Mench M.M. Fuel Cell Engines
Подождите немного. Документ загружается.


c04 JWPR067-Mench January 28, 2008 17:28 Char Count=
130 Performance Characterization of Fuel Cell Systems
Total exothermic release
available for work
(electrical or chemical)
Extent of reaction
Initial “Push”
Metastable State I
Metastable State II
“Activated complex”
η
a
Figure 4.8 Schematic of reaction coordinate pathway for given reaction.
the initial state which corresponds to nonreacted fuel and oxidizer, the reactants are at a
metastable equilibrium with a higher energy than the product state. Under the driving force
of a voltage over potential at the electrode (v/a), the reactant is moved along the reaction
coordinate toward an activated complex state, where the reactant is partially converted to
product. This state corresponds to the elementary charge transfer reaction step discussed
in Chapter 2. The intermediate species at this state is highly reactive and cannot remain
in a stable condition. This transition-state-activated complex separates the reactant from
the product states along the reaction coordinate. As the reaction moves to completion, the
activated complex moves to a product state, and a net chemical energy conversion occurs
as the products reach a final metastable equilibrium. The difference between the initial
reactant and final product chemical energy is the enthalpy of reaction, which is converted
to electrical work and heat in an electrochemical reaction.
Now, consider an individual electrode, initially at equilibrium. For example, consider
the global HOR:
Reduced
H
2
↔2H
+
+2e
−
←−−−−−−−−→ Oxidized (4.7)
At equilibrium (open circuit), this reaction actually proceeds in both directions across
the anode double layer, with no net reaction in either direction. Some hydrogen is being
oxidized, and an equivalent amount is being reduced. This is analogous to a frictionless
pendulum, shown in Figure 4.9. At equilibrium, the pendulum swings back and forth with
Figure 4.9 Mechanical pendulum analogy to describe electrode reaction and equilibrium.

c04 JWPR067-Mench January 28, 2008 17:28 Char Count=
4.2 Region I: Activation Polarization 131
Figure 4.10 Schematic of pendulum analogy of small net anodic current.
no net motion in either direction. The energy in the system is conserved and oscillates
between potential energy and kinetic energy, just as the species at the electrode will
oscillate between oxidized and reduced hydrogen, with no net change in the reactants.
This equilibrium current exchange is termed the exchange current density i
o
, and is an
important parameter discussed in the following sections.
Next, consider moving the electrode out of equilibrium and into a condition of net
hydrogen oxidation and current generation. This reaction produces a net flow of electrons
which are transported to the external circuit. For a very low current, a small overpotential is
required to drive the net reaction in the anodic direction. As the level of current is increased
beyond some equilibrium exchange value, the overpotential required to sustain the reaction
rate is greatly increased. Consider again the frictionless pendulum analogy; in order to
leave equilibrium and move to a net anodic reaction, if the pendulum were reflected at
the midpoint in the oscillation by a perfectly elastic barrier, as shown in Figure 4.10, the
pendulum would rise back to the initial height in the anodic direction to conserve the system
energy. The net result is a small anodic current.
In order to push the pendulum beyond the level allowed by the initial system oscillation
energy, additional external energy must be input to the pendulum, as illustrated in Figure
4.11. In this analogy, the additional work input is converted to a higher height of the
pendulum in the anodic direction and a greater net reaction (i.e., more current).
Figure 4.11 Schematic of pendulum analogy of larger net anodic current and energy input.
