Martin P.M. Handbook of Deposition Technologies for Films and Coatings, Third Edition: Science, Applications and Technology
Подождите немного. Документ загружается.

Thin Film Nucleation, Growth, and Microstructural Evolution 571
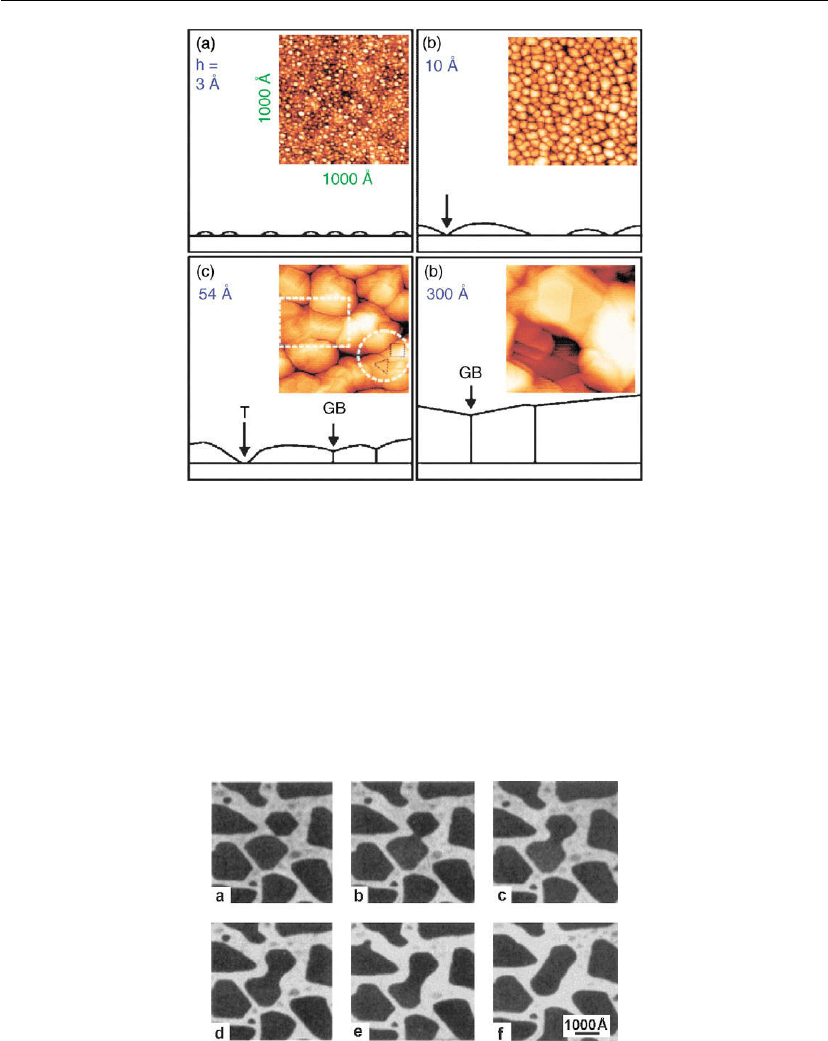
Figure 12.13: STM images (1000 × 1000
˚
A
2
) of Ag layers deposited by MBE on a-Si substrates to
thicknesses of (a) 3, (b) 10, (c) 54, and (d) 300
˚
A. The deposition rate was R = 0.08
˚
A/s.
Schematic cross-sectional representations are also shown in which T and GB symbolize trenches
and grain boundaries, respectively. (Adapted from [51].)
The initial contact point is at corner facets of the two islands. The driving force for
coalescence is a reduction in surface energy by curvature-driven diffusion causing the islands
to become taller and more compact. (A simple geometric calculation of the change in total
surface area due to the coalescence of two half-sphere-shaped islands into one larger
Figure 12.14: A series of TEM images from a movie file showing coalescence of two Au islands on
MoS
2
(0002) during in situ evaporative deposition at 400
C: (a) arbitrary t = 0, (b) t = 0.06 s, (c)
0.19 s, (d) 0.5 s, (e) 1.06 s, and (f) 6.19 s. (Adapted from [52, 53].)
