Mattingly J.D., Heiser W.H., Pratt D.T. Aircraft Engine Design
Подождите немного. Документ загружается.

336 AIRCRAFT ENGINE DESIGN
In Eqs. (9.12-9.15)
ni
is the mass-specific mole number of the ith species (i =
1, NS); T
is the temperature; p is the mixture mass density, ~j and ot~j are the
stoichiometric coefficients of species i (i = 1,
NS)
in reaction j (j = 1, J J) as
a reactant and as a product species, respectively;
kj
and
k_j are
the forward and
reverse rate constants in the modified Arrhenius rate expressions for
Rj and R_j,
which in turn are the forward and reverse rates of the jth reaction
(j = 1, J J). NS
is
the total number of distinct chemical species in the gas mixture, and
JJ
is the total
number of independent chemical reactions prescribed in the reaction mechanism.
A typical form for
kj
is given in Table 9.3.
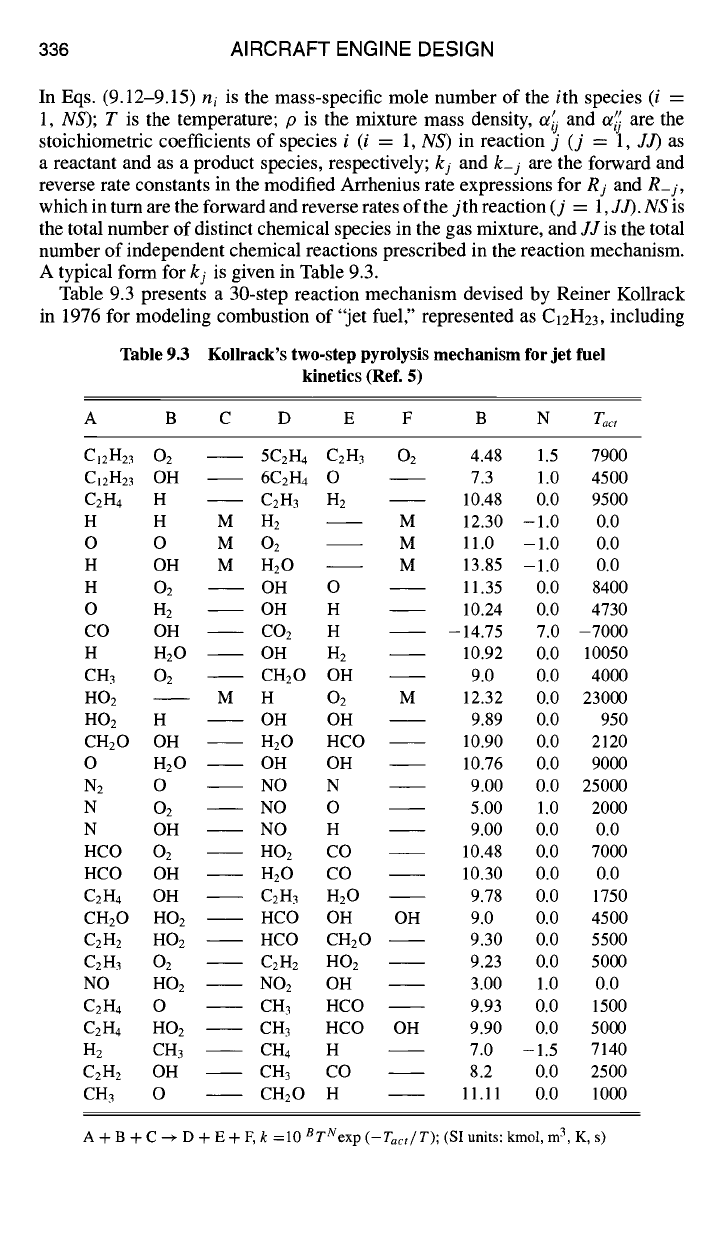
Table 9.3 presents a 30-step reaction mechanism devised by Reiner Kollrack
in 1976 for modeling combustion of "jet fuel," represented as C12H23, including
Table 9.3 Kollrack's two-step pyrolysis mechanism for jet fuel
kinetics (Ref. 5)
A B C D E F B N Tact
CI2H23 02 5C2H4 C2H3 02 4.48 1.5 7900
C12H23 OH
6C2H4 O 7.3 1.0 4500
C2H4
H
C2H3 H 2
10.48 0.0 9500
H H M H2 M 12.30 -1.0 0.0
O O M 02 M 11.0 -1.0 0.0
H OH M H20 M 13.85 -1.0 0.0
H 02 OH O 11.35 0.0 8400
O H2 OH H 10.24 0.0 4730
CO OH CO2 H - 14.75 7.0 -7000
H H20 OH H2 10.92 0.0 10050
CH3 02 CH20 OH 9.0 0.0 4000
HO2 M H 02 M 12.32 0.0 23000
HO2 H OH OH 9.89 0.0 950
CH20 OH H20 HCO 10.90 0.0 2120
O H20 OH OH 10.76 0.0 9000
N2 O NO N 9.00 0.0 25000
N O2 NO O 5.00 1.0 2000
N OH NO H 9.00 0.0 0.0
HCO O2 HO2 CO 10.48 0.0 7000
HCO OH
H20
CO 10.30 0.0 0.0
C2H4 OH C2H3 H20 9.78 0.0 1750
CH20 HO2 HCO OH OH 9.0 0.0 4500
C2H2 HO2 HCO CH20 9.30 0.0 5500
C2H3 02
C2H2 HO2 9.23 0.0 5000
