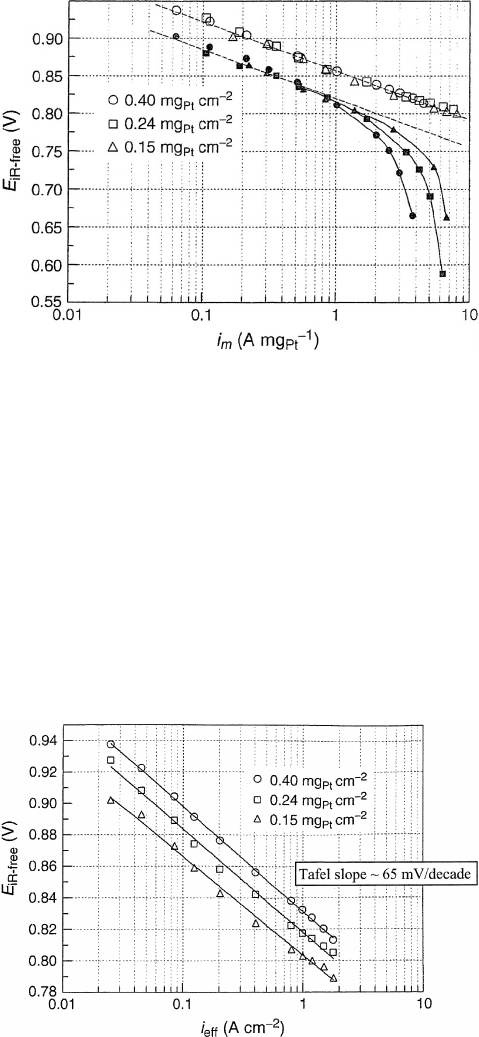
Figure 4.23 Experimental Tafel plot of cell voltage versus current, corrected for fuel cell ohmic
and other losses, so that only cathode polarization losses are remaining. The results are normalized
to platinum loading. Results with open circles are with humidified oxygen, and closed circles are
with humidified air. The dashed line represents the Tafel slope behavior. Note that for all loadings the
Tafel slope for oxygen reduction on platinum is the same but deviates from this behavior under mass-
limiting behavior. Also note that the vertical axis is ohmic corrected fuel cell voltage, not electrode
overpotential, so the voltage falls with increasing current density. (Reproduced with permission from
[9].)
Figure 4.23), since the opposing branch of the BV equation can no longer be neglected,
and the Tafel approximation is not valid. At very high current density, deviation is observed
experimentally due to concentration polarization effects. An experimental Tafel slope for
the ORR in a humidified H
2
/O
2
fuel cell is shown in Figure 4.24. The data in Figure 4.24
were extracted from the polarization curves of fully humidified PEFCs operating on pure
Figure 4.24 Tafel plot for ORR current–overpotential curve. Data for a H
2
–O
2
PEFC at 80
◦
C
with different cathode catalyst loadings. The slope of the line (Tafel slope) for all conditions is
approximately 0.65 mV/decade. (Reproduced with permission from [9].)

c04 JWPR067-Mench January 28, 2008 17:28 Char Count=
4.2 Region I: Activation Polarization 151
Linearized
kinetics
region
Tafel kinetics
region
Slope breakoff
due to mass
transfer effects
log i
η
activation
i
o
Figure 4.25 Region of applicability for sinh BV simplification.
oxygen and hydrogen at high stoichiometry. After correcting for measured ohmic losses,
one can measure the kinetics of a fuel cell in situ; however, careful ohmic correction and
pure, fully humidified reactants are needed to avoid concentration and other unaccounted
losses. For an electrode ex situ, Tafel measurements can be accomplished with the use of a
reference electrode and galvanostat/potentiostat to impose a desired voltage or current on
the working electrode.
Simplified Butler–Volmer Equation 3: Butler–Volmer Equation with Identical Charge
Transfer Coefficients–sinh Simplification A very nice simplification can be made to
the BV model if the anodic and cathodic charge transfer coefficients at the electrode are
equivalent (i.e., α
c
= α
a
). In this case, no approximation is needed, and a new form explicit
in η and mathematically equivalent to the original BV model can be written. This model is
valid over all regions of the electrode polarization, as shown in Figure 4.25.