c04 JWPR067-Mench January 28, 2008 17:28 Char Count=
132 Performance Characterization of Fuel Cell Systems
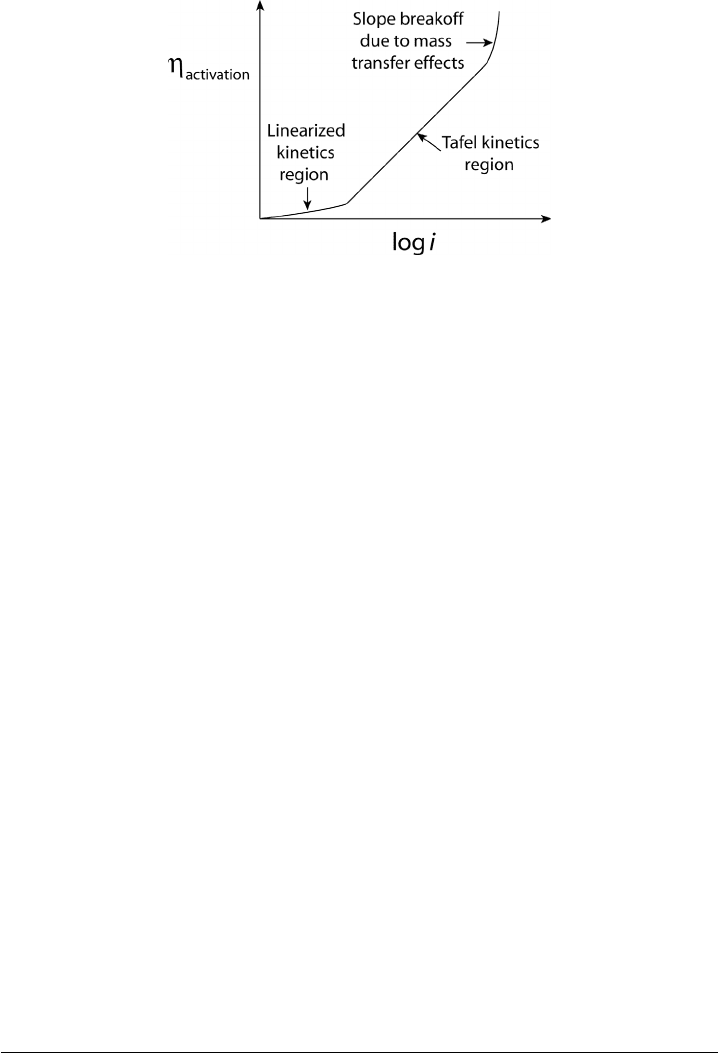
Figure 4.12 Schematic of activation overpotential with respect to current.
With the pendulum analogy in mind, we can now examine the typical electrode activa-
tion polarization behavior, as shown in Figure 4.12. At low current density, the activation
overpotential η
activation
required to maintain a net reaction rate in a given direction is small.
Beyond a threshold value in current density related to the equilibrium reaction exchange
rate of the electrode, the additional polarization requiredfor increasing current is greatly
increased. The exchange current density i
o
is of preeminent importance in the activation
over potential for a given electrochemical reaction rate, as it is a measure of the effec-
tiveness of the electrode in promoting the electrochemical reaction and is the electrode
reaction exchange at equilibrium. At zero net cell current density, the electrode current is
the exchange current density. The higher the exchange current density at a given electrode,
the lower the overall activation polarization losses for a given current.
4.2.1 Butler–Volmer Model of Kinetics
1
In this section, we derive a general expression to describe activation polarization losses at a
given electrode, known as the Butler–Volmer (BV) kinetic model. The BV model is not the
only (or necessarily the most appropriate) model to describe a particular electrochemical
reaction process. Nevertheless, it is a classical treatment of electrode kinetics that is widely
applied to study and model a majority of the electrode kinetics of fuel cells. The BV
model describes an electrochemical process limited by the charge transfer of electrons,
which is appropriate for the ORR, and in most cases the HOR with pure hydrogen. The
fundamental assumption of the BV kinetic model is that the reaction is rate limited by a
single electron transfer step, which may not actually be true. Some reactions may have
two or more intermediate charge transfer reactions that compete in parallel or another
intermediate step such as reactant adsorption (Tafel reaction from Chapter 2) may limit
the overall reaction rate. Nevertheless, the BV model of an electrochemical reaction is
standard fare for a student of electrochemistry and can be used to reasonably fit most fuel
cell reaction behavior.
