Martin P.M. Handbook of Deposition Technologies for Films and Coatings, Third Edition: Science, Applications and Technology
Подождите немного. Документ загружается.

Vacuum Polymer Deposition 551
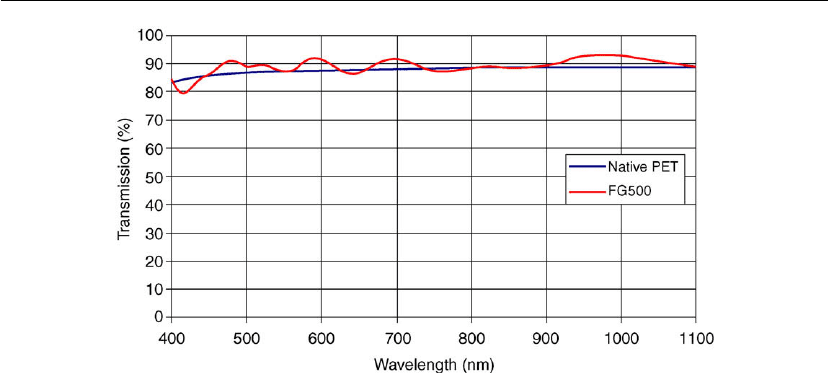
Figure 11.11: Transmission spectrum of ultrabarrier coating [10].
Figure 11.11 for the Barix
TM
coating marketed by Vitex Systems [14]. Without the ITO
conductive electrode, transmission on PET is ∼ 90%.
11.5.2 Nanolaminate Coatings
This hybrid deposition process is also used to deposit polymer/oxide and polymer/metal
nanolaminate coatings, which are free-standing structures consisting of hundreds to thousands
of alternating polymer/inorganic layers [15]. The nanolaminate can be transparent, used for
lightweight windows, or opaque, used for capacitors. In addition to increased functionality,
a major advantage of these coatings is their very low surface roughness and low optical
scattering. The multilayer plastic substrate can include additional layers, including
scratch-resistant layers, antireflective coatings, antifingerprint coatings, antistatic coatings,
conductive coatings, transparent conductive coatings, and barrier coatings, to provide
functionality to the substrate.
11.5.3 Advanced Applications
Advanced applications for multilayer VPD coatings include contaminant-resistant coatings for
flat panel displays, fabrics, fibers, and yarns; corrosion-resistant coatings to replace the toxic
heavy metals presently used (e.g. chromium); reduction of VOC release; and antibacterial
coatings [13]. Contaminant-resistant coatings are based on acrylate formulations containing
between 49% and 65% fluorine, which significantly decreases surface energies and increases
hydrophobicity. These coatings are being developed for antismudge flat panel displays and
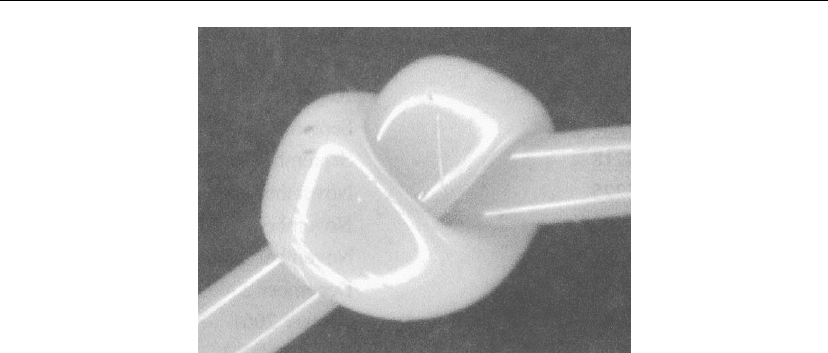
windows, increasing chemical resistance for yarns and fabrics. A picture of a coated fiber is
shown in Figure 11.12 [13].
552 Chapter 11
Figure 11.12: Microscope picture of fiber with corrosion-resistant coating [13].
Heavy metals, particularly chromium (Cr
VI
) and mercury, have known toxicity. In addition,
volatile organic compounds (VOCs) and chlorofluorocarbons (CFCs) can be released into the
atmosphere during deposition of polymer coatings. Because PVD coatings are cross-linked
using UV radiation, virtually no VOCs or CFCs are released during the deposition process.