If α
c
= α
a
= α, we can rearrange the BV equation into a general form:
i
cell
= i
o
exp
αF
R
u
T
η
− exp
−αF
R
u
T
η
= i
o
exp
x
−exp
−x
where x =
αFη
R
u
T
(4.47)
From mathematics
sinh
(
x
)
=
1
2
(exp
x
−exp
−x
) (4.48)
Comparing Eqs. (4.47) and (4.48), we can show that
i
cell
= 2i
o
1
2
exp
αF
R
u
T
η
− exp
−
αF
R
u
T
η
(4.49)
= 2i
o
sinh
αF
R
u
T
η
(4.50)
c04 JWPR067-Mench January 28, 2008 17:28 Char Count=
152 Performance Characterization of Fuel Cell Systems
Taking the inverse, we can show our desired expression:
η =
R
u
T
αF
sinh
−1
i
cell
2i
o
(4.51)
Summary of Butler–Volmer Kinetics and Useful Simplifications
1. General kinetics, applicable under all current density conditions; the general BV
expression for η (solve numerically):
i
cell
= i
o
exp
α
a
F
R
u
T
η
− exp
−α
c
F
R
u
T
η
(4.52)
2. Low polarization, facile kinetics, linearized BV approximation (explicit η expres-
sion):
η =±
i
i
o
R
u
T
(
α
a
+ α
c
)
F
(4.53)
3. High polarization, Tafel approximation (explicit η expression):
η =
R
u
T
α
j
F
ln
i
i
o
(4.54)
4. Both regions, α
a
= α
c
sinh simplification (explicit η expression):
R
u
T
αF
sinh
−1
i
cell
2i
o
= η (4.55)
Example 4.4 Selection of Proper Butler–Volmer Kinetic Model Solve for the most
appropriate symbolic expression for the activation overpotential at each electrode in the
given examples.
SOLUTION Case 1: Solid Oxide Fuel Cell On the anode, a pure hydrogen feed at
1000
◦
C is used with low losses. The cathode shows high losses but you can assume that
α
a,c
= α
c,c
:
At the anode: simplified model, facile kinetics, since no information on the charge transfer
coefficient is given:
i
cell
= i
o,a
α
a,a
+ α
c,a
F
R
u
T
η
a
η
a
=
i
cell
i
o,a
R
u
T
α
a,a
+ α
c,a
F
At the cathode: simplified model, sinh simplification, since α
a,c
= α
c,c
:
i
cell
= i
o,c
× 2sinh
αF
R
u
T
η
c
R
u
T
αF
sinh
−1
i
cell
2i
o,c
= η
c
c04 JWPR067-Mench January 28, 2008 17:28 Char Count=
4.2 Region I: Activation Polarization 153
Case 2: Direct Methanol Fuel Cell At the anode and cathode, there are significant
polarizations:
At the anode: simplified model, Tafel kinetics:
i
cell
= i
o,a
exp
α
a,a
F
R
u
T
η
a
⇒ η
a
=
R
u
T
α
a,a
F
ln
i
cell
i
o,a
At the cathode: simplified model, Tafel kinetics:
i
cell
= i
o,c
−exp
−
α
c,c
F
R
u
T
η
c
⇒
|
η
c
|
=
R
u
T
α
c,c
F
ln
i
cell
i
o,c
Case 3: Hydrogen PEFC At the anode the exchange current density is very high
with α
a,a
= α
a,c
. At the cathode i
o
∼ 1 × 10
−7
A/cm
2
and α
c,c
= α
a,c
:
At the anode: simplified model, linearized kinetics (we should check this assumption, as in
Example 4.1, with more information):
i
cell
= i
o,a
α
a,a
+ α
c,a
F
R
u
T
η
a
η
a
=
i
cell
i
o,a
R
u
T
α
a,a
+ α
c,a
F
At the cathode: simplified model, sinh simplification:
i
cell
= i
o,c
2sinh
αF
R
u
T
η
c
R
u
T
αF
sinh
−1
i
cell
2i
o,c
= η
c
COMMENTS: The sinh simplification is generally preferred to the Tafel or lineraized BV
approximation if appropriate, since it is mathematically equivalent to the full BV equation.