572 Chapter 12
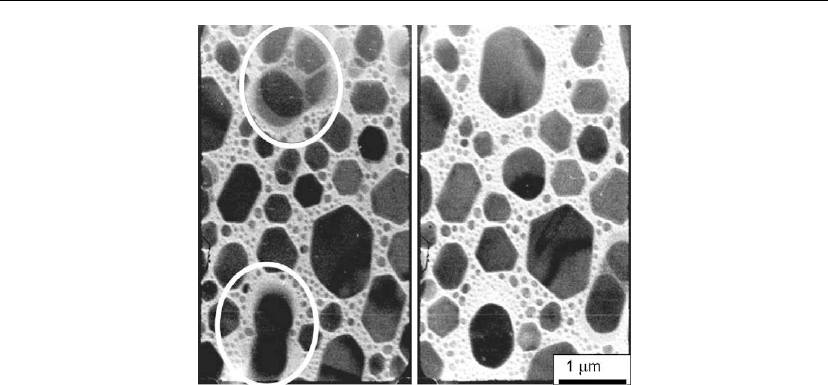
Figure 12.15: In situ plan-view TEM micrographs obtained during the growth and coalescence of
In islands deposited at 5
˚
A/s on amorphous C substrates at T
s
= 540
C. The time lapse between
obtaining the left and right images is 0.3 s. Note the denuded zones surrounding the coalesced
islands. (From [54].)
half-sphere islands, ignoring the difference in interface area, yields a decrease in surface
energy of ∼ 26%). Over the next ∼ 6 s from image (b) to image (f), the coalesced island
relaxes back toward a hexagonal shape. During this process, the total projected area on the
substrate decreases leading to the formation of a cluster denuded zone around the coalesced
island, favoring additional secondary nucleation.
Plan-view TEM images showing denuded areas surrounding coalesced islands [54] are
reproduced in Figure 12.15. The images, separated by 0.3 s, were obtained in situ during
evaporation of In on an amorphous C substrate at T
s
=40
◦
C. The deposition rate was 5
˚
A/s.
Coalescence is often described as occurring in a liquid-like manner, although electron
diffraction results show that it is generally a solid-state reaction. Liquid coalescence has,
however, been reported for films grown at high T
s
/T
m
ratios [55] due to freezing point
depression associated with small clusters [56]. Section 12.4.2 contains a more detailed
discussion of coalescence kinetics using an analytical model to describe in situ STM results
during the simpler case of 2D homoepitaxial growth, in this case for TiN(001) and TiN(111)
layers grown by reactive magnetron sputter deposition in a UHV system.
In the early stages of deposition, when the fractional surface coverage is still relatively low,
composite islands can relax to a pseudomorphic crystallographic shape following coalescence,
as illustrated in Figure 12.15, and the islands are still single crystals. This process, termed
complete coalescence as mutual island misorientation is eliminated via recrystallization (grain

Thin Film Nucleation, Growth, and Microstructural Evolution 573
boundary diffusion), ultimately leads to an as-deposited average grain size which is much
larger than average island sizes prior to the onset of coalescence. The driving force is the
resulting decrease in the total grain boundary interfacial length. For metals, grain boundary
tensions are of the order of one-third of the corresponding surface tensions [57]. However, as
continued coalescence of larger islands occurs, recrystallization kinetics become too slow at
lower growth temperatures and the composite islands are polycrystalline with trapped
large-angle grain boundaries. In the final stages of coalescence, holes and channels are left in
an otherwise continuous film. These voids are eventually filled by secondary nucleation, island
growth, and further coalescence.
The average thickness at which three-dimensionally nucleated films become continuous
depends on the choice of film and substrate materials, supersaturation, contamination, and T
s
.