1
Instructors may wish to skip to Eq. (4.35) and assign the derivation of the Butler–Volmer kinetics to advanced
undergraduate or graduate students.
c04 JWPR067-Mench January 28, 2008 17:28 Char Count=
4.2 Region I: Activation Polarization 133
In a fuel cell, at each electrode, there is an equilibrium reaction that can be written as
AO
+
+ ne
−
k
f
k
b
BR
→= Reduction (cathodic)
←= Oxidation (anodic)
(4.8)
where
Ĺ The stoichiometric coefficients of the rate-limiting elementary charge transfer reac-
tion at a given electrode are A and B. The elementary reaction should be distinguished
from the global reaction occurring at a given electrode, as discussed in Chapter 2.
The elementary charge transfer reaction is the intermediate reaction responsible for
the charge transfer. Although the BV model assumes a single charge transfer reaction
step, there can be several charge transfer steps occurring in parallel. The BV model
can still accommodate this, as will be discussed.
Ĺ At each electrode in the fuel cell at open circuit condition, an equilibrium as in
Eq. (4.8) is occurring with no net current through the circuit (recall the equilibrium
pendulum of Figure 4.9). That is, both the anode and the cathode have completely
separate equilibrium reactions occurring, linked only by the net charge transfer
through the circuit. At open circuit, there is no net current flowing through the
electrodes, and the anode and cathode reactions are independent.
For a given purely chemical reaction, we can change the temperature and pressure to affect
the reaction rate. For an electrochemical reaction, there is an additional factor: the electrode
overpotential across the double layer. The overpotential at the electrode surface controls the
direction and rate of the net reaction. When net current is drawn, an overpotential at each
electrode forces the electrode reactions out of the equilibrium condition and toward the
desired direction. At the anode, the electrode potential becomes higher than its equilibrium
potential (see Figure 4.5), resulting in a net oxidation reaction. At the cathode, the electrode
potential becomes lower than its equilibrium potential, resulting in a net reduction reaction.
Figure 4.13 shows this on a reaction coordinate. At the initial surface electrode potential
φ
1
, the forward reaction is not favored. At φ
3
, the reaction is now favored because the
final energy state is below the initial energy state. For electrochemical reaction circuits,
spontaneous galvanic (exothermic) reactions can be reversed simply by applying an external
potential to change the polarity of the electrodes. This is the principle of the reversible fuel
cell discussed in Chapter 1.
Consider an electrode at state φ
1
in Figure 4.14. To induce this electrode to have a net
spontaneous reduction reaction, we must go from φ
1
to φ
3
. Although the potential energy
of the electrode is increased to promote this reaction and support charge transfer across
the double layer of the electrode, the actual surface overpotential relative to the SHE will
decrease (see Figure 4.5), since we are moving toward a cathodic reduction reaction product
in this example.
Symmetry Factor We have added nF(φ
3
– φ
1
) in electrical potential to the system. Only
a fraction of this energy will go toward reducing the activation energy of the cathodic
(reduction) reaction at the electrode. In a general case, the fraction of the additional energy

c04 JWPR067-Mench January 28, 2008 17:28 Char Count=
134 Performance Characterization of Fuel Cell Systems
Energy
Reactants Products
Extent of Reaction
φ
1
φ
2
φ
3
is endothermic for the forward reaction
is exothermic for the forward reaction
is the surface potential (V) of the electrode
φ
1
φ
3
φ
Figure 4.13 Schematic of activation overpotential with current at given electrode.
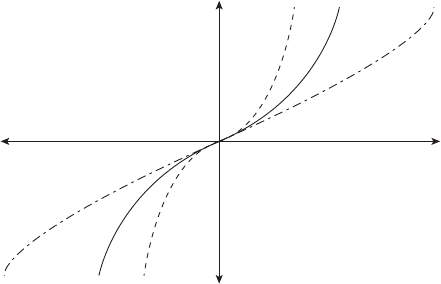
imparted to the electrode that promotes the reduction reaction is called β, the symmetry
factor:
0 <β<1 (4.9)
If β = 1 (see Figure 4.15), the additional overpotential at the electrode goes completely
toward promoting the reduction reaction. If β = 0 (see Figure 4.16), all of the additional
potential is applied toward promotion of the anodic oxidation reaction. In the particular
case shown in Figure 4.15, all of the additional voltage potential increases the oxidized
state energy, and none is applied to lower the reduced state energy. In practice, β varies for
a given electrode and reaction and lies between 0 and 1 but is not necessarily 0.5, since
certain catalysts promote oxidation of a given species more readily than others.