NO HO2 NO2 OH 3.00 1.0 0.0
C2H4 O CH 3
HCO 9.93 0.0 1500
C2H4 HO2 CH 3
HCO OH 9.90 0.0 5000
H2 CH3 CH4 H 7.0 -1.5 7140
C2H2
OH
CH3
CO 8.2 0.0 2500
CH3 O CH20 H 11.11 0.0 1000
A + B + C ~ D + E + F, k =10 B TNexp
(-Tact~T);
(SI units: kmol, m 3, K, s)

DESIGN: COMBUSTION SYSTEMS 337
production of NOx (Ref. 5). The first two reactions in Table 9.3 are not elementary
physico-chemical reactions, but are "global" reactions, devised to represent a very
great number of complex and largely unknown steps in the pyrolysis of the fuel
molecules to smaller molecular fragments. In Table 9.3 the symbol M stands for
"third body," meaning any species acting as a gas-phase catalyst.
For adiabatic and no-work reaction, conservation of static enthalpy, Eq. (9.10),
constitutes an algebraic constraint on Eqs. (9.12) to (9.15). The mass density p in
Eqs. (9.13-9.15) is determined from the temperature and pressure by the equation
of state for an ideal gas, p = P/(RTnm), where k is the universal gas constant
and nm is the sum of the mole numbers, nm "-- ~NS 1 hi.
Constant-pressure batch reaction. Consider an initially quiescent uniform
mixture of fuel and air in a shock tube. At time zero a shock wave passes quickly
through the mixture, rapidly raising the pressure and temperature well above the
ignition limits. The experiment is designed so that the pressure remains constant
until chemical equilibrium is approached. Because there is no mass convection,
Eq. (9.12) simplifies to
dni
-----j~(nk, T) i,k=l,NS (9.16)
dt
The subsequent events leading to release of sensible thermal energy occur in
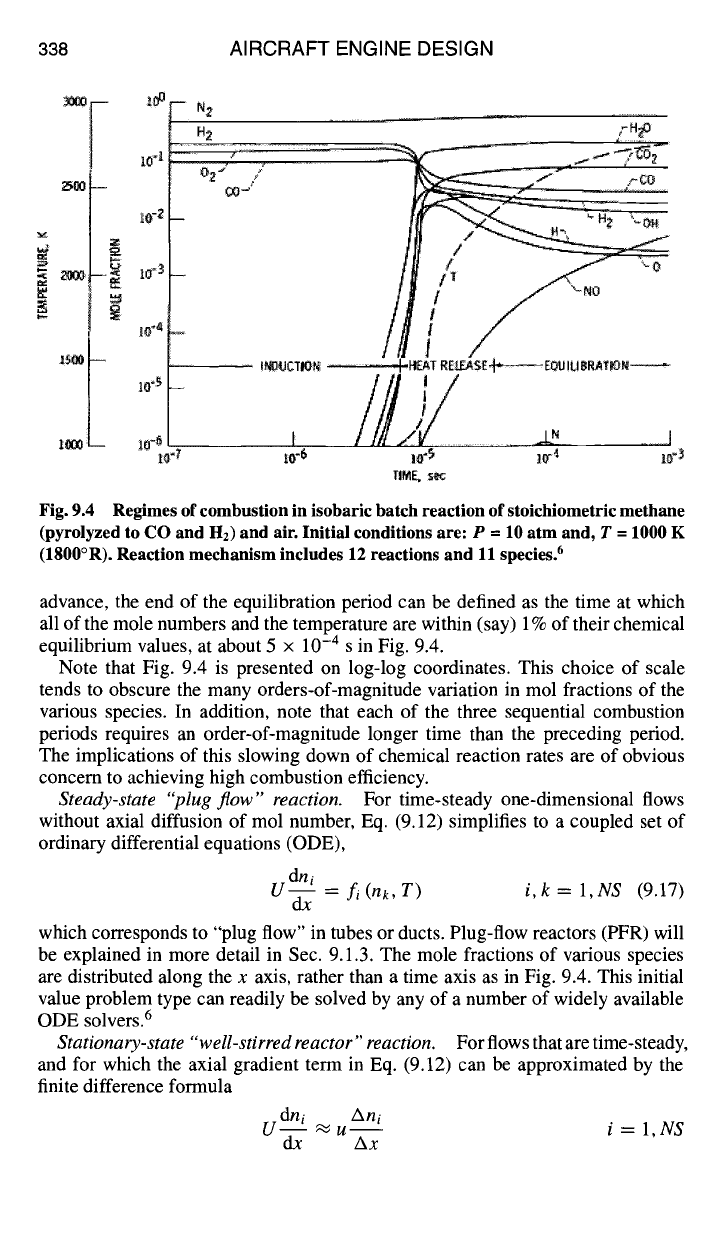
three distinctly different chemical-physical periods, as illustrated in Fig. 9.2. These
three periods or regimes are called the induction,heat release, and equilibration
regimes.