Corrosion inhibitors, such as benzotriazole and imidazole, can be added to the monomer mix.
These technologies exploit enhanced barrier properties for corrosion control to reduce the need
for effective corrosion inhibitors. It is postulated that an optimum balance exists between those
properties that enhance inhibitor effectiveness and those that enhance physical barrier. These
organic compounds bond with the metal surface and form a barrier that prevents or retards
oxidation. On the other hand, the rosin-based coatings adhere to the metal substrate but do not
chemically bond to it. Efficient, non-toxic, corrosion-resistant coatings will reduce
environmental impact during manufacture and use, decrease costs, and extend the service life
of the equipment.
Antibacterial coatings are being developed that incorporate biocide additives, such as silver,
into the hydrophobic/oleophobic contaminant-resistant materials discussed previously. The
modified coatings combine abrasion resistance, stain resistance, and antibacterial properties in
a single polymer coating, with excellent potential for applications in the carpet and textile
industries.
References
[1] J.D. Affinito et al., in: Society of Vacuum Coaters 39th Annual Technical Conference Proceedings (1996) 392.
[2] J.D. Affinito et al., in: Society of Vacuum Coaters 41st Annual Technical Conference Proceedings (1998) 220.
[3] J.D. Affinito et al., in: Society of Vacuum Coaters 40th Annual Technical Conference Proceedings (1997) 210.
[4] N. Inagaki, Plasma Surface Modification and Plasma Polymerization, Technomic, Basel, Switzerland (1996).

Vacuum Polymer Deposition 553
[5] J.D. Affinito et al., Method of making molecularly doped composite polymer material, US Patent 6,909,230.
[6] J.D. Affinito et al., Method of making molecularly doped composite polymer material, US Patent 6,613,395.
[7] A. Yializis, G.L. Powers, D.G. Shaw, IEEE Trans. Components Hybrids Manuf. Technol. 13 (1990) 66.
[8] M.E. Gross et al., in: Society of Vacuum Coaters 46th Annual Technical Conference Proceedings (2003) 89.
[9] P.M. Martin et al., in: Society of Vacuum Coaters 39th Annual Technical Conference Proceedings (1996) 187.
[10] M.G. Mikhael, A. Yialszis, in: Society of Vacuum Coaters 48th Annual Technical Conference Proceedings
(2006) 663.
[11] P.M. Martin, W.D. Bennett, C.H. Henager, in: Society of Vacuum Coaters 50th Annual Technical Conference
Proceedings (2007) 643.
[12] M.E. Gross et al., in: Society of Vacuum Coaters 49th Annual Technical Conference Proceedings (2006) 139.
[13] A. Yializis et al., in: Society of Vacuum Coaters 41st Annual Technical Conference Proceedings (1998) 477.
[14] G.L. Graff et al., in: Society of Vacuum Coaters 43rd Annual Technical Conference Proceedings (2000) 397.
[15] P.M. Martin et al., Multilayer plastic substrates, US Patent 6,962,671.

CHAPTER 12
Thin Film Nucleation, Growth,
and Microstructural Evolution:
An Atomic Scale View
J.E. Greene
Materials Science and Physics Departments and the Frederick Seitz Materials Research
Laborator y, University of Illinois, Urbana, Illinois 61801, USA
12.1 Introduction 554
12.2 Nucleation and the Early Stages of Film Growth 555
12.2.1 Thermodynamic Descriptions of Experimental Results 558
12.2.2 Kinetic Descriptions of Experimental Results 565
12.3 Three-Dimensional Nucleation and Growth 569
12.3.1 Nucleation and Early Growth 569
12.3.2 Three-Dimensional Island Coalescence 570
12.4 Two-Dimensional Nucleation and Growth 573
12.4.1 Nucleation and Early Growth 574
12.4.2 Two-Dimensional Island Coalescence 581
12.5 Stranski–Krastanow Nucleation and Growth 583
12.5.1 S-K Mechanism and Examples 584
12.6 Structural Evolution of Polycrystalline Films at the Nanoscale and Microscale 592
12.6.1 Elemental Polycrystalline Films and Structure-Zone Models 593
12.6.2 Multicomponent and Multiphase Film Growth 599
12.6.3 Ion Irradiation Effects 602
12.7 Conclusions 616
12.1 Introduction
Understanding nucleation and growth at the atomic scale is fundamental to both the science
and the technology of thin films. The increasingly stringent requirements of sophisticated thin
film applications, processing technologies, and devices provide a strong impetus for obtaining
ever better control over the dynamics of processes which govern the nanoscale chemistry and
structure of as-deposited layers. The primary deposition variables determining nucleation and
growth kinetics, microstructural evolution, and, hence, the physical properties of films are: the
Copyright © 2010 Peter M. Martin. Published by Elsevier Inc.