This is clear from the combination of Eqs (12.7) and (12.10). Films which display a dense
population of small islands during the initial stages of deposition will become continuous at a
relatively low average film thickness, typically a few tens to a few hundreds of
˚
A. However,
films consisting of a low areal density of large islands during the early stages of deposition will
exhibit an island structure which persists up to relatively large average film thicknesses.
In addition to large-angle grain boundaries, polycrystalline films grown at low T
s
/T
m
often
exhibit a high density of other mechanical defects such as dislocations, dislocation loops,
twins, stacking faults, and low-angle boundaries. Dislocations are generated to partially relax
stresses produced in continuous films due to film/substrate lattice parameter and thermal
expansion mismatch. Contamination can also play an important role by preferential adsorption
on different surface facets and step edges and by inhibiting island reorientation and
recrystallization during coalescence [8].
12.4 Two-Dimensional Nucleation and Growth
Referring again to Eq. (12.6), 2D Frank–van der Mewre growth is energetically favorable
when a
2
r
2
γ
s−v
≥ a
1
r
2
γ
f−v
+ a
2
r
2
γ
s−f
(see schematic diagram in the lower right of Figure
12.3). The simplest case, corresponding to the equality sign, is homoepitaxial growth in the
absence of contamination. However, deposition of, for example, a low-melting-point (hence
low γ) metal such as Cd on the higher melting-point (higher γ) metal W also fulfills the
condition since γ
s−f
in this case is relatively small. Wagner and Vooerhoeve [58] used a
combination of TPD measurements as a function of T
s
and overlayer coverage θ
Cd
with
replication TEM to study nucleation and the initial stages of metal MBE in UHV. They found
that the desorption energy E
d
of Cd on W is greater than that for Cd on Cd. On clean
polycrystalline W substrates, E
d
decreases from an initial value of 2.2 eV (averaged over all W
grain orientations) at low coverages to a value approximately equal to the heat of sublimation
of bulk Cd, 1.2 eV, at θ
Cd
∼ 4 ML. Thus, the Cd–W binding energy is much larger than that of
Cd–Cd. It is therefore not surprising that TEM results show Cd exhibits local epitaxy and
574 Chapter 12
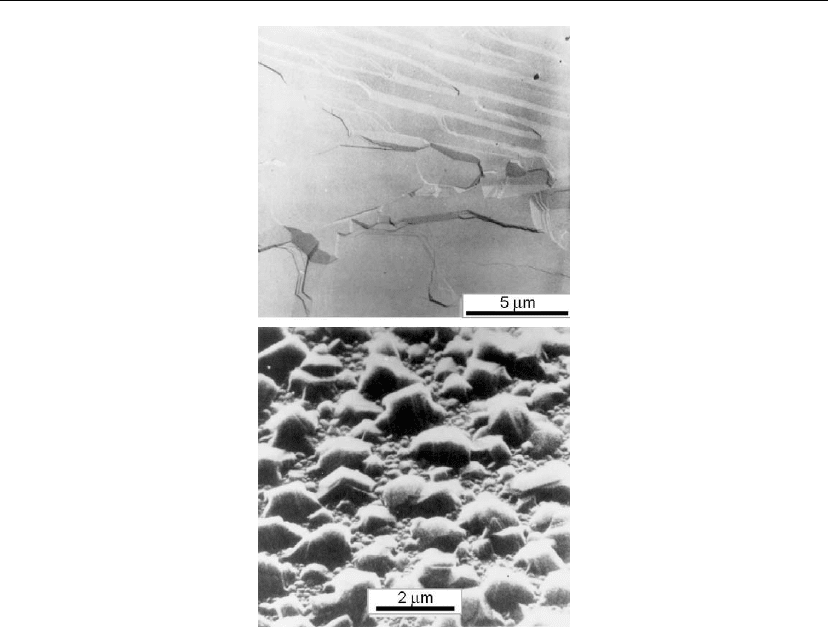
Figure 12.16: Electron microscopic images of Cd layers, θ
Cd
∼ 500 ML, grown at T
s
= 100
Con
clean (upper) and O
2
-contaminated (lower) polycrystalline W substrates. (From [58, 59].)
replicates each W grain. However, the introduction of a small amount of oxygen (less than a
monolayer) dramatically alters the nucleation mode to 3D (the surface tension of gas–metal
compounds is much less than that of metals [27], as briefly discussed in Section 12.3), and
inhibits epitaxial growth [59]. The images in Figure 12.16 very graphically illustrate the effect
of oxygen contamination for layers grown at T
s
= 100
◦
C.