The induction period is the time interval immediately following some form of
homogeneous bulk ignition. In the homogeneous (completely micromixed) case
under consideration, ignition occurs as a result of shock compression, but such
ignition occurs only if fuel and air are micromixed to flammable proportions
(0.2 < ~b < 2.0). During the induction period, the mole numbers of reaction in-
termediates or chain carriers, such as O, H, OH, HO2, and H202, increase by
many orders of magnitude from near-zero values in the initial mixture. During
this period, the coupling with the enthalpy equation Eq. (9.10) is very weak, so
that the induction process is essentially isothermal as well as adiabatic, that is,
no sensible energy is released. When the intermediate species have reached some
critical value of concentration sufficient to begin to react with fuel and oxygen
molecules, the process of releasing sensible thermal energy can begin. Therefore,
the induction period ends when the mixture temperature begins to rapidly increase.
In the methane/air example illustrated in Fig. 9.4, the induction time (also called
the ignition delay time) is about 8 x 10 -6 s.
During the heat release period, very rapid changes in temperature and species
mol numbers occur. During this period, the species equations and the energy
conservation equations are all very strongly coupled. The heat release period ends
when the reaction intermediates have all passed their peak values, at about 1 x 10 -5 s
in Fig. 9.4.
The equilibration period begins when all species mole numbers begin a decaying-
exponential approach toward their respective equilibrium values. The equilibration
process does not have a clearly defined termination, because of the asymptotic
nature of the approach to the chemical equilibrium state. However, because equi-
librium values of temperature and species concentration can be determined in
338
°F
:@
I0 q
N2
AIRCRAFT ENGINE DESIGN
H2
~Hp
IV~
--
~ -- ~ l~ 3 __
I
1500 ~ t
10~5
_
I~0-
10-~1~ q
0 2 ~
/
CX)J"
J
IT
t ~-N0
!
I~UC'TIOt¢
T
REUSE-l*'
EOUIUi~R,AT~I~
//I
I ////]J/
...........
.A N
........ I
10-6 10-~ tp-~
iV~
TIME.
sec
Fig. 9.4 Regimes of combustion in isobaric batch reaction of stoichiometric methane
(pyrolyzed to CO and H2) and air. Initial conditions are: P --- 10 atm and, T = 1000 K
(1800°R). Reaction mechanism includes 12 reactions and 11 species. 6
advance, the end of the equilibration period can be defined as the time at which
all of the mole numbers and the temperature are within (say) 1% of their chemical
equilibrium values, at about 5 × 10 -4 s in Fig. 9.4.
Note that Fig. 9.4 is presented on log-log coordinates. This choice of scale
tends to obscure the many orders-of-magnitude variation in mol fractions of the
various species. In addition, note that each of the three sequential combustion
periods requires an order-of-magnitude longer time than the preceding period.
The implications of this slowing down of chemical reaction rates are of obvious
concern to achieving high combustion efficiency.
Steady-state "plug flow" reaction.
For time-steady one-dimensional flows
without axial diffusion of mol number, Eq. (9.12) simplifies to a coupled set of
ordinary differential equations (ODE),
U~--~ = fi (nk, T) i, k = 1, NS
(9.17)
which corresponds to "plug flow" in tubes or ducts. Plug-flow reactors (PFR) will
be explained in more detail in Sec. 9.1.3. The mole fractions of various species
are distributed along the x axis, rather than a time axis as in Fig. 9.4. This initial
value problem type can readily be solved by any of a number of widely available
ODE solvers. 6
Stationary-state "well-stirred reactor" reaction.
For flows that are time-steady,
and for which the axial gradient term in Eq. (9.12) can be approximated by the
finite difference formula
u~i
Ani
i = 1, NS
~UAx
dx
DESIGN: COMBUSTION SYSTEMS 339
So that Eq. (9.12) can be rewritten as
(ni - n~)
pUA-- -- pf~(nk, T) i,k = l,NS
(9.18)
AAx
where n* refers to the upstream or entry value of
n i
into the control volume of
volume V =
AAx and pAu
= rn. With this notation the resulting well-stirred
reactor (WSR) equations become 4
rn
v(ni - n~) = pfi(nk, T) i, k = 1, NS
(9.19)
Note that Eq. (9.19) can also be derived directly from an elementary control
volume mass balance.