All rights reserved.
554

Thin Film Nucleation, Growth, and Microstructural Evolution 555
film material, the flux J and kinetic energy E of species incident at the growing surface, the
growth temperature T
s
, the flux of contaminants, and the substrate material, surface
cleanliness, crystallinity, and orientation.
The kinetic energy of the incident flux during film growth by thermal evaporation, for which
E ∼ 100–200 meV, is determined by the temperature of the evaporant source. In contrast,
typical average sputtered atom ejection energies range from 5 to 10 eV, which is of the order
of, or higher than, bond energies in solids. In addition, energetic ions and fast atoms
neutralized and reflected from the target during plasma or ion-beam sputter deposition have
been shown to be useful in controllably altering the composition, chemistry, and structure of
as-deposited layers through trapping, preferential sputtering, enhancing adatom mobilities,
and dynamic collisional mixing [1–13].
This chapter is organized in the following manner. Section 12.2 deals with nucleation and the
early stages of film growth. Thermodynamic and kinetic models are developed in Sections
12.2.1 and 12.2.2, respectively, and compared with experimental results. Three-dimensional
(3D), two-dimensional (2D), and Stranski–Krastanow (S-K) ‘quantum dot’ growth modes are
discussed sequentially in Sections 12.2.3, 12.2.4, and 12.2.5. Microstructure development is
reviewed in Section 12.6, beginning with elemental films and their classification by structure
zone diagrams (Section 12.6.1), then moving to multicomponent and multiphase film growth
(Section 12.6.2). The chapter ends with a discussion of the role of low-energy ion irradiation
(Section 12.6.3) for manipulating the dynamics of structural (and physical property)
development including defect formation/annihilation, surface roughening/smoothening
mechanisms, the evolution of preferred orientation, and synthesis of self-organized 3D
nanostructures with unique properties.
12.2 Nucleation and the Early Stages of Film Growth
Nucleation on a substrate surface corresponds to a phase transition in which vapor or liquid
phase atoms are deposited to coverages θ yielding sufficiently high 2D spreading pressures
that local density fluctuations in the 2D gas give rise to the formation of stable clusters
(nuclei). ‘Stable’ in this sense refers to clusters which have a higher probability to grow than to
dissociate. There are three primary modes of film growth on substrates [14, 15], as illustrated
schematically in Figure 12.1. During 3D, or Volmer–Weber, island growth, stable clusters
develop into 3D islands which in turn coalesce to form a continuous film. This type of growth
occurs when the adatoms are much more strongly bound to each other than to the substrate as
is often the case for metal films on insulators or contaminated substrates (weakly interacting
film/substrate interfaces with high interfacial energy densities). 2D layer-by-layer, or
Frank–van der Merwe, growth corresponds to the case in which adatom–adatom binding
energies are equal to, or less than, those between the adatoms and the substrate. In addition to
the obvious case of homoepitaxy on a clean substrate, there are numerous examples of 2D
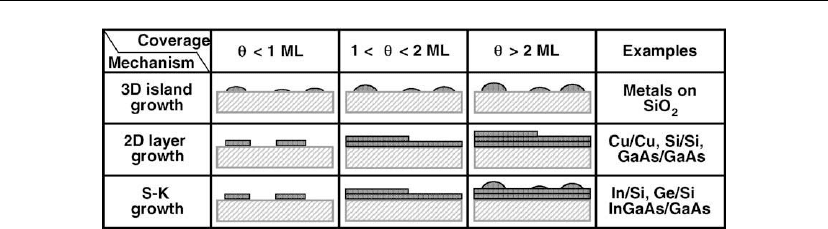
556 Chapter 12
Figure 12.1: Schematic representations of three solid-film growth modes on substrates. θ is the
overlayer coverage in monolayers.