12.4.1 Nucleation and Early Growth
Representative STM images showing 2D clusters of Fe deposited by MBE on Fe(001) with
J
Fe
= 1.4 × 10
13
cm
−2
s
−1
to constant coverages θ
Fe
= 0.07 ML as a function of T
s
between 20
and 356
◦
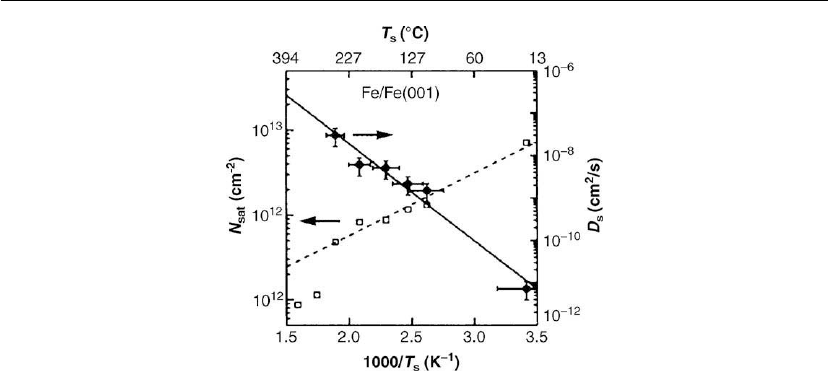
C were shown in Figure 12.5. Saturation cluster densities N
sat
at T
s
= 20–250
◦
C, for
which i* = 1, are plotted in Figure 12.17 [60]. The results were fitted using the scaling
relationship N
sat
∝ (J/D
s
)
1/3
∝ (1/D
s
)
1/3
(see discussion in Section 12.2.2) to obtain
E
s
= 450 ± 80 meV with D
o
= 7.2 × 10
−4
cm
2
s
−1
. The resulting Fe adatom surface diffusion
Thin Film Nucleation, Growth, and Microstructural Evolution 575
Figure 12.17: Temperature dependence of saturation Fe island densities N
sat
(open squares) on
Fe(001), obtained from direct STM measurements, and Fe adatom diffusion constants D
s
(solid
squares) obtained from rate equation analyses of N
sat
vs T
s
. The solid lines are least squares fits
to the data at T
s
≤ 250
C. (Adapted from [60].)
constants D
s
(T
s
) are also plotted in Figure 12.17. Note that the open data points at T
s
> 250
◦
C
are well below the dashed line, corresponding to expected values for N
sat
(T
s
), owing to the
onset of significant island loss by coarsening (Ostwald ripening) [61].
Figure 12.18 shows representative in situ STM images of 2D single-atom-high islands of a
two-component system, TiN, grown on TiN(001) substrates with atomically flat average
terrace widths L ∼ 600
˚
A, by reactive magnetron sputtering from a Ti target in purified N
2
at
P = 3 mtorr [62]. The experiments were carried out in a multichamber UHV system at
temperatures T
s
of (a) 510, (b) 600, and (c) 800
◦
C using a Ti flux J
Ti
= 1.6 × 10
14
cm
−2
s
−1
.
The TiN islands, corresponding to coverages θ
TiN
= 0.5 ± 0.06 ML, are dendritic since at these
relatively low temperatures (T
s
/T
m
= 0.22, 0.25, and 0.30), adatom kinetic energies are too low
to overcome the binding energy at island steps and edge diffusion is small compared to terrace
diffusion. Nevertheless, the islands, which are in the saturation coverage regime, still exhibit
the local four-fold symmetry expected for NaCl cubic structures. Increasing T
s
to 890
◦
C
results in the formation of square 2D TiN(001) islands, bounded by side and corner facets, as
shown in Figure 12.19 [63].