The WSR model for combustion will be further explained in Sec. 9.1.3. For
present purposes, it is sufficient to just point out that WSR solutions have features
that are markedly and significantly different from PFR solutions. For one, the
WSR equations are a coupled set of nonlinear algebraic equations, rather than
ordinary differential equations, and therefore require different numerical methods
for their solution. In addition, WSR solutions feature multiple stationary states, and
decisions must be made as to which of those solutions are desireable or achievable
in practice. The real-world implications of these features will be dealt with in
Sec. 9.1.3.
The AEDsys program KINETX solves the WSR equations using the Kollrack
mechanism given in Table 9.3.
9.1.3 Combustion Stability and Flameholding
It has long been known that it is possible to sustain a self-propagating (that is,
continuously self-igniting) flame in a steady-flow combustor, even when the veloc-
ity of reactants entering greatly exceeds the turbulent flame speed of a stoichiomet-
ric mixture of fuel and air at combustor entrance temperatures and pressures. For
example, while the fastest measured turbulent flame speeds for hydrocarbon-air
combustion are in the range 10-15 ft/s, combustor entry airspeeds are typically
200-300 ft/s:
How is it possible for the mean combustor flow velocity to be more than one
order of magnitude greater than the highest known turbulent flame speed? This is
made possible by the insertion of a flameholder, which may be a "bluff body" or
"vee-gutter," or some other form of flow blockage, into the stream. 3'7 The separated
flow in the aerodynamic wake behind the flameholder causes the flow to recirculate
from downstream to upstream, so that some fraction of the reactants can reside
for a sufficiently long time to mix with hot product gases, thereby sustaining a
continuous combustion reaction.
9.1.3.1 Chemical reactor theory.
The interplay of mixing and chemical
processes that enable flameholding in a wake region can be better understood
by using chemical engineering concepts called reaction engineering or chemical
reactor theory, s
Steady-flow chemical reactors may be represented by a spectrum of mixing
models, varying between two idealized extremes: the zero-dimensional WSR and
340 AIRCRAFT ENGINE DESIGN
the one-dimensional PFR. In theory, the two extreme reactor types may be char-
acterized by means of a hypothetical tracer-response experiment, in which a step
function concentration of tracer substance is introduced into the feed stream at
some instant, and the subsequent concentration of tracer at reactor exit (the re-
sponse) is monitored. The WSR response to such an input step function would
be a decaying-exponential rise in tracer concentration, with no time delay from
inlet step change to the initial rise of response signal at exit. The PFR response
would be simply the appearance of the same step change at exit, precisely one
mean residence time later. The negative time derivative of the response function
to a step input function is called the residence time distribution function and can
be shown to be the probability density for a fluid particle, having entered at time
to, to exit at time (t - to). s
PFRs offer no conceptual difficulties the concept is familiar, because all steady-
flow systems feature an inflow at one end and outflow at the other, so that one can
readily envision a "plug" of fluid entering and leaving without axial mixing.
The WSR concept is somewhat less familiar. The WSR is an idealization of
flow systems in which the flow stream is recirculated in some way to promote
gross mixing of products, reactants, and reaction intermediates. The physical re-
actor being modeled is usually referred to as a backmixed or jet-stirred reactor. In
terms of turbulent mixing theory, the WSR assumption implies that the gross re-
circulating flow velocity is so great, and the turbulent diffusion across streamlines
so rapid, that every entering fluid particle has instantaneous access to every other