The activation energy (polarization) required to promote charge transfer across the
double layer for the reduction reaction at an electrode can be shown as
E
a2,c
= E
a1,c
+ βnF(φ
2
− φ
1
) (4.10)
For a reduction reaction, the sign of φ
2
is more negative than φ
1
(Figure 4.5), so φ
2
− φ
1
is negative.
For the oxidation reaction on the electrode, 1 − β is the anodic symmetry factor, the
fraction of the polarization energy which promotes the oxidation reaction at the electrode,
and the activation energy (polarization) required to promote charge transfer across the
Energy
Oxidized state Reduced state
Extent of reaction
φ
1
φ
2
φ
3
Transition state
E
a3
Figure 4.14 Activation overpotential increase to initiate reaction at given electrode.

c04 JWPR067-Mench January 28, 2008 17:28 Char Count=
4.2 Region I: Activation Polarization 135
Energy
Extent of Reaction
φ
1
φ
2
Oxidized State Reduced State
nF φ
2
−
φ
1
()
Ea
1, c
+
nF φ
2
−
φ
1
()
=
Ea
2, c
= cathodic reaction activation energy
Figure 4.15 Symmetry factor β = 1.
double layer for the oxidation reaction at an anode can be shown as
E
a2,a
= E
a1,a
− (1 − β)nF(φ
2
− φ
1
) (4.11)
So when we decrease the electrode potential, the activation energy for reduction goes down
by βn
c
F(φ
2
−φ
1
), and the oxidation activation energy is increased by
(1 − β)nF(φ
2
− φ
1
) (4.12)
Returning to the elementary charge transfer reaction,
AO
+
+ ne
−
k
f
k
b
BR (4.13)
For each reaction, k
f
and k
b
, a commonly used model for the rate equation is an Arrhenius
expression:
k = k
ref
exp
−E
a
R
u
T
(4.14)
where the reference reaction rate (k
ref
) and the reaction activation energy (E
a
) are different
for k
f
and k
b
, the forward and reverse reactions at the electrode, respectively.
Energy
Extent of Reaction
φ
1
φ
2
Oxidized State Reduced State
nF φ
2
−
φ
1
()
No help in promoting
reduction reaction
Ea
1, c
=
Ea
2,c
Figure 4.16 Symmetry factor β = 0.
c04 JWPR067-Mench January 28, 2008 17:28 Char Count=
136 Performance Characterization of Fuel Cell Systems
A rate equation for the electrochemical reaction can be written considering Faraday’s
law and the reaction rate constant:
|r|=
mol
s · cm
2
=
i
nF
= k
[C]
γ
exp
−E
a
R
u
T
=
dC
n
dt
(4.15)
where γ is the reaction order of the elementary electron transfer step, r is the rate of
consumption/formation of species, and C is the concentration of species in reaction of
interest in units of moles per cubic centimeter. For an ideal gas,
C =
P
R
u
T
(4.16)
Taking the anodic (oxidation) reaction branch of Eq. (4.13),
BR
k
b
−−−−−−−→ AO
+
+ ne
−
(4.17)
Then the oxidation rate of the elementary charge transfer reaction can be written as
|r
a
|=
mol
s · cm
2
=
i
a
n
a
F
= k
a
[C
R
]
P
a
exp
−E
a,a
−
(
1 − β
)
nF
(
φ − φ
ref
)
R
u
T
(4.18)
Let the reference potential for the anode and cathode electrodes (ø
ref
) be 0 V (SHE potential)
|r
a
|=
i
a
n
a
F
= k
a
[C
R
]
P
a
exp
(
1 − β
)
nF(φ)
R
u
T
(4.19)
where we have absorbed E
a,a
, the oxidation reaction reference activation energy, into the
k
a
term to become k
a
, the anode reaction rate constant.