The number densities of the TiN(001) islands shown in Figure 12.18 are described as a
function of deposition conditions by Eq. (12.17),
N
sat
= 0.2[4JN
s
/ν
s
]
i
∗
/(i
∗
+2)
exp[(E
b
+ i
∗
E
s
)/(i
∗
+ 2)kT
s
]or
L
n
∝ (ν
s
/J)
i
∗
/(2i
∗
+4)
exp[ − (E
b
+ i
∗
E
s
)/(2i
∗
+ 4)kT
s
]
(12.18)

576 Chapter 12
Figure 12.18: In situ STM image of 0.50 ± 0.06 ML TiN layers deposited at temperatures T
s
with
J
Ti
=1.6× 10
14
cm
−2
s
−1
on atomically flat TiN(001) terraces. T
s
and image sizes are: (a) 510
C
and 700 × 700
˚
A
2
, (b) 600
C and 1700 × 1700
˚
A
2
, and (c) 800
C and 2000 × 2000
˚
A
2
.
(From [62].)
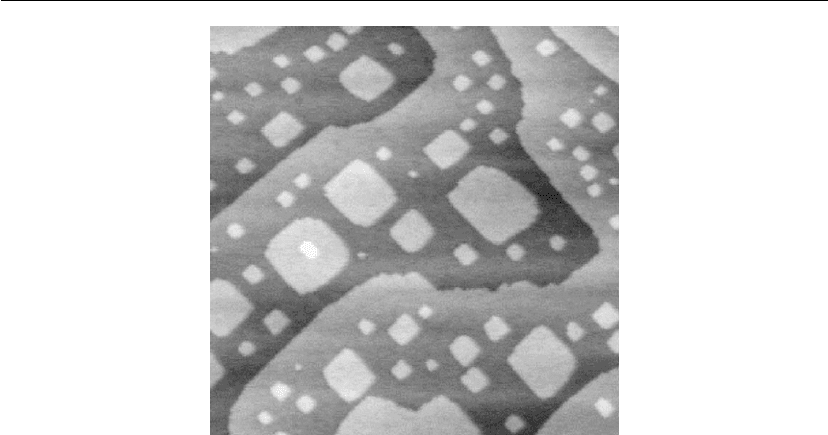
Thin Film Nucleation, Growth, and Microstructural Evolution 577
Figure 12.19: STM images of a 0.15 ML TiN layer deposited at T
s
= 850
C on atomically flat
TiN(001) terraces. Image size: 1200 × 1450
˚
A
2
. (From [63].)