fluid particle. This assumption, however, does not require that mass or energy
transfer caused by molecular diffusion will necessarily occur between the fluid
particles. WSRs may therefore be subdivided into two important subclasses:
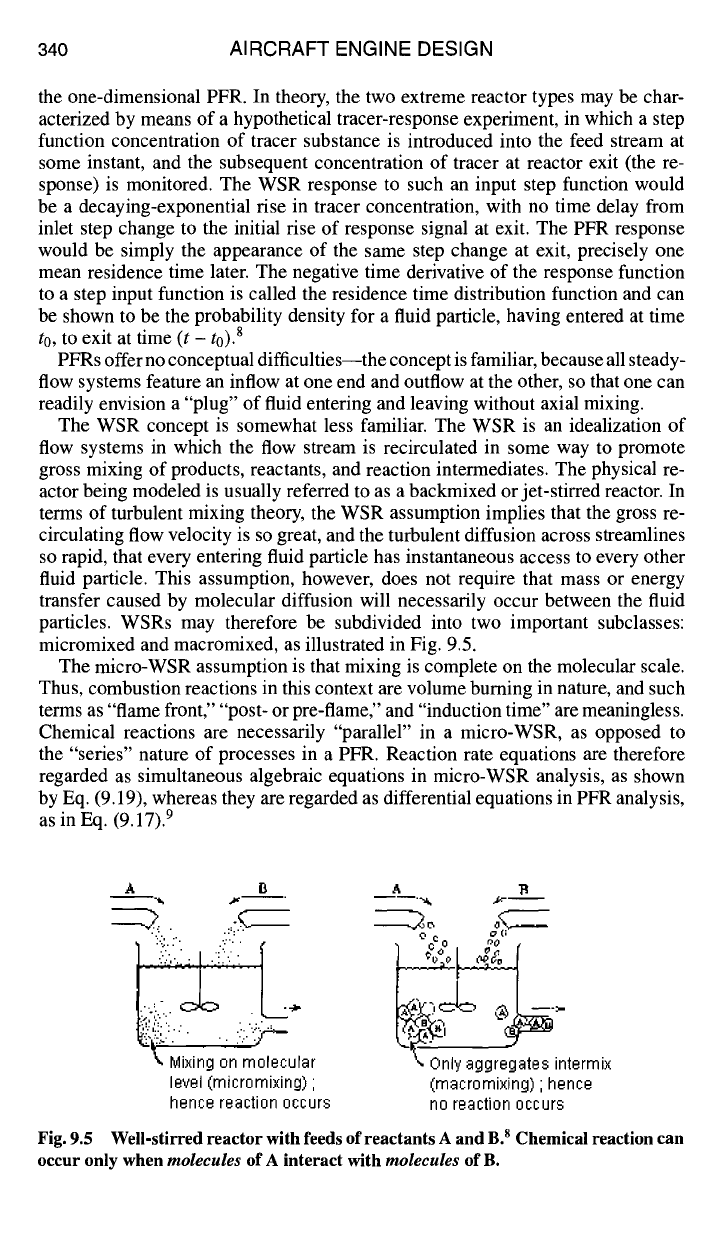
micromixed and macromixed, as illustrated in Fig. 9.5.
The micro-WSR assumption is that mixing is complete on the molecular scale.
Thus, combustion reactions in this context are volume burning in nature, and such
terms as "flame front," "post- or pre-flame," and "induction time" are meaningless.
Chemical reactions are necessarily "parallel" in a micro-WSR, as opposed to
the "series" nature of processes in a PFR. Reaction rate equations are therefore
regarded as simultaneous algebraic equations in micro-WSR analysis, as shown
by Eq. (9.19), whereas they are regarded as differential equations in PFR analysis,
as in Eq. (9.17). 9
A B A T~
---~ ~ ...... -.~ ~---
~ Mi×ing on molecular
level (micromixing) • (macromixing) ; hence
hence reaction occurs no reaction occurs
Fig. 9.5 Well-stirred reactor with feeds of reactants A and B. s Chemical reaction can
occur only when molecules of A interact with molecules of B.

DESIGN: COMBUSTION SYSTEMS 341
The macro-WSR model assumes mixing to be complete on a scale small by
comparison with reactor dimensions, but large on a molecular scale. The small
macrovolumes are assumed to retain their individual molecular integrity, acting
as little batch reactors, but are uniformly distributed throughout the vessel instan-
taneously upon entry. The theoretical decaying-exponential response curve that
characterizes a WSR cannot in principle discriminate between the micro- and
macromixed condition.
The micro-WSR concept has meaning only for homogeneous or single-phase
flow systems, whereas the macro-WSR concept has utility when dealing with two-
phase flows. In this case the macrovolume may represent a liquid droplet or a solid
particle. For homogeneous fluid flow the macrovolumes may represent idealized
turbulent eddies or"turbules." In the context of homogeneous gas flows, the micro-
WSR assumption is equivalent to assuming that turbulence is generated, cascades
through the diminishing spectrum of eddy sizes, and dissipates--all this on a
timescale very small compared with the mean residence time. The macro-WSR
assumption corresponds to assuming that the turbulent eddies enter in the feed
stream already formed and reside in the reactor in too short an interval to undergo
coalescence, dispersion, or dissipation prior to exiting the reactor. In designing and
analyzing both combustors and afterburners, both mixing models will be used. In
the micromixed reacting zones shown as cross-hatched areas in Figs. 9.1 and
9.2, combustion chemical reactions will be assumed to occur as in a micromixed
WSR. In the dilution zone of the main burner, on the other hand, because no further
chemical reactions occur the flow can be assumed to be macromixed.
The Bragg criterion.
A self-stabilized, steady-state, steady flow combustion
system, such as that in a gas turbine engine, requires two distinct subregions,
known as the primary and secondary zones. An idealized main burner with a WSR
primary zone and a PFR secondary zone is known as a "Bragg combustor" and is
illustrated in Fig. 9.6.
As the actual primary zone is a region of intense backmixing or recirculation,
it is idealized or modeled as a WSR, within which inflowing reactants are mixed
with previously burned gases that are continuously recirculated within the primary
zone. The effluent from the primary zone, being a mixture of reactants, products,
and reaction intermediates, is necessarily at a combustion efficiency of (typically)
less than 80%.
The main function of the secondary zone, which is idealized as a PFR, is to allow
sufficient convective residence time or "stay time" for the primary zone effluent
to burn out (approach 100% combustion efficiency) before exiting the combustor.