heteroepitaxy in metal/metal (e.g. Cd on W), metal/semiconductor (Fe on GaAs), and
semiconductor/semiconductor (Si
1−x
Ge
x
on Si) systems. However, for heteroepitaxial growth,
the continuously increasing thickness-dependent strain energy will eventually lead to the
initiation of one or more relaxation mechanisms (e.g. misfit dislocation formation and/or
surface roughening) at ‘critical’ layer thicknesses.
The third growth mode, predicted by Stranski and Krastanow (S-K) in 1938 [16],isa
combination of the first two. In this case, after initially forming one or more 2D monolayers of
a heterostructure, further layer growth becomes energetically unfavorable and 3D islands form.
Other commonly used terms for this growth mode are islanding and strain-induced roughening.
The transition from 2D to 3D growth is driven by the elastic strain energy E
elas
∝ ε
2
h, where
ε =(a
f
− a
s
)/a
s
is the lattice parameter mismatch between the film (a
f
) and the substrate (a
s
)
and h is the film thickness [17]. The transition occurs when the increase in the total film strain
energy due to the growth of an additional layer is larger than the increase in surface energy
required to form 3D islands which can partially relax via dilation as illustrated in Figure 12.2.
Thus, the growth transition is driven by a decrease in total system energy [18, 19].
An example of an S-K system is In/Si(001)2×1 in which three In layers deposited at
T
s
=70
◦
C(T
s
/T
m
= 0.8; T
m
is the In melting point in K) by molecular beam epitaxy (MBE)
grow two-dimensionally and the next layer forms 3D islands which continue to evolve [20].
The archetype S-K model system is Ge/Si(001) owing to the interest in Si
1−x
Ge
x
/Ge(001)
quantum dot electronics. For this case, the transition is also said to occur after approximately
three monolayers (MLs) of growth, but detailed in situ scanning tunneling microscopy (STM)
studies show that lines of Ge dimer vacancies begin to form at Ge coverages θ
Ge
<1MLand
orthogonal cross-hatch dimer vacancy rows start to form at ∼ 2ML[21, 22]; both are
strain–relaxation mechanisms driven by the fact that the lattice constant of Ge is 4.2% larger
than that of Si, giving rise to the formation of a large compressive stress σ
c
.
The left side of Figure 12.3 schematically illustrates the essential features leading to
nucleation on a substrate. An incident flux of film species must first become thermally
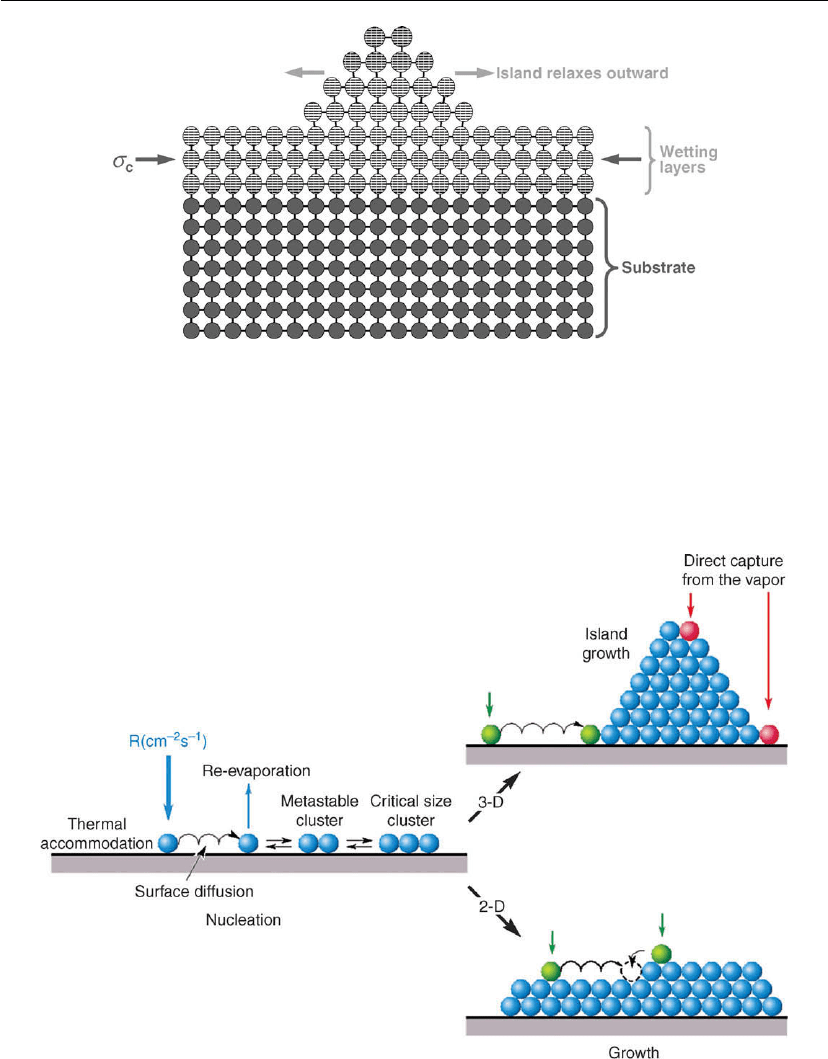
Thin Film Nucleation, Growth, and Microstructural Evolution 557
Figure 12.2: Schematic representation of strain-driven Stranski–Krastanow ‘quantum dot’
