Mench M.M. Fuel Cell Engines
Подождите немного. Документ загружается.

c03 JWPR067-Mench December 18, 2007 1:59 Char Count=
120 Thermodynamics of Fuel Cell Systems
5. N. Laurendeau, Statistical Thermodynamics: Fundamentals and Applications, Cambridge Uni-
versity Press, New York, NY, 2005.
6. T. E. Springer, T. A. Zawodzinski, and S. Gottesfeld, J. Electrochem. Soc., Vol. 138, No. 8, pp.
2334–2341, 1991.
7. S. Turns, An Introduction to Combustion: Concepts and Applications, 2nd ed., McGraw-Hill,
New York, 2000.
8. J. Newman, Electrochemical Systems, 2nd ed., Prentice-Hall, Englewood Cliffs, N. J., 1991.

c04 JWPR067-Mench January 28, 2008 17:28 Char Count=
4
Performance Characterization
of Fuel Cell Systems
Q: When will we see the first production fuel-cell vehicle from Toy-
ota? A: A realistic date is 2010. There are still a few technical and
infrastructural difficulties to overcome. Problems include space
and the safe storage of hydrogen on board the vehicle. In terms of
the fuel cell stack itself, however, we have found most of the answers.
—Katsuaki Watanabe, President of Toyota Motor Company, in
a March 2006 interview in Automobile Magazine
4.1 POLARIZATION CURVE
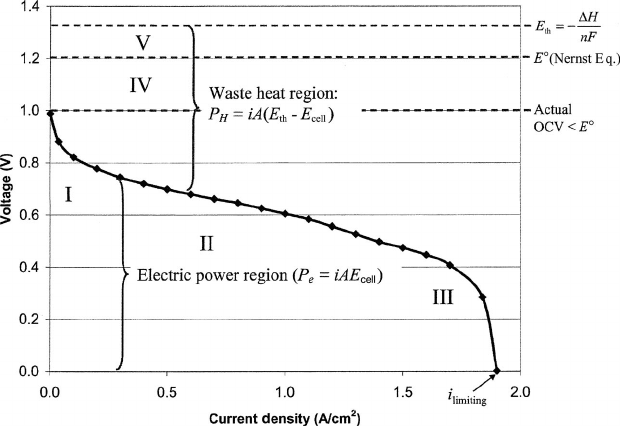
Figure 4.1 is an illustration of a typical polarization curve for a fuel cell with negative
entropy of reaction, such as the hydrogen–air fuel cell, showing five regions of interest
labeled I–V. The polarization curve, which represents the cell voltage–current relationship,
is the standard figure of merit for evaluation of fuel cell performance. Voltage versus
current density, scaled by geometric electrode area, is typically shown, so that the results
are scalable between differently sized cells. Returning to the five regions labeled on the
polarization curve of Figure 4.1:
Ĺ The losses in region I are dominated by the activation (kinetic) overpotential at the
electrodes.
Ĺ The losses in region II are dominated by the ohmic polarization of the fuel cell. This
includes all electrical and ionic conduction losses through the electrolyte, catalyst
layers, cell interconnects, and contacts.
Ĺ The losses in region III are dominated by the concentration polarization of the fuel
cell, caused by mass transport limitations of the reactants to the electrodes.
Ĺ The losses in region IV represent the departure from the Nernst thermodynamic
equilibrium potential. This loss can be very significant and can be due to undesired
species crossover through the electrolyte, internal currents from electron leakage
through the electrolyte, or other contamination or impurity.
Ĺ The losses in region V represent the departure from the maximum thermal voltage,
a result of entropy change which cannot be engineered. Figure 4.1 is shown for a
fuel cell with negative S. If the entropy change is positive, the Nernst voltage is
actually greater than the thermal voltage, and the heat generation by entropy change
is negative, as discussed in Chapter 3.
121
Fuel Cell Engines
Copyright © 2008 by John Wiley & Sons, Inc.
Matthew M. Mench
c04 JWPR067-Mench January 28, 2008 17:28 Char Count=
122 Performance Characterization of Fuel Cell Systems
Figure 4.1 Typical polarization curve for fuel cell with significant kinetic, ohmic, concentration,
and crossover potential losses.