For the reduction reaction rate at the cathode
|
r
c
|
=
i
c
n
c
F
= k
c
[C
O
]
P
c
exp
−βnF(φ)
R
u
T
(4.20)
Some derivations also absorb the electron transfer number n into β as
nβ = α
c
(4.21)
where α
c
is known as the cathodic charge transfer coefficient, and
n(1 − β) = α
a
(4.22)
where α
a
is known as the anodic charge transfer coefficient.
It is important to note that n is the number of electrons transferred in the elementary
charge transfer step. This is very different from the global reaction n we defined for
c04 JWPR067-Mench January 28, 2008 17:28 Char Count=
4.2 Region I: Activation Polarization 137
Faraday’s law and in the Nernst equation. Typically, n here is less than 2. Based on the
single charge transfer reaction step used in Eq. (4.8), the value of n should be an integer.
However, experimentally this value is often determined to be a noninteger, which can be
true if more than one reaction is acting as a limiting charge transfer step in parallel. In this
case, the BV model can still be applied to model an electrode’s reaction kinetics, but the
value of n determined experimentally will be a noninteger value, a result of the cumulative
effect of the multiple charge transfer reaction steps.
Charge Transfer Coefficient From the definition of the cathode charge transfer coef-
ficient α
c
, it physically represents the fraction of additional energy that goes toward the
cathodic reduction reaction at an electrode. It can also be thought of as a symmetry coeffi-
cient of the electrode reaction. In terms of the pendulum analogy shown in Figure 4.9, the
pendulum does not have to be balanced. If the pendulum is tilted sideways with respect to
the ground reference, additional work input to the system will asymmetrically add potential
energy to the maximum height on each swing. Many reactions tend toward symmetry, so
with no information it is usually reasonable to assume a value of 0.5 = α
c
. The effect of
the charge transfer coefficient on the electrode polarization symmetry is shown in Figure
4.17. The negative values of overpotential correspond to the cathode polarization, and the
positive values of polarization correspond to the anode reaction. The vertical axis corre-
sponds to the current density. For a charge transfer coefficient of n × 0.5, polarization
affects the anodic and the cathodic reaction at a given electrode equally, and the curve is
symmetric around the axis. For a cathodic charge transfer coefficient that is n × 0.75, the
polarization required for the cathodic reduction reaction for a given current is much less
than the polarization required for the anodic oxidation. Obviously, a cathode with α
c
> n ×
0.5 would be preferred for the cathode. However, as an engineering design parameter, there
is little we can do to alter this value in practice for a given electrode, and other parameters
we can affect through engineering, such as the exchange current density i
o
, have a much
greater impact.
η
i
α
c
= 0.75n
α
c
= 0.50n
α
c
= 0.25n
α
c
= 0.75n
α
c
= 0.50n
α
c
= 0.25n
Positive
Positive
Negative
Negative
Figure 4.17 Effect of charge transfer coefficient on symmetry of current–overpotential curves.
(Reproduced from [1].)
c04 JWPR067-Mench January 28, 2008 17:28 Char Count=
138 Performance Characterization of Fuel Cell Systems
Note that we are still not considering the entire fuel cell yet! We are discussing
a single electrode with simultaneously occurring oxidation and reduction reactions. For
nonequilibrium operating conditions, we desire a net current density. For a net oxidation
current density at the anode we consider the net oxidation reaction rate:
r
net,anodic
= r
a
−r
c
=
i
a
n
a
F
−
i
c
n
c
F
=
i
nF
= k
a
[C
R
]
P
a
exp
α
a
F(φ)
R
u
T
− k
c
[C
O
]
P
c
exp
−α
c
F(φ)
R
u
T
(4.23)
At open circuit, the fuel cell net current density is zero, but the rates of exchange r
a
and r
c
are not zero but equal. We can solve for this resting exchange current density i
o
:
i
o
nF
= r
a
= r
c
= k
a
[C
R
]
P
a
exp
α
a
Fφ
◦
R
u
T
= k
c
[C
O
]
P
c
exp
−
α
c
Fφ
◦
R
u
T
(4.24)
where ø
◦
is the equilibrium potential (OCV) at i = 0. If we rearrange Eq. (4.24) and take
the natural log of both sides,
φ
◦
=
RT
nF
ln
k
c
k
a
−
RT
nF
ln
C
Pr
r
C
Po
o
or E(OCV) = E
o
(
T
)
−
RT
nF
ln
C
νr
r
C
νo
o
(4.25)
which is the Nernst equation! This makes sense, since any kinetic theory must reduce to
the thermodynamic theory at equilibrium. So at i
net
= 0, we are at equilibrium, and the
expected maximum voltage is determined from the Nernst equation, as we have already
shown in Chapter 3.