In aircraft gas turbine design, where combustor volume, weight, and frontal
area are limited by airframe considerations, this question naturally arises: "Given
J
Air WSR
--1
Fig. 9.6 Bragg combustor, an idealized gas turbine combustor with WSR primary
zone and PFR secondary zone.
342 AIRCRAFT ENGINE DESIGN
a fixed axial distance and/or spatial volume within which combustion must take
place, what is the optimal division of this space between primary and secondary
zones?" The answer, arrived at first through chemical reactor modeling and later
verified in practice, was summarized in the late 1940s by S.L. Bragg, a pioneer in
British gas turbine development, in what has become known as the Bragg criterion:
The maximum overall combustor efficiency occurs when the primary zone is
operating at incipient blowout, lo
This is not intuitively obvious, as the smallest value of primary zone combustion
efficiency occurs at incipient blowout! However, at incipient blowout the chemical
reaction rate in the primary zone is at its maximum value, and also the primary
zone effluent has its highest concentration of reaction intermediates. In addition,
the maximum secondary zone volume provides the greatest convective stay time
possible to promote near-complete combustion at secondary zone exit. However,
in actual practice combustors are never operated at incipient blowout because
experience shows that incipient blowout means eventual blowout!
Blowout.
To illustrate these flameholding concepts, a greatly simplified ver-
sion of the atom-balance equation [Eq. (9.5)], will be employed, in which it is
assumed that, for fuel-lean mixtures only (4~ < 1), the excess air remaining after
CO2 and H20 are formed is simply present as a diluent:
79
~CxHyq- (x~- 4) {O2q- -~N2 }
/
--+ x~bCO2 + 2~bHzO + (1 + Y ) (1 - ~b)O2 + ~N2 (9.20)
Also, for incomplete combustion, the atom-balance equation Eq. (9.20) may be
further generalized as
79 N /
~)Cxny-~-(x "~Y)
02"~- ~ 2 /
~e(l-~cx~y+xeEco2+~eEH2O+ +~) (l-ee~o2+~i 2 /
(9.21)
where e is the "fraction reacted" or combustion reaction progress variable, also
commonly known as the combustion efficiency.
When e = 1 in Eq. (9.21), the maximum value of T, the adiabatic flame tem-
perature
TAFt,
is realized. This temperature is determined by the energy balance
equation, Eqs. (9.9) and (9.10), which may be very well approximated by the linear
equation
dpfsthpR
TAFT "~ Ti "q- -- -- Ti -}- (b
ATma x (9.22)
Cp
where ATmax is the maximum temperature rise when both ~b = 1 and e = 1.
The static temperature T and the reaction progress variable e can be similarly
represented by the linear equation
T=Ti+e(TAvr-Ti)=Ti+e(qSfsth'~--------~R]
= Ti + eq~ATmax (9.23)
\ Cp /
DESIGN: COMBUSTION SYSTEMS 343
The volumetric mass rate of consumption of the fuel (Ibm fuel/s-ft 3) can be
expressed by a modified Arrhenius equation for the overall combustion reaction
RRf = MfAe-Tact/T[CxHy][02]
Ibm fuel/s-ft 3 (9.24)
where
My =
12x + y is the apparent molecular weight of the fuel, A is the
pre-exponential factor,
Taa
is the activation temperature of the reaction, and the
square-bracket terms denote the concentration (mols per unit volume) of fuel and
of oxygen, respectively.
The concentrations of fuel and oxygen may be expressed in terms of their
respective mol fractions Y, the mixture static temperature T, static pressure P,
molar density~, and the universal gas constant/~, by
[CxHy] =
PYGHy = YGHy
and
[02] =
PYo: = Yo:
The volumetric mass rate of disappearance of the fuel may then be rewritten as
RR f = My Ae- Tact/T YG l-b Yo2
(9.25)
The sum of the product mol numbers on the right-hand side (RHS) of Eq. (9.21)
is given by
100 (X+ 4) +¢[1 + (4- 1) e ] (9.26)
~Np = 21-
SO that the mole fractions of fuel and oxygen may be written from the RHS of
Eq. (9.21) as
¢(1 - e) (x + Y) (1 - Ce)
Ycx Hy
--
E Np and Yo2 = Z Np
respectively. With these subsitutions for mole fractions, Eq. (9.25) becomes
Ref--=Mf(x+4) dpAe-Tact/Z( P )2
RT-ENp
(1 - e)(1 - Ce) (9.27)
With the substitution of Eq. (9.26) for
ENp
and Eq. (9.23) for T, Eq. (9.27)
can be written with only the reaction progress variable e as an independent variable.