Also, we should note the concentration effect is ignored here but can significantly affect
the exchange current density through Eq. (4.31).
Example 4.5 Activation Polarization Loss Calculation Given the table below, solve for
the following:
(a) Using the appropriate kinetics, calculate the anodic activation overpotential (η
a,a
)
at 0.5 A/cm
2
.
(b) Using the appropriate kinetics, calculate the cathodic activation overpotential at
0.5 A/cm
2
.
Parameter Value
Temperature 363 K
n
a
(elementary charge transfer step) 1
n
c
(elementary charge transfer step) 1.2
β
a,a
0.5
β
a,c
0.5
E
◦
(T , P) 1.15 V
i
o,a
1.5 A/cm
2
i
o,c
0.005 A/cm
2

c04 JWPR067-Mench January 28, 2008 17:28 Char Count=
154 Performance Characterization of Fuel Cell Systems
SOLUTION (a) Calculate the anodic activation overpotential at 0.5 A/cm
2
(η
a,a
). The
linearized BV equation can be used since α
i
Fη/R
u
T < 0.15. Also, since in this case α
a,a
=
α
a,c
= βn
a
, sinh can be used:
R
u
T
αF
sinh
−1
i
cell
2i
o,a
= η
a,a
=
8.314 × 363
0.5 × 96,485
sinh
−1
0.5
2 · 1.5
= 0.0104 V
(b) Calculate the cathodic activation overpotential at 0.5 A/cm
2
(η
a,c
). The Tafel BV
approximation can be used since α
i
Fη/R
u
T > 1.2:
η
c
=
R
u
T
α
c,c
F
ln
i
cell
i
o,c
=
8.314 × 363
0.5 × 1.2 × 96,485
ln
0.5
0.005
= 0.24 V
COMMENTS: The number of electrons transferred in the elementary charge transfer
step at the cathode, n
c
, can be a noninteger value if derived experimentally since there can
be more than one charge transfer reaction in parallel. The kinetic polarization voltages in
this example are typical relative to one another. That is, the ORR losses usually dominate
activation losses when pure hydrogen is used as the fuel.
Model Development Returning to our ultimate goal of this chapter to analytically model
the polarization curve, we now have an expression that includes the starting equilibrium
voltage from the Nernst equation (the top of the “waterfall”) and the departure from the
waterfall resulting from activation overpotential on the anode and cathode:
E
cell
= E
◦
(T , P) − η
a,a
−
η
a,c
Can now solve
−η
r
− η
m,a
−
η
m,c
− η
x
(4.56)
Next, we need to add ohmic, concentration, and other polarization losses before our model
is complete.
4.2.3 Langmuir and Tekmin Model of Kinetics
2
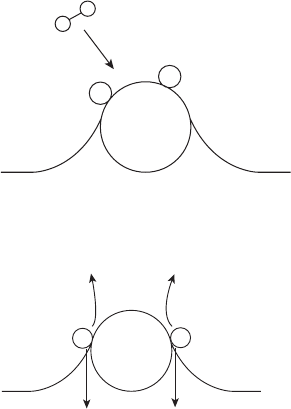
For an electrochemical reaction to take place, the reacting species must first undergo
adsorption or chemisorption onto the reacting surface followed by an intermediate reaction
to an activated complex, as depicted in Figure 4.26. For example, consider the following
HOR mechanism intermediate reactions:
H
2
2
(
H − M
)
ad
(4.57)
(
H − M
)
ad
H
+
+ e
−
(4.58)
For the charge transfer to take place at the catalyst surface, the reacting species is first
adsorbed onto the catalyst, in this case dissociating in the process into separately adsorbed
hydrogen atoms. Here, M indicates the adsorbing catalyst surface. After adsorption, the
charge transfer reaction can take place.