It is important to note that regions I–III in Figure 4.1 of dominant kinetic, ohmic, or
mass transfer polarizations are not discrete. That is, all modes of loss contribute throughout
the entire current range. For example, ohmic losses occur whenever there is current but
only dominate losses in region II. As another example, although the activation overpotential
dominates in the low-current region I, it still contributes to the cell losses at higher current
densities where ohmic or concentration polarization dominate. Thus, each region shown in
Figure 4.1 is not unique and separate, and all losses contribute throughout the operating
current regime.
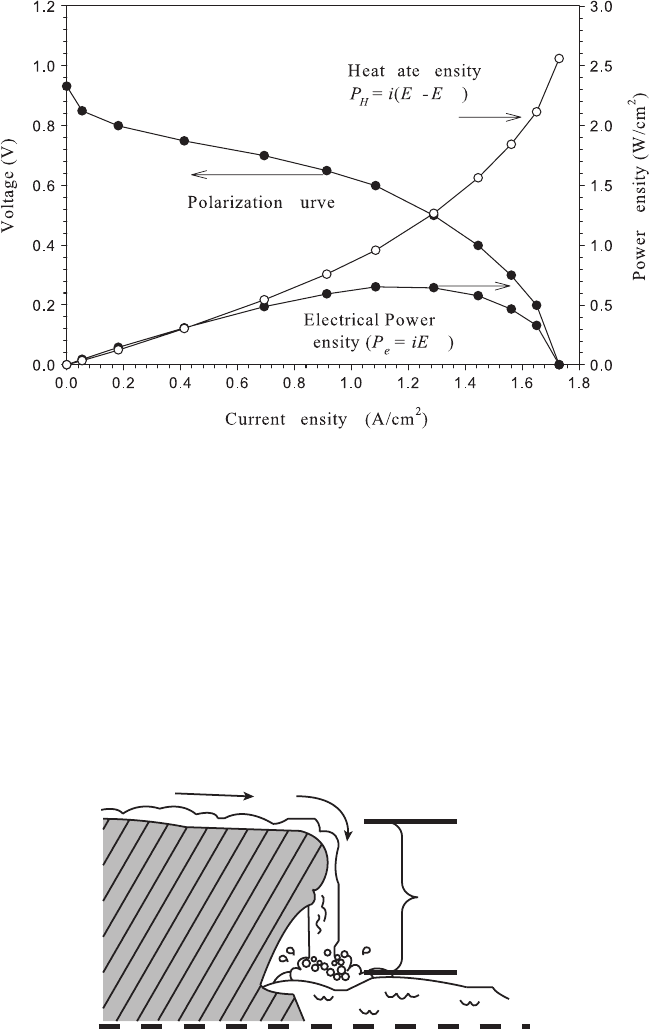
Also shown in Figure 4.1 are the regions of electrical and heat generation. The actual
electrical and heat generation rates are shown on Figure 4.2. The electrical power generated
is the cell current multiplied by the fuel cell voltage, where the heat generation rate is
the cell current multiplied by a different voltage, the voltage departure from the thermal
voltage. Since the thermodynamically available energy not converted to electrical energy is
converted to heat, the thermodynamic efficiency can be qualitatively observed by compar-
ing the relative magnitude of the voltage potential converted to waste heat and to electrical
power.
It should be noted that voltage loss, polarization, and overpotential are all interchange-
able terms and refer to a voltage loss. In general, the operating voltage of a fuel cell can be
represented as the departure from ideal voltage caused by the various polarizations:
E
cell
= E
◦
(T , P) − η
a,a
−|η
a,c
|−η
r
− η
m,a
−|η
m,c
|−η
x
(4.1)
where E
◦
(T,P) is the theoretical equilibrium open-circuit potential of the cell, calculated
from the Nernst equation. The activation overpotentials at the anode and cathode are
c04 JWPR067-Mench January 28, 2008 17:28 Char Count=
4.1 Polarization Curve 123
c
d
˝
˝
d
, i
cell
cellth
d
d
r
Figure 4.2 Illustration of polarization curve, waste heat generation, and useful electrical power
generation rate density.
represented by η
a,a
and η
a,c
, respectively. The polarization in region IV results from
crossover of fuel and oxidizer through the electrolyte or internal short circuits in the
cell. This departure from the Nernst equilibrium voltage (η
x
) can be modeled as a result
of crossover current. The ohmic (resistive) polarization is shown as η
r
. The concentration
(mass transfer) polarization at the anode and cathode are represented as η
m,a
and η
m,c
, respec-
tively. Throughout the remainder of the chapter, we will discuss each of these losses in detail.