Note the limiting values of
RRf
in Eq. (9.27) for varying values of the reaction
progress variable e. Clearly,
RRf
approaches zero as e approaches 1. However,
note that
RRf
does not approach zero as e approaches zero, but rather to a small
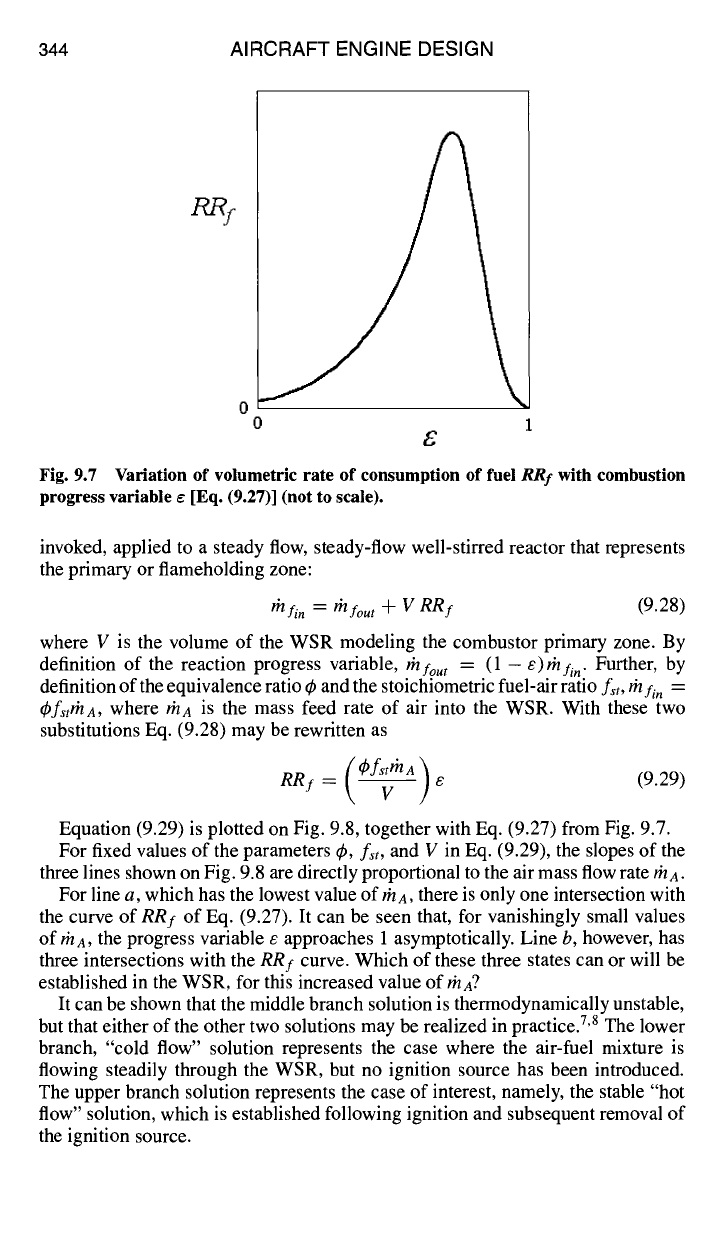
positive value. Equation (9.27) is plotted on Fig. 9.7.
From inspection of Fig. 9.7, it can be seen that there is an optimal value of
e between 0 and 1 (and corresponding temperature between T/ and
TAFT)
for
which
RRf
is a maximum. As one might guess, a design goal for afterburners and
combustor primary zones is to achieve as nearly as possible this maximum value
of
RRT
at some location in the combustion device.
The next question to be addressed is: Which operating point on Fig. 9.7 will be
realized in practice? To answer this, the principle of conservation of fuel mass is
344 AIRCRAFT ENGINE DESIGN
RRf
0
0
E
Fig. 9.7 Variation of volumetric rate of consumption of fuel
RRf
with combustion
progress variable e [Eq. (9.27)] (not to scale).
invoked, applied to a steady flow, steady-flow well-stirred reactor that represents
the primary or flameholding zone:
i?l fi n = 171fout -~- V RR f
(9.28)
where V is the volume of the WSR modeling the combustor primary zone. By
definition of the reaction progress variable,
r'nfout
= (1 -e)rhfi
..