where L
n
is the nucleation length. L
n
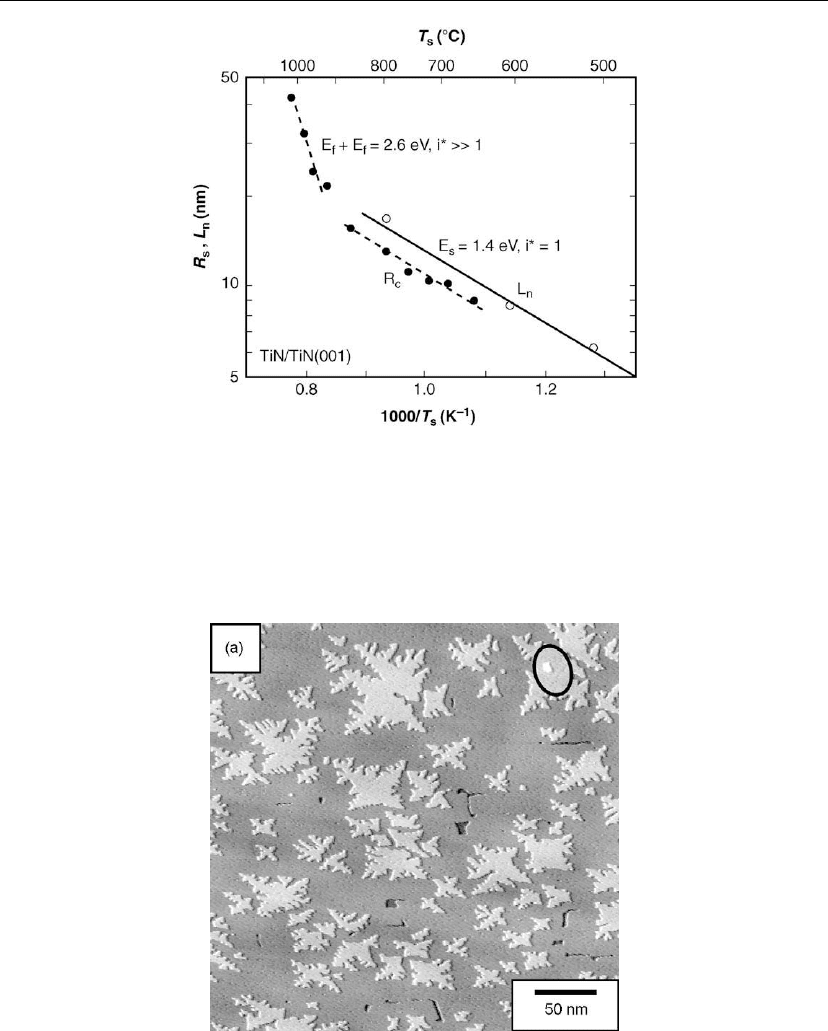
values were determined experimentally by in situ
high-temperature STM and the results are plotted vs T
s
in Figure 12.20. The total areas over
which L
n
was measured include > 150 islands at each temperature; the upper limit for T
s
is
800
◦
C in order to avoid L
n
approaching the average terrace width. Over this temperature
range, i* = 1 and Eq. (12.18) becomes
L
n
∝ [(ν
s
/J)exp(−E
s
/kT
s
)]
1/6
(12.19)
From the T
s
-dependent L
n
data shown in Figure 12.20, a least squares fit using Eq. (12.19)
yields a surface diffusion activation energy E
s
= 1.4 ± 0.1 eV with a prefactor ν
s
=10
13±1
s
−1
.
As a parallel approach to extracting the characteristic length for homoepitaxial nucleation on
TiN(001), the characteristic island size R
c
necessary to nucleate a second layer was also
measured as a function of T
s
. Karr et al. [64] and Kodambaka et al. [63, 65] have shown that
TiN(001) has a relatively small Ehrlich barrier for adspecies diffusing over step edges. In this
case, R
c
can be extracted from measurements of the fraction f of islands of size R which
exhibit second layer nucleation using an analytical relationship developed by Tersoff et al.
[66], in which
f ={1 − exp[ − (R/R
c
)
8
]} (12.20)
R
c
is primarily dependent on the smallest island dimension since adatom diffusion is isotropic
on a square lattice. Figure 12.21 shows a second layer island with an inscribed ellipse
578 Chapter 12
Figure 12.20: The solid data points (dashed lines) correspond to the characteristic TiN(001)
island sizes R
c
required for nucleation of an upper layer and the open data points (solid line) are
the measured nucleation lengths L
n
on large open terraces as a function of the deposition
temperature T
s
. (From [62].)
Figure 12.21: STM image of an 80
˚
A thick TiN/TiN(001) layer grown at T
s
= 920
C with
J
Ti
=1.6× 10
14
cm
−2
s
−1
. A second layer island is inscribed with an ellipse. Image size:
3400 × 3400
˚
A
2
. (From [62].)