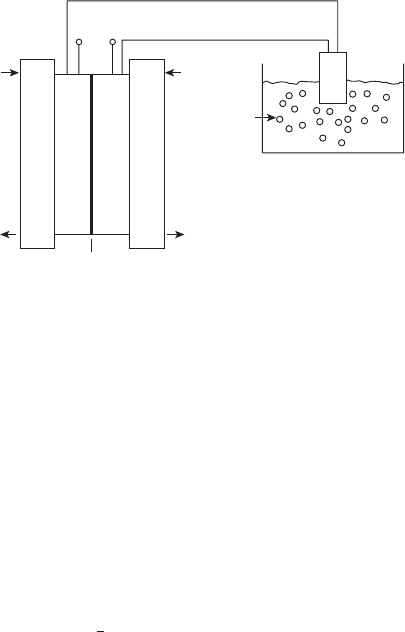
We return to the waterfall analogy of Chapter 2, shown in Figure 4.3. In Chapter 2, we
did not yet know how to calculate the expected cell voltages, and the waterfall analogy was
E
cathode
E
anode
Flow rate over falls ∝ current
Standard reference: standard hydrogen electrode (SHE = 0.0 V)
E
cell
= E
cathode
- E
anode
Figure 4.3 Waterfall analogy illustrating voltage potential at each electrode and for the fuel cell.
c04 JWPR067-Mench January 28, 2008 17:28 Char Count=
124 Performance Characterization of Fuel Cell Systems
Pt
E
cell
+
-
E
anode
E
cathode
H
2
in
H
2
out
Air out
Air in
Electrolyte
Anode
Cathode
Standard reference
electrode (SRE)
H
2
bubbles
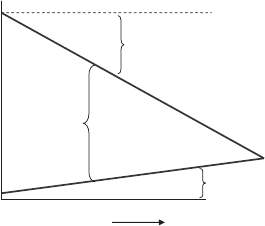
Figure 4.4 Illustration of measurement of cell and individual electrode voltage in a fuel cell.
used to illustrate the concepts of the current and potential for reaction. Now that we know
how to calculate the maximum expected equilibrium cell potential (the Nernst voltage),
we can use this as the starting point for the operating fuel cell voltage. Recall that the
Nernst potential is calculated for a condition of pure thermodynamic equilibrium, which is
only approached at open-circuit conditions. With any current drawn, there will be ohmic,
activation, and mass transfer losses. Figure 4.4 is shown to illustrate the connection between
the waterfall and the fuel cell. The cell voltage E
cell
is the measured potential difference
between the anode and cathode and is a measure of the potential to do electrical work. From
experience, we know that if a fuel and oxidizer are mixed and combusted, some heat will
be released that is the reaction enthalpy:
1
2
O
2
+ H
2
−−−−−→H
2
O + H (4.2)
From the thermal voltage from Chapter 3, E
th
=−H/nF, we see that this thermal voltage
potential is indeed what would occur if all of the thermal energy released in the combustion
reaction were instead converted into voltage potential instead.
As discussed in Chapter 3, for comparison purposes, some baseline voltage must be
defined. The condition of zero-volt potential has been assigned to a platinum electrode
with pure hydrogen flow at STP conditions. This reference potential is called the standard
hydrogen electrode (SHE), and all other voltage potentials are relative to this baseline.
This is similar to defining an arbitrary “zero” reference point in potential-energy-related
problems. Returning to Figure 4.4, if we measured the voltage potential between a standard
hydrogen reference electrode and the cathode, the resulting voltage could be positive or
negative relative to the SHE. For the cathode in a galvanic cell, the potential must only
be greater than the anode. Strictly speaking, neither the anode or cathode must be positive
relative to the SHE.
We can solve for the theoretical OCV or the “initial height of the waterfall” from
the tools of thermodynamics discussed in Chapter 3. The next step is to understand what
happens when we move the fuel cell out of equilibrium. When we leave equilibrium and
begin to draw current, the system suffers various polarization losses as shown in Eq. 4.1,
and the cell voltage decreases.
c04 JWPR067-Mench January 28, 2008 17:28 Char Count=
4.1 Polarization Curve 125
V
i
E
cell
η
cathode
will be negative, as the electrode potential
decreases, the potential for reduction reaction increases.
For an H
2
/air system, η
cathode
is greater than η
anode
η
anode
.