formation above an initial wetting layer. Here, the film lattice constant is larger than that of the
substrate (i.e. a
f
> a
s
) and the layer is under compressive stress σ
c
.
Figure 12.3: Schematic representation of processes leading to nucleation and 3D (upper right)
and 2D (lower right) film growth.

558 Chapter 12
accommodated with the substrate. For a given adatom, this typically occurs within a few
vibrational periods (even for sputtered atoms incident with several eV). The adatoms then
diffuse on the surface to interact with other adatoms or desorb (depending on film/substrate
materials and T
s
). At sufficiently low temperatures, a fraction of the clusters which are formed
continue to grow in size (the rest dissolve back into the 2D gas) and become islands which
eventually impinge on, and coalesce with, their neighbors to form a continuous film. Note that
at high deposition rates R (in units of atoms/s) or low deposition temperatures such that
R >> N
s
D
s
, where N
s
is the substrate surface site number density (of the order of 10
15
cm
−2
for
metals and semiconductors, depending on material and orientation) and D
s
is the adatom
surface diffusivity, the film is amorphous. This occurs since the adatoms do not have sufficient
time to diffuse to low-energy sites before they are buried by subsequently deposited adatoms.
Covalently and ionically bonded materials have low packing densities with strong bond
directionality and are thus easily deposited in the amorphous state. Metals, however, exhibit
much higher surface diffusivities and are considerably more difficult to obtain in the
amorphous state. However, metallic glasses can be formed from deep eutectic alloys using
special techniques to provide extremely fast cooling rates, ≥ 10
6
K/s [23, 24].
12.2.1 Thermodynamic Descriptions of Experimental Results
Based on Figure 12.3, the minimum thermodynamic requirements to obtain net deposition
would appear to be that the condensate pressure P in the gas phase is at least equal to its
equilibrium vapor pressure P
vp
over the solid. Actually, however, the supersaturation ratio
ζ = P/P
vp
must be much larger than one since small clusters have much higher vapor pressures
than the corresponding bulk material due to their high surface-to-volume ratios. In analyzing
film growth experiments, it is more convenient to use ζ = J/J
vp
where the particle flux J is
related to P via the kinetic definition of pressure:
J(cm
−2
s
−1
) = 3.513×10
22
P/(mT )
1/2
(12.1)
in which m is the molecular weight in amu, T is the gas temperature in K, and P is in torr.