In BV modeled reactions, the intermediate charge transfer steps are rate limiting, and
the adsorption is comparatively facile and rapid. However, this is not always the case. In
2
This section can be skipped without loss of continuity.
c04 JWPR067-Mench January 28, 2008 17:28 Char Count=
4.2 Region I: Activation Polarization 155
H
H
H
2
Catalyst
Surface
Ionomer
(a)
Step 1: Dissociative
chemisorption
of hydrogen
H
H
Catalyst
surface
Ionomer
H
+
H
+
Step 2:
Electro-oxidation
(To cathode)(To cathode)
e
–
e
–
(To Bipolar plate)
(b)
Figure 4.26 Schematic of electrochemical reaction and absorption.
some situations, the rate-limiting step can be the surface adsorption or chemisorption of the
reacting species onto the catalyst sites. Certain situations in fuel cells can be characterized in
this fashion. Specifically, this can be important where a catalyst poison interferes with the de-
sired reaction. As an example, when hydrogen containing part-per-million levels of carbon
monoxide is introduced into the anode of a low-temperature PEFC, the carbon monoxide
electro-oxidative desorption from the catalyst is very slow relative to the carbon monoxide
adsorbtion and the hydrogen electro-oxidation reactions. The key rate-limiting reaction is
believed to be the electrochemical oxidative desorption of CO from the catalyst site [11].
H
2
O + (M − CO) −−−−−→M + CO
2
+ 2H
+
+ 2e
−
(4.59)
Other similar cases exist, such as direct electro-oxidation of alcohols such as methanol
and formic acid in low-temperature fuel cells. In these cases, an alternative to the
BV formulation that accounts for the limiting adsorption and charge transfer steps is
appropriate. Two common models for a surface adsorption limited reaction are the
Langmuir and Temkin kinetics. In the simpler Langmuir model, the surface adsorption
rate constant is independent of surface coverage. In the Temkin model, the adsorption rate
constant is modeled as a function of the surface coverage of adsorbed species. In both
models, a two-step reaction mechanism is assumed [6]:
1. A surface adsorbtion/desorbtion reaction, one or both of which are rate determining.
2. An electrochemical reaction responsible for ion exchange and current generation
that is assumed to be much faster than the adsorbtion/desorbtion step. In addition,
the reaction is assumed to occur so fast that the reverse reaction is assumed to be
negligible.

c04 JWPR067-Mench January 28, 2008 17:28 Char Count=
156 Performance Characterization of Fuel Cell Systems
This two-step mechanism can be expressed as
R + M
k
1 f
k
1b
(
R − M
)
ad
(rate limiting) (4.60)
(
R − M
)
ad
k
2 f
−−−−−→ R
+n
+ ne
−
(relatively fast) (4.61)
Where k
1f
, and k
1b
represent the adsorbtion and desorption reactions (which can be physical
or chemical), respectively, and R represents the molar gas-phase concentration of the
reacting species. The k
2f
reaction represents the fast-forward ion charge transfer reaction of
the adsorbed species responsible for the current generation, and it is assumed the reverse
reaction is negligible. It should be noted that other species or parallel reactions can also be
included in the above formulation methodology. If θ represents the fraction of the available
catalysis sites that the adsorbed species occupies, we can then write
dθ
dt
= k
1 f
y
R
P
(
1 − θ
)
I
−k
1b
θ
II
−k
2 f
θ
III
(4.62)
Term I represents the adsorption of species R onto the remaining noncovered reaction sites.
If θ becomes 1, representing uniform surface coverage, the adsorption obviously stops. The
partial pressure of species R (y
R
P) is also included in Eq. (4.62) since the adsorption from
the gas phase is proportional to the concentration in the gas phase.