E
cathode
E
anode
Figure 4.5 Representation of individual electrode potentials as function of current density. Note
that the polarization behavior at each electrode will likely not be linear with current density, as shown
for simplicity.
At the anode at equilibrium, the oxidation reaction is balanced with the reduction
reaction:
Ox + ne
−
↔ Re (4.3)
As illustrated in Figure 4.5, when we move the fuel cell out of equilibrium and produce
a net oxidation at the anode and a net reduction at the cathode, polarization moves the
anode toward a greater oxidation potential, or higher voltage relative to the SHE. That is,
as current increases, the anode becomes more positive to promote oxidation of the fuel.
This makes sense, considering a more positive electrode will develop a greater attractive
force to separate electrons from the reactive species (oxidation process). On the other
hand, the cathode voltage is reduced as current increases to promote the oxidizer reduction
reaction. There is a cost associated with this promotion of reaction rate (current): As
the anode potential is increased to promote fuel oxidation and the cathode potential is
decreased to promote oxidizer reduction, the resulting overall fuel cell voltage (E
cell
)is
decreased. Relating to the waterfall analogy, the effect of drawing current is to raise the
bottom of the waterfall and lower the top of the waterfall, so that the distance between
the two is decreased and the potential voltage of the cell is reduced. Eventually, as the
losses increase with increasing current, the potential difference between the electrodes
will reach zero, and no additional current can be drawn. This is the limiting current,
i
limiting
shown in Figure 4.1. The limiting current is a result of the combined effect of all
polarizations in the system, which include be ohmic, kinetic, mass transfer, and crossover or
shorting.
Modeling the Fuel Cell Performance Curve: Zero-Dimensional Steady-State Model We
seek to develop a basic fuel cell model that can predict the polarization curve as a function
of engineering parameters. That is, we seek expressions for the terms in Eq. (4.1):
E
cell
= E
◦
(T , P) − η
a,a
−|η
a,c
|−η
r
− η
m,a
−|η
m,c
|−η
x
(4.4)
In this chapter, a single-phase zero-dimensional steady-state model will be developed that
describes the individual regions of the polarization curve. Obviously, this is a starting
point and should be considered as a general learning tool. Much more complex models

c04 JWPR067-Mench January 28, 2008 17:28 Char Count=
126 Performance Characterization of Fuel Cell Systems
are developed in the literature, including multidimensional, multiphase effects with various
length and time scales.
In the following sections, we will develop fundamental, analytical and emperical
models to describe each of the losses in Eq. (4.4). By the end of the chapter, the student
will be able to model fuel cell performance and generate an entire polarization curve for
any fuel cell system.
4.2 REGION I: ACTIVATION POLARIZATION
Activation polarization, which dominates losses at low current density, is the voltage
overpotential required to overcome the activation energy of the electrochemical reaction
on the catalytic surface. From Eq. (4.1)
E
cell
= E
◦
(T , P) − η
a,a
−|η
a,c
|
Activation
polarization
−η
r
− η
m,a
−|η
m,c
|−η
x
(4.5)
The activation polarization at the anode and cathode are shown as η
a,a
and η
a,c
, respec-
tively. Physically, the activation polarization represents the voltage loss required to ini-
tiate the reaction. In a somewhat similar fashion, consider a purely chemical reaction
between gasoline vapor and air in a combustion chamber. There needs to be some ig-
nition energy input to the system to enable the spontaneous reaction to proceed. In an
electrochemical system, this manifests as voltage losses, which decrease the original max-
imum potential energy represented by the theoretical open-circuit potential of the fuel
cell.
Electrical Double Layer In addition to an analytical expression for the activation po-
larization at an electrode which we will develop in this chapter, an understanding of the
microscopic process occurring at the electrode during charge transfer is also important.
A very natural question often arises when discussion of the activation overvoltage is first
introduced: What is the physical nature of the activation polarization and how exactly does
the charge transfer reaction proceed?
