Martin P.M. Handbook of Deposition Technologies for Films and Coatings, Third Edition: Science, Applications and Technology
Подождите немного. Документ загружается.

Thin Film Nucleation, Growth, and Microstructural Evolution 561
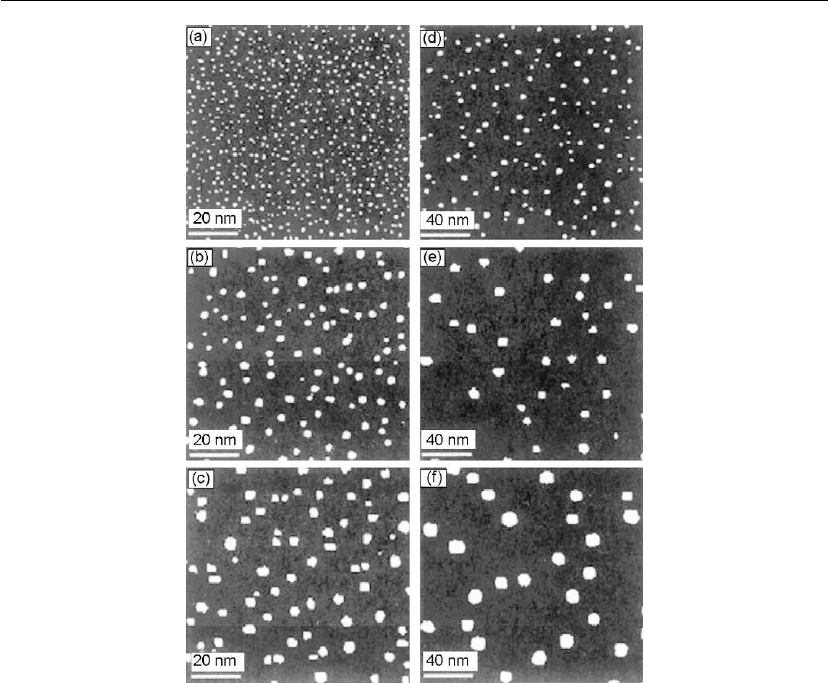
Figure 12.5: In situ STM images of Fe islands deposited on Fe(001) by MBE with a flux
J
Au
=1× 10
13
cm
−2
s
1
to a coverage θ
Fe
= 0.07 ML at temperatures T
s
= (a) 20, (b) 108, (c) 163,
(d) 256, (e) 301, and (f) 356
C. (From [29].)
lengths L
n
) at the same coverage θ. An example is shown in Figure 12.5 for the 2D
nucleation and growth of Fe on Fe(001). In these experiments, Fe was deposited at a flux
J = 1.4 × 10
13
cm
−2
s
−1
in ultrahigh vacuum (UHV) by MBE to a coverage θ
Fe
= 0.07 ML at
temperatures T
s
= (a) 20, (b) 108, (c) 163, (d) 256, (e) 301, and (f) 356
◦
C [29]. The images
were obtained by in situ STM. Similarly, r* also increases with decreasing flux J, i.e.
decreasing ζ at constant T
s
.
In the above discussion, homogeneous nucleation theory was employed, for illustration
purposes, to describe the heterogeneous nucleation of Au/NaCl(001). This corresponds to the
unrealistic case of nucleating a spherical particle with a contact angle of 180
◦
on a solid
surface (i.e. zero film/substrate interaction). The actual situation is, of course, much more
complex. Nevertheless, most thermodynamic descriptions of heterogeneous 3D nucleation
562 Chapter 12
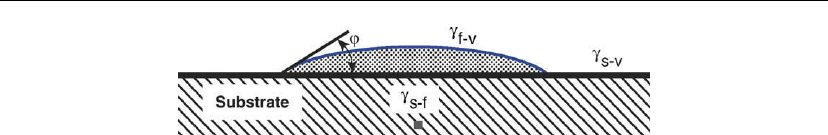
Figure 12.6: Schematic representation of a hemispherical cap-shaped island on a solid substrate.
The γ terms are interfacial energies per unit area and the subscripts s, f, and v symbolize the
substrate, film, and vapor phases. The wetting angle ϕ is related to the interfacial energies
through Young’s equation: γ
s−v
= γ
f−s
+ γ
f−v
cosϕ.
from the vapor phase are extensions of homogeneous nucleation theory using the capillarity, or
droplet, model [30–32]. More sophisticated treatments are also available [15, 33, 34], but all
thermodynamic models suffer from several shortcomings. The most important one is that
values for surface energies, interface energies, formation energies, etc., which must be entered
into the final equations are usually unknown for small clusters and, hence, bulk values, which
can be very different, are used. Strongly size-dependent properties (e.g. reduced cohesive
energies and melting points, increased vapor pressures, and the collapse of continuous
densities of electronic states into discrete atom-like levels) are precisely the reason that
nanostructures are so interesting. Thermodynamic models also generally employ convenient,
but often unrealistic, geometries to represent nuclei that, on crystalline substrates which
provide a template for preferential adatom diffusion directions, have shapes reflecting the
underlying crystallography. Examples are one-dimensional Si and Ge clusters on Si(001)2×1
[35]. Nevertheless, as noted above, the capillarity model has the advantage that it captures
much of the essential physics of the nucleation process.