Term II represents the desorption of the adsorbed species from the reactive surface
and decreases the surface coverage. Term III is the forward electrochemical ionization
reaction responsible for current flow. From inspection of Eq. (4.62), in the case of an
adsorption-limited reaction, the kinetic limiting current density should be the maximum
possible adsorption rate where the surface coverage θ becomes zero, or
i
lim
∝ k
1 f
y
R
P
(
1 − θ
)
− k
1b
θ − k
2 f
θ ∝ k
1 f
y
R
P (4.63)
The constant of proportionality is the electrons per mole of reactant, nF,or
i
lim
= nFk
1 f
y
R
P (4.64)
so that, for an adsorption-controlled reaction, the limiting current density is linearly pro-
portional to the gas-phase partial pressure, and adsorption rate constant k
1f
can be either
assumed to be constant (Langmuir model) or a function of the surface coverage (Temkin
model). Here, we will show solution for the Langmuir model. The reader is referred to
advanced electrochemistry texts for additional details on Temkin kinetics [e.g., 12].
The overall electrochemical reaction rate can be shown as
i = nFk
2 f
θ (4.65)
Using this two-step model, which can be expanded to include other intermediate steps
as well, a simple formulation for the electrode overpotential current relationship at a
given electrode can be developed. If we assume that the reaction rate constants involved
are independent of the surface coverage of reactant R, then we can derive the Langmuir
kinetics model solution. With constant rate constants in Eq. (4.62), at steady state we can
c04 JWPR067-Mench January 28, 2008 17:28 Char Count=
4.3 Region II: Ohmic Polarization 157
show that
dθ
dt
= 0 = k
1 f
y
R
P
(
1 − θ
)
− k
1b
θ − k
2 f
θ (4.66)
Then, solving for surface coverage, we can show, from algebraic manipulation, that
θ =
k
1 f
y
R
P
k
2 f
+ k
1b
+ k
1 f
y
R
P
(4.67)
Physically, this shows the surface coverage fraction is simply the ratio of the adsorption
reaction to the total sum of parallel reactions. Then the current density i becomes
i = nFk
2 f
θ = nFk
2 f
k
1 f
y
R
P
k
2 f
+ k
1b
+ k
1 f
y
R
P
(4.68)
Now we seek to relate this expression to the overpotential required to produce that current
density at the electrode of interest, so that we can predict electrode overpotential as a
function of current density in a similar fashion as with the BV approach for electron-
transfer-limited reactions. The electrochemical reaction rate constant k
2f
can be written
as
k
2 f
1
s
=
i
o
nF
exp
αnF
R
u
T
η
(4.69)
where i
o
is the exchange current density at θ = 1. Using Eq. (4.68), we can achieve our
desired result, an explicit expression for electrode overpotential as a function of current
density and other measurable parameters for an absorption-limited electrochemical reaction
under the Langmuir model:
η =
R
u
T
αnF
ln
i
i
o
nF
k
1b
+ k
1 f
y
R
P
nRk
1 f
y
R
P − i
(4.70)
The reaction rates required for solution would be determined from existing literature or
from direct experimental results.
4.3 REGION II: OHMIC POLARIZATION
At moderate current densities, a primarily linear region is evident on the polarization curve
in Figure 4.1. In region II, reduction in voltage is dominated by internal ohmic losses
(η
r
) through the fuel cell, resulting in the nearly linear behavior, although activation and
concentration polarization in this region are still present. The ohmic polarization can be
represented as
η
r
= iA
n
k=1
r
k
(4.71)
where each r
k
value is the area-specific resistance of individual cell components, including
the ionic resistance of the electrolyte, and the electric resistance of bipolar plates, cell

c04 JWPR067-Mench January 28, 2008 17:28 Char Count=
158 Performance Characterization of Fuel Cell Systems
I
= Linear path of ion travel
A = Cross-sectional area
ρ=
RA
I
= Ω-m
Figure 4.27 Schematic of ion travel in conductor.
interconnects, and contact resistance between mating parts and any other cell components.
For most fuel cell types at the beginning of operating life, ohmic polarization is dominated
by ionic conductivity in the main electrolyte and in the catalyst layers. Electronic resistance
is typically relatively low, unless there are aging or assembly/contact issues.