A large surface-to-volume ratio also leads directly to the requirement that clusters must be
greater than a minimum critical size in order for growth to occur. This is easy to understand in
the simple case of homogeneous nucleation such as the formation of an embryonic ice particle
in water cooled below the liquid freezing point T
m
=0
◦
C. At temperatures less than T
m
,
solidification lowers the Gibbs free energy per unit volume G
V
. However, the formation of a
solid cluster increases the system free energy G by introducing an interfacial surface area
between the ice cluster and the surrounding liquid. Surfaces and interfaces are 2D defects in
infinite 3D crystals and their production requires the expenditure of energy. The difference
between the decrease in G
V
, which varies as r
3
(r is the cluster radius) and the increase in
interfacial energy, which varies as r
2
, results in a free energy activation barrier for nucleation.
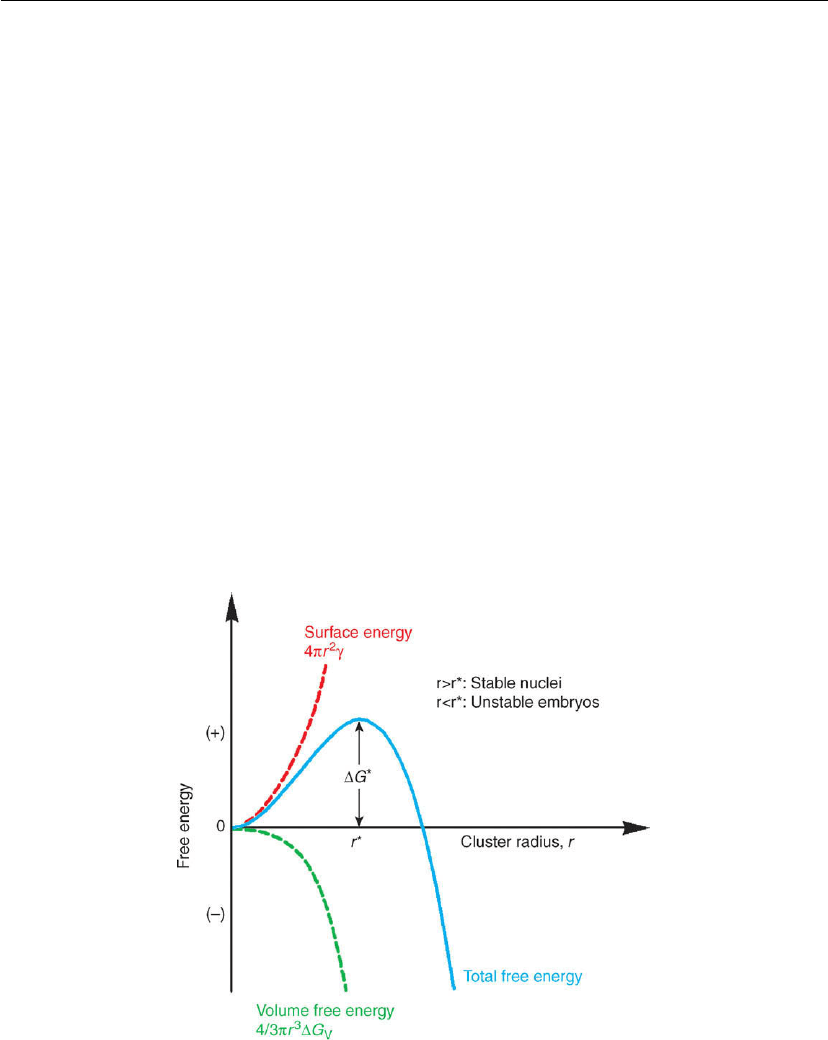
Thin Film Nucleation, Growth, and Microstructural Evolution 559
That is, the incipient clusters must reach a critical size before the volume term overcomes the
surface term and stable nuclei are formed.