Between an electrode and the electrolyte, there exists a complex structure known as the
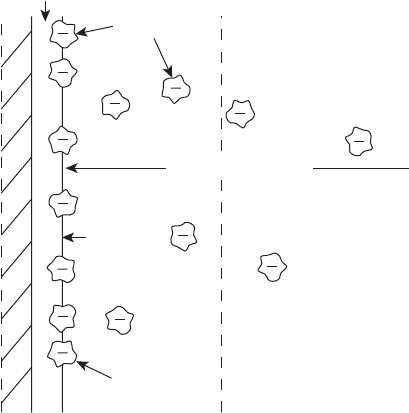
electrical double layer. At the electrode surface and in the adjacent electrolyte, a buildup
of charge occurs, as illustrated in Figure 4.6. The sign of the charge along the electrode
surface depends on the electrode. At the anode, the potential is lower than the surrounding
electrolyte, so the there is a buildup of negative charge along the surface of the catalyst
and a positive charge in the surrounding electrolyte forming the double-layer structure.
The double layer consists of a complex structure including an inner Helmholtz plane (IHP)
that exists along the electrical centers of specifically adsorbed reactant ions (e.g., adsorbed
hydrogen at the anode of a hydrogen fuel cell). In general, the sum of charges on the solution
sides must be equal to that on the electrode side, regardless of the sign of the ionic charge
in the IHP. Beyond the IHP, an outer Helmholtz plane (OHP) is formed along the locus
of the centers of the nearest layer of solvated (hydrated) ions in the electrolyte. Beyond
the OHP, other solvated ions in the electrolyte can also interact with the catalyst surface
through long-range electrostatic interaction in the diffuse layer. All three layers form the
double layer which is typically less than 10 nm deep into the electrolyte.
c04 JWPR067-Mench January 28, 2008 17:28 Char Count=
4.2 Region I: Activation Polarization 127
Diffuse double layer
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
Helmholtz layer
Ionic excess
Helmholtz
surface
Adsorbed ions
Figure 4.6 Schematic of electrical double layer. (Reproduced from [1].)
At the electrolyte–electrode interface, the buildup of charge occurs across the double
layer. The ionic species charge transfer reaction is driven by this potential difference. The
voltage difference required to drive a given electrochemical reaction rate (current) across
the catalyst–electrolyte interface is the source of the activation overpotential. That is, for
a given current, a certain potential buildup across the double layer is required to force the
charge transfer. The role of the catalyst is to enable reaction to occur with a low buildup
of charge. To illustrate the high driving force for reaction generated, consider a typical
oxidation activation overvoltage of ∼0.2 V at an electrode over an estimated double-layer
distance of 10 nm. The electric field strength is an amazing 2.0 × 10
8
V/cm, which
would be equivalent to 20,000 MV across 1 m distance! No wonder the reaction takes
place.
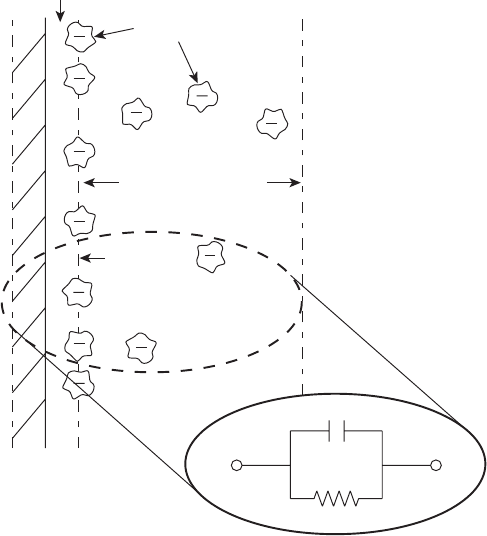
The discontinuity of charge physically behaves like a capacitor, as illustrated in Figure
4.7. Typical capacitances are on the order of 5–20 mF/cm
2
[2]. The fuel cell (and other
electrochemical systems) can therefore be modeled as a resistance–capacitance RC circuit
(R being the ohmic drop and charge transfer resistance in the circuit). Actually, the whole
fuel cell can be modeled as a multiple resistance and capacitor network. The science of the
use of an equivalent electrical circuit analogy to describe electrode charge transfer processes
is an active area of research [3] and is considerably more complicated than shown here. The
concepts, however, can be used to glean information about the electrode dynamics, charge
transfer resistance, and other quantities, as discussed in Chapter 8.
Activation Polarization Activation polarization losses are highly nonlinear with current
and manifest as a sharp initial drop in cell voltage from open-circuit conditions followed by
diminishing additional losses as the current is increased through ohmic and concentration
polarization dominated regions, as shown in region I of Figure 4.1.
c04 JWPR067-Mench January 28, 2008 17:28 Char Count=
128 Performance Characterization of Fuel Cell Systems
Diffuse double layer
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
+
Helmholtz layer
Ionic excess
Helmholtz
surface
R
C
Figure 4.7 Schematic of electrical double layer with electrical circuit analogy. (Adapted from [1].)