Electronic and Ionic Resistance To solve ohmic resistance problems, some basic tools
are required. First, Ohm’s law can be written
V = IR = iAR (4.72)
where R is the resistance, measured in units of ohms ( = Js/C
2
), and A is the electrode
geometric surface area. The resistance is a function of the geometry of the conducting
material. The resistivity ρ is an intrinsic property of a material related to the resistance
through the cross-sectional area of ion travel, A, and the linear path length of ion travel, l,
as shown in Figure 4.27.
Resistance and resistivity are terms typically used to describe ohmic drop in materials
that have significant resistance. For materials with very low ionic resistivity, the inversely
related conductance G and conductivity σ are commonly used. By definition
σ = conductivity =
1
ρ
= 1/ m = S/m (4.73)
G = conductance =
1
R
=
−1
= S (4.74)
and Ohm’s law, Eq. (4.72), can be shown as
V = iA
l
σ A
=
il
σ
= iρl (4.75)
Table 4.3 shows some typical conductivity values for selected fuel cell materials. Note
the dominance of the ionic conductivity of the electrolyte. The functional dependence of
electrolyte conductivity for various fuel cells is covered in Chapter 5.
From a basic understanding of Ohm’s law, we can identify the critical factors governing
these ohmic losses in a fuel cell:
1. Material Conductivity The electrolyte and catalyst layer should have the highest
possible ionic conductivity and all other components including the catalyst layer
should have the highest possible electrical conductivity.

c04 JWPR067-Mench January 28, 2008 17:28 Char Count=
4.3 Region II: Ohmic Polarization 159
Table 4.3 Typical Conductivity/Resistivity Values for Selected Fuel Cell Materials
Component
Typical Bulk
Through-Plane
Conductivity σ
i
or σ
e
Typical
Thickness Functional Dependencies
PEFC electrolyte σ
i
= 10 S/m (hydrated) 50–200 µm Temperature, water content
SOFC electrolyte σ
i
= 1–10 S/m
(>800
◦
C)
10–300 µm Temperature, dopants
(conductivity through oxygen
vacancies)
AFC electrolyte σ
i
on order of 1–100
S/m at operating
temperature
0.5–2.0 mm Ion concentration, temperature,
charge number on ion, dielectric
constant of solution, mobility,
viscosity, degree of ion
dissociation, other liquids
MCFC electrolyte σ
i
on order of 1–100
S/m at operating
temperature
0.5–2.0 mm See AFC electrolyte
PAFC electrolyte σ
i
on order of 1–100
S/m at operating
temperature
0.5–2.0 mm See AFC electrolyte
PEFC bipolar plate
(graphite)
σ
i
= 5000–20,000 S/m 2–4 mm each Oxide film (corrosion), materials,
coatings
PEFC gas diffusion
layer (GDL)
σ
i
= 10,000 S/m
(much less in plane)
100–300 µm Approximately constant
PEFC catalyst layer ∼1–5 S/m 5–30 µm Morphology, Nafion and carbon
loading, age
Contact resistances
for cell
Very low if built well;
resistance ∼30
m·cm
2
Not available,
use area as
contact area
Compression, pressure,
temperature, age (corrosion),
number of cycles, and others,
current collector total landing
area
Total cell resistance
(based on active
cell area)
Total resistance < 100
m·cm
2
Not available,
use cell
superficial
active area
See above
2. Material Thickness The ohmic losses are directly proportional to the distance
traveled by the current. In addition to a compact design, this is the reason the
electrolyte and other components are manufactured to be as thin as other constraints
will allow.
Example 4.6 Estimate Total Fuel Cell Resistance Given the experimental polarization
data for a SOFC at 700
◦
C from [13], estimate the ionic resistance of the electrolyte. Is this
a maximum or a minimum value for the actual ohmic resistance of the electrolyte?
SOLUTION To estimate the ohmic resistance of the electrolyte under these conditions,
we can take the slope of the main linear region of the polarization curve. In doing so, we
are assuming that the activation and concentration polarization are minor. Although not