The net free energy associated with the formation of a solid spherical cluster in an otherwise
homogeneous fluid is
G = 4πr
2
γ + 4πr
3
G
V
/3 (12.2)
where γ is the interfacial energy per unit area. An expression for the critical cluster size r*is
obtained by setting the derivative d(G)/dr = 0 and solving to yield
r
∗
=−2γ/G
V
(12.3)
In modern literature, the critical size is generally represented as i* in units of atoms; thus, the
smallest stable nucleus is of size (i* + 1) atoms. Substituting Eq. (12.3) into (12.2) yields the
nucleation activation barrier:
G
∗
= 16γπ
3
/3(G
V
)
2
(12.4)
The factors 2 and 16π
3
/3 in Eqs (12.3) and (12.4) derive from geometry, i.e. the
assumption here of spherical nuclei. The important results are that r* ∝ γ/G
V
and
G* ∝ γ
3
/(G)
2
. Thus, as illustrated in Figure 12.4, nucleation is a simple stability problem
Figure 12.4: Schematic diagram showing total system free energy vs the radius r of a spherical
nucleus in a homogeneous fluid. γ is the solid/liquid interfacial energy per unit area and G is the
Gibbs free energy per unit volume of the liquid-to-solid phase transition.

560 Chapter 12
(r
2
in the energy cost term vs r
3
in the energy gain term) and an elementary self-assembly
process.
To take this example a step further in order to estimate r*, consider evaporating Au onto a
NaCl(001) substrate at room temperature. Using Eq. (12.3) for r*, the strategy is to derive an
expression for G
V
beginning with the combined first and second laws of thermodynamics,
d(G) = VdP − SdT (12.5)
where V and S are the system volume and entropy, respectively. Since thin films are generally
deposited at constant T = T
s
, the last term in (12.5) is ignored. For film growth in vacuum, the
ideal gas law, PV = NkT, is a reasonable approximation. N/V is the number density of Au atoms
in the incident flux and k is Boltzmann’s constant. Substituting V = NkT/P into (12.5) yields:
d(G)
T
= NkT (dP/P) (12.6)
Consider the addition of one more adatom to a cluster on a substrate which is maintained at T
s
.
Since G
V
is just G/, where is the adatom volume, d(G
V
)
T
=[kT
s
/](dP/P), or
G
v
= [kT
s
/]n(P/P
vp
) = [kT
s
/]n(J/J
vp
) = [kT
s
/]n(ζ) (12.7)
ζ = J/J
vp
is the Au atom supersaturation. Substituting Eq. (12.7) into (12.3) yields
r
∗
= 2γ/kT
s
n(ζ) (12.8)
A reasonable value for J
Au
during in situ transmission electron microscopy (TEM) studies of
Au evaporation on NaCl(001) is 1 × 10
13
cm
−2
s
−1
(∼ 0.01 ML/s) [25]. This corresponds, from
Eq. (12.1) with m
Au
= 196.97 amu and T
s
= 300 K, to a deposition pressure P
Au
∼ 7 × 10
−8
torr.
The extrapolated vapor pressure P
vp
of Au at 300 K is ∼ 10
−30
torr [26]. Thus, ζ in this
hypothetical experiment is ∼ 7 × 10
22
. Substituting this, together with
Au
= 12.51
˚
A
3
and the
energy cost for covering the low surface tension NaCl substrate (γ
NaCl
= 0.014 eV/
˚
A
2
) with Au
(γ
Au
= 0.088 eV/
˚
A
2
) [27] into Eq. (12.8) yields r* = 1.37
˚
A which corresponds approximately
to i* = 1. Thus, the smallest stable Au cluster under these deposition conditions is (i*+1)=2
atoms, consistent with experimental results.
The good agreement in the above example, however, is mostly fortuitous, as easily shown by
simply changing T
s
, which also changes ζ through the term P
vp
. At 400 K, Eq. (12.8) yields
r* = 1.83
˚
A, which is larger than r*atT
s
= 300 K, but much less than the experimental result
(i* + 1) = 4 atoms. Nevertheless, Eq. (12.8) does show the correct trends: r*(T
s
) increases with
T
s
since the cohesive energy of clusters decreases as 1/r [28]; thus, larger clusters are
required to avoid thermal dissociation at higher deposition temperatures. In addition, based
purely on kinetic arguments, adatom mobilities are higher at elevated T
s
leading to longer
surface mean free paths and, therefore, a smaller density of larger clusters (longer nucleation