Different activation losses occur at each electrode. In fact, the reactions at each elec-
trode are only linked through conservation of charge. That is, the current passed through
the anode must equal the current through the cathode:
|
i
cell
|
=
|
i
a
|
=
|
i
c
|
(4.6)
where we have used absolute values to avoid conflicts with sign conventions for the direction
of current. Although the net current is identical at each electrode, the polarization losses
required to achieve this level of current on each electrode are independent. One can imagine
the resistance to reaction at an electrode as a reaction friction, that may be different at the
anode and cathode. The activation polarization losses are influenced by the following:
1. Reaction Mechanism In general, the more complex a reaction mechanism, the
greater the overpotential required to break the chemical bonds and generate cur-
rent. For example, the HOR is less complex and involves fewer intermediate steps
than the methanol electrooxidation, so that for the same current the overpotential
for methanol oxidation is greater than for hydrogen oxidation. There are steric
(geometric) and other factors involved as well.
2. Catalyst Type A poor choice of catalyst will require a greater polarization to enable
the electrochemical reaction at that electrode to proceed. Each electrochemical
reaction has different preferred catalysts, so that there is no single perfect catalyst.
c04 JWPR067-Mench January 28, 2008 17:28 Char Count=
4.2 Region I: Activation Polarization 129
Generally, for low-temperature reactions, noble metal catalysts such as platinum
work well, and for higher temperature fuel cells, less expensive metals such as
nickel and other alloys can be used.
3. Catalyst Layer Morphology The microstructure of the catalyst has a strong effect
on the overall effectiveness of the catalyst. From the generic fuel cell description
of Chapter 2, the catalyst structure is highly three dimensional, and the potential
reaction locations are limited to those with immediate access to ionic and electronic
conductors, catalyst, and reactant gas. Maximization of this triple phase boundary
area will reduce the activation polarization losses for a given current density. A
catalyst layer with very low triple-phase boundary area density will have reduced
number of available reaction sites and reduced performance.
4. Operating Parameters Electrochemical reactions are catalyzed by increased tem-
perature, just like chemical reactions (think about the chemical reaction analog: a
heated mixture of gasoline vapor and air will react more readily than a cold mix-
ture). Since the molecules participating in the reaction have a higher kinetic energy
with increased temperature, the probability of collisions, as well as the fraction of
collisions resulting in reaction, is strongly related to temperature. Other thermody-
namic operating parameters such as pressure can have an effect as well, although
temperature generally has the strongest impact.
5. Impurities and Poisons The presence of any impurities or catalyst poisons in the
reacting flow can have a highly deleterious effect on performance. Some impurities
such as carbon monoxide and sulfur dioxide can reduce performance dramatically
for certain fuel cells, even in levels as low as parts per million (ppm) or parts per
billion (ppb). Each catalyst and fuel cell has different poisons. For instance, carbon
monoxide is a serious poison for low-temperature PEFCs but can be oxidized as a
fuel in high-temperature MCFCs and SOFCs.
6. Species Concentrations The species dependence on the expected Nernst voltage is
a result of the equilibrium thermodynamic effect. During the highly nonequilibrium
electrochemical reaction process, there is also a concentration effect on the acti-
vation polarization. As the reacting species become more sparse, the double-layer
polarization required to attract sufficient reactants increases. In the extreme case,
no reaction can take place across the double layer if there is no reactant available.
7. Age The catalyst performance of a given fuel cell can change significantly over the
operating lifetime of the fuel cell. This is generally a result of physical morphological
or chemical changes in the catalyst. The catalyst with the highest initial reactivity
may not be the best choice for a given application if the performance over time is
not stable.
8. Service History The service history of the fuel cell, including environment, load
cycling, and voltage history, has an effect on the performance of a fuel cell. Dynamic
load cycles can accelerate degradation, as discussed in Chapter 7.
The positive side of all of these activation loss dependencies is that most of them can be
engineered to some degree to reduce losses and increase efficiency.
To move from an equilibrium state and draw useful current, a net reaction must
occur. Figure 4.8 is a schematic of the reaction coordinate for a galvanic (exothermic)
electrochemical (or chemical) reaction, as described by transition state theory [4]. At