Further, by
definition of the equivalence ratio 4> and the stoichiometric fuel-air ratio
fs, rh fin =
4~fstrhA,
where /?/A is the mass feed rate of air into the WSR. With these two
substitutions Eq. (9.28) may be rewritten as
RRI=(~)~
(9.29)
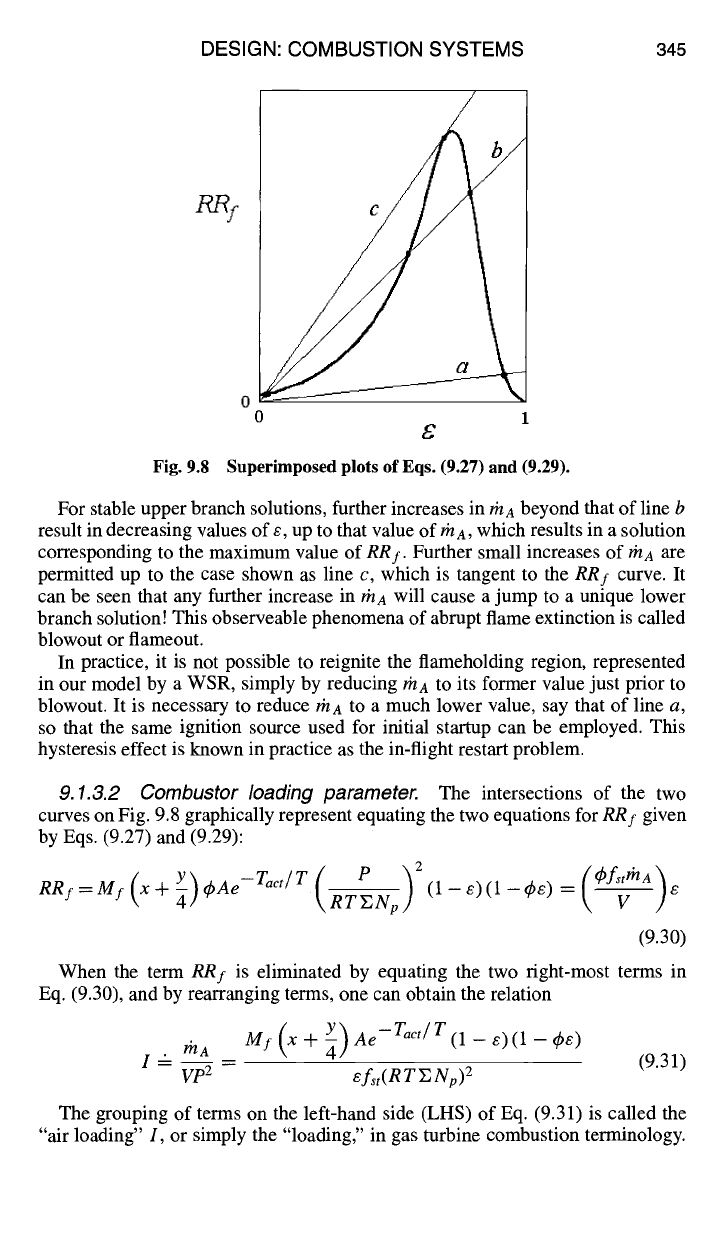
Equation (9.29) is plotted on Fig. 9.8, together with Eq. (9.27) from Fig. 9.7.
For fixed values of the parameters ~b,
fst,
and V in Eq. (9.29), the slopes of the
three lines shown on Fig. 9.8 are directly proportional to the air mass flow rate rhA.
For line a, which has the lowest value of rnA, there is only one intersection with
the curve of
RRf
of Eq. (9.27). It can be seen that, for vanishingly small values
of rnA, the progress variable e approaches 1 asymptotically. Line b, however, has
three intersections with the
RRI
curve. Which of these three states can or will be
established in the WSR, for this increased value of thA?
It can be shown that the middle branch solution is thermodynamically unstable,
but that either of the other two solutions may be realized in practice. 7,8 The lower
branch, "cold flow" solution represents the case where the air-fuel mixture is
flowing steadily through the WSR, but no ignition source has been introduced.
The upper branch solution represents the case of interest, namely, the stable "hot
flow" solution, which is established following ignition and subsequent removal of
the ignition source.
DESIGN: COMBUSTION SYSTEMS 345
/LRy
Fig. 9.8
0
£
Superimposed plots of Eqs. (9.27) and (9.29).
For stable upper branch solutions, further increases in
#/A
beyond that of line b
result in decreasing values of e, up to that value of rha, which results in a solution
corresponding to the maximum value of
RRf.
Further small increases of/~/A
are
permitted up to the case shown as line c, which is tangent to the
RRf
curve. It
can be seen that any further increase in rha will cause a jump to a unique lower
branch solution! This observeable phenomena of abrupt flame extinction is called
blowout or flameout.
In practice, it is not possible to reignite the flameholding region, represented
in our model by a WSR, simply by reducing
#/a to
its former value just prior to
blowout. It is necessary to reduce/hA to a much lower value, say that of line a,
so that the same ignition source used for initial startup can be employed. This
hysteresis effect is known in practice as the in-flight restart problem.
9.1.3.2 Combustor loading parameter.
The intersections of the two
curves on Fig. 9.8 graphically represent equating the two equations for
RR I
given
by Eqs. (9.27) and (9.29):
RRf=Mz(x+~)¢Ae-T"¢t/T(RT-~Np)
(1-e)(1-q~s)=, -- ~--
(9.30)
When the term
RR[
is eliminated by equating the two right-most terms in
Eq. (9.30), and by rearranging terms, one can obtain the relation
I--
l~'lA -- mf(x + 4) Ae-T"ct/T(1-s)(1-(pe)
(9.31)
VP 2
efst(R T E
Np)2
The grouping of terms on the left-hand side (LHS) of Eq. (9.31) is called the
"air loading" I, or simply the "loading," in gas turbine combustion terminology.