Thin Film Nucleation, Growth, and Microstructural Evolution 579
representing island size R defined as one-half the minor axis of the largest ellipse which fits
though the rough edges of the first layer island. Both R and f (R) were measured for > 100
islands at each growth temperature and R
c
determined from Eq. (12.20). R
c
results are plotted
in Figure 12.20 as a function of T
s
over the range from 650 to 1010
◦
C. The increase in the
slope at T
s
> 865
◦
C is due to an increase in the size of the smallest stable cluster, as discussed
below.
In the low temperature regime for which i* = 1, the characteristic island size R
c
is given by
[62]
R
c
=
192
π
1/6
ν
JN
s
exp
−E
s
kT
s
1/6
(12.21)
A least squares fit of the R
c
(T
s
) data in Figure 12.20 between 650 and 865
◦
C yields
E
s
= 1.4 ± 0.1 eV with ν
s
=10
13±1
s
−1
for nucleation on growing islands, in agreement with the
above results for nucleation on open terraces.
Identifying the diffusing species is fundamental to understanding atomic processes occurring
during nucleation. For TiN(001), there are several possible candidates including Ti, N, and
TiN
x
(x =1,2,3,...) admolecules. Using ab initio density functional theory (DFT), the
calculated Ti surface diffusion activation energy on TiN(001), 0.35 eV, is found to be a factor
of four smaller than the E
s
value measured by experiment [62]. Petrov et al. [67] showed that
there is a significant flux of atomic N (including N
2
+
ions which dissociate upon collision)
incident at the growing film surface during reactive magnetron sputter deposition of Ti in pure
N
2
. DFT results reveal that as N atoms diffuse and encounter each other on TiN(001), N
2
forms with a binding energy of 2.1 eV, but with an adsorption energy of only 0.2 eV with
respect to gas-phase N
2
. Thus, N
2
desorbs at near kinetic rates. Moreover, the calculated N
diffusion barrier is 0.95 eV, significantly smaller than the E
s
= 1.4 eV value obtained
experimentally. The authors therefore conclude, consistent with additional DFT calculations,
that TiN
x
(x = 1, 2, 3) admolecules are the primary diffusing species rather than Ti or N
adatoms. TiN
4
is unstable and dissociates to TiN
2
admolecules and N
2
. Under high N supply
rate conditions, as in these experiments, TiN
2
and/or TiN
3
are expected to be the primary
diffusing species, while a reduction in the N supply increases the coverage of Ti and TiN
adspecies at the expense of TiN
2
and TiN
3
leading to a change in nucleation kinetics, as
demonstrated below, by decreasing the N
2
partial pressure in an Ar/N
2
mixture.
The results in Figure 12.20 reveal a dramatic increase in the rate of change in R
c
vs T
s
at T
s
> 865
◦
C. This could be due to either a change in the nature of the primary diffusing species or
a change in the critical cluster size. Assuming that i* remains constant, the E
s
value extracted
from Figure 12.20 in the high T
s
regime is 8.4 eV, larger than all TiN
x
admolecule adsorption
energies and therefore not physically reasonable. Thus, i* must increase over the T
s
range
580 Chapter 12
between 865 and 945
◦
C. For T
s
> 945
◦
C, the linear relationship between ln(R
c
) and 1/T
s
is
well fitted with i* 1 for which R
c
is given by
R
c
= 2
v
JN
s
−(E
s
+ E
c
)
kT
s
1/2
(12.22)
where the cluster formation energy E
c
= E
b
/i*. A least squares fit to the 945 ≤ T
s
≤ 1010
◦
C
data in Figure 12.20 results in (E
s
+ E
c
) = 2.6 ± 0.2 eV with ν =10
13±1
s
−1
. Assuming that the
primary diffusing species remains constant over the temperature range of these experiments
(i.e. E
s
= 1.4 eV), E
c
= 1.2 eV.