Now, consider the overpotential at an electrode, η, which represents a departure from
this equilibrium potential:
η = φ − φ
o
(4.26)
Plug this into Eq. (4.23), and we can show that
r
net
=
i
nF
= k
a
[C
R
]
P
a
exp
α
a
F(φ)
R
u
T
− k
c
[C
O
]
P
c
exp
−α
c
F(φ)
R
u
T
(4.27)
and the net current density in the oxidation (anodic) direction, i
net
, at a single electrode can
be shown as
i
net
= nFk
a
C
P
a
R
exp
α
a
F
R
u
T
η +
R
u
T
nF
ln
k
c
k
a
+
R
u
T
nF
ln
C
Pc
o
C
Pa
R
−nFk
c
C
P
c
O
exp
−
α
c
F
R
u
T
η +
R
u
T
nF
ln
k
c
k
a
+
R
u
T
nF
ln
C
Pc
o
C
Pa
R
(4.28)
c04 JWPR067-Mench January 28, 2008 17:28 Char Count=
4.2 Region I: Activation Polarization 139
Simplifying yields
i
net
= nFk
(1−β)
c
k
(−β)
a
C
(1−β) P
c
O
C
β P
a
R
exp
α
a
F
R
u
T
η − exp
−α
c
F
R
u
T
η
(4.29)
At open-circuit conditions, i
net
= 0, but each component of anode/cathode current must
equal the exchange current density i
o
. So the first term in brackets at OCV (η = 0) is the
oxidation branch at the electrode, and the second term is the reduction branch. At η = 0,
each branch must be at the exchange current density, so that i
o
– i
o
= i
net
= 0. So we see
that the term outside the brackets in Eq. (4.29) is really the exchange current density.
We can now rewrite our standard BV model of kinetics for an individual electrode:
i
net
= i
o
exp
α
a
F
R
u
T
η − exp
−α
c
F
R
u
T
η
(4.30)
If
i
o
= i
o,ref
C
C
∗
γ
= i
o
C
C
∗
γ
(4.31)
where α
a
and α
c
refer to the anodic and cathodic charge transfer coefficients at the electrode,
respectively. This is to be applied at each electrode. For example, for the anode
i
cell
= i
net,anode
= i
o,a
exp
α
a,a
F
R
u
T
η
a
− exp
−α
c,a
F
R
u
T
η
a
(4.32)
For the cathode
i
cell
= i
net,cathode
= i
o,c
exp
α
a,c
F
R
u
T
η
c
− exp
−α
c,c
F
R
u
T
η
c
(4.33)
By conservation of charge, i
a
= i
c
= i
cell
, so that
i
o,a
C
H
2
,surface
C
∗
H
2
γ
H
2
exp
α
a,a
F
R
u
T
η
a
− exp
−α
c,a
F
R
u
T
η
a
= i
o,c
C
O
2
,surface
C
∗
O
2
γ
O
2
exp
α
a,c
F
R
u
T
η
c
− exp
−α
c,c
F
R
u
T
η
c
(4.34)
In words, this means that the net current at the cathode and anode is conserved, providing
the linkage between the two electrodes. The overpotential at each electrode will adjust to