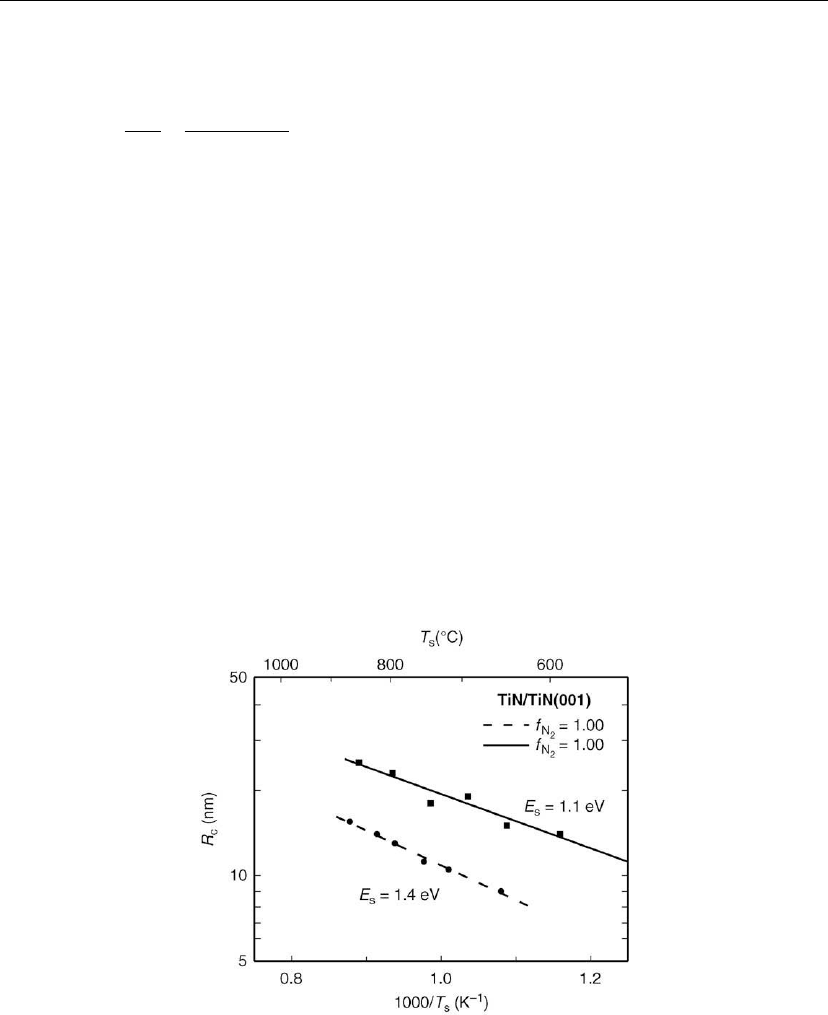
Wall et al. [68] tested the above conclusions concerning the rate controlling diffusing species
during nucleation and early film growth of homoepitaxial TiN(001) by reducing the N
2
fraction in N
2
/Ar mixtures while maintaining the total pressure and the total Ti flux constant at
P = 3 mtorr and J
Ti
= 1.6 × 10
14
cm
−2
s
−1
, respectively. Typical R
c
vs T
s
results, fitted using Eq.
(12.21), are shown in Figure 12.22. The smallest stable cluster size remains a dimer, but E
s
decreases substantially from 1.4 eV with f
N2
= 1 to 1.1 eV with f
N2
= 0.1. The frequency
prefactor ν is 10
13±1
s
−1
in both cases. Decreasing f
N2
results in a factor of two increase in R
c
due to lower steady-state N coverages favoring TiN
x
adspecies with lower x values and, hence,
higher surface diffusivities for the same TiN coverages θ
TiN
.
Figure 12.22: The characteristic TiN island size R
c
required for nucleation of an upper layer during
TiN(001) homoepitaxial growth (J
Ti
=1.6× 10
14
cm
−2
s
−1
) on TiN(001) as a function of the
deposition temperature T
s
in mixed Ar/N
2
atmospheres with N
2
gas fractions f
N2
=0.10() and
1.00 (䊉). E
s
is the surface diffusion barrier extracted from Eq. (12.21). (Adapted from [68].)