To proceed, assume that a hemispherical 3D cluster of mean dimension r and contact angle ϕ
forms on a solid surface as in Figure 12.6. The cluster has surface area a
1
r
2
exposed to the
vapor phase, a contact area a
2
r
2
with the substrate, and a volume a
3
r
2
where the a
i
terms are
functions of geometry. The total free energy of the cluster with respect to dissociation is
G = a
1
r
2
γ
f−v
+ a
2
r
2
γ
s−f
− a
2
r
2
γ
s−v
+ a
3
r
3
G
V
(12.9)
γ
f−v
is the positive free energy per unit area associated with the formation of a new surface
between the film material f and the vapor phase v; γ
s−f
is the substrate/film interfacial energy
per unit area; and the term a
2
r
2
γ
s−v
accounts for the disappearance of free substrate surface
due to cluster formation. Note that Eq. (12.9), like Eq. (12.2), corresponds to a stability
problem characterized by r
2
vs r
3
. Thus, we again take the derivative with respect to r and set
it equal to zero to obtain r*,
r
∗
=−2(a
1
γ
f−v
+ a
2
γ
s−f
− a
2
γ
s−v
)/3a
3
G
V
(12.10)
Thin Film Nucleation, Growth, and Microstructural Evolution 563
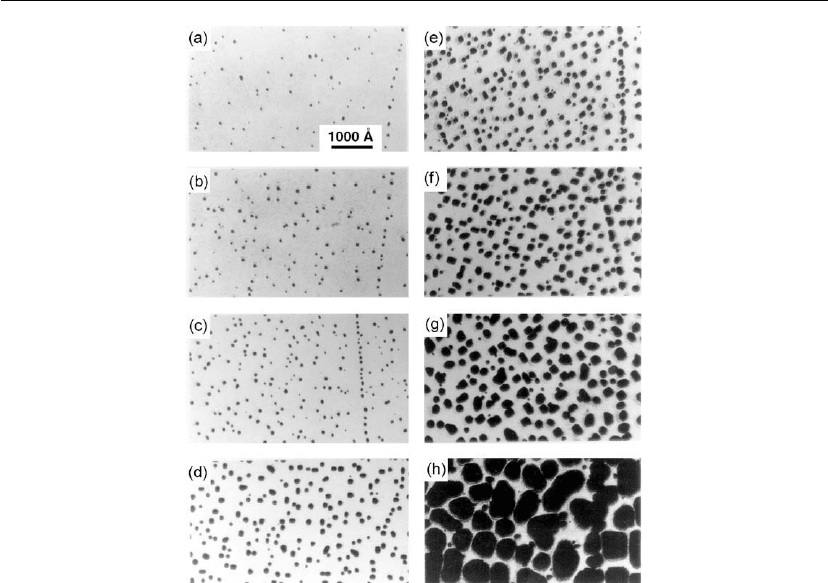
Figure 12.7: Replication transmission electron micrographs of Au islands deposited on NaCl(001)
substrates cleaved in situ in UHV as a function of evaporation time: (a) 0.5, (b) 1.5, (c) 4, (d) 8,
(e) 10, (f) 15, (g) 30, and (h) 85 minutes. The deposition rate R and growth temperature T
s
were
1 × 10
13
cm
−2
s
−1
and 250
C, respectively. (Adapted from [25].) The non-random island arrays,
along nearly straight lines, in frames (a)–(e) arise owing to preferential nucleation at low-angle
grain boundaries created during substrate preparation by in situ substrate cleavage via a
guillotine.
and substitute r* into Eq. (12.9) to yield
G
∗
= 4(a
1
γ
f−v
+ a
2
γ
s−f
− a
2
γ
s−v
)
3
/27a
2
3
G
2
V
(12.11)
While the equations for r* and G* are more complex than (12.3) and (12.4), averaging the
surface energy density (surface tension) terms still yields r* ∝ γ/G
V
and G* ∝ γ
3
/(G)
2
.
Figure 12.7 is a series of TEM images of Au clusters obtained during in situ UHV experiments
in which an evaporated Au flux J
Au
=1× 10
13
cm
−2
s
−1
is incident on cleaved NaCl(001)
substrates, maintained at 250
◦
C, for times ranging up to 85 minutes [25]. During the early
stages – frame (a) corresponding to t = 0.5 minute – deposition kinetics are primarily
controlled by nucleation. Coalescence dominates in frames (g) and (h), t = 30 and 85 minutes,
564 Chapter 12
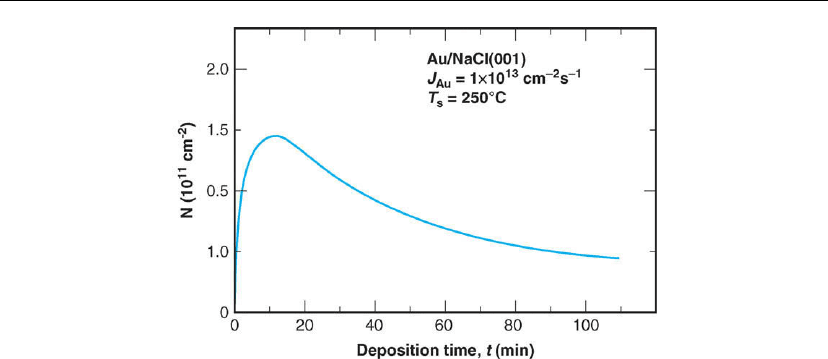
Figure 12.8: Au island number density on NaCl(001) as a function of deposition time. The MBE
deposition flux J
Au
and growth temperature T
s
were 1 × 10
13
cm
−2
s
−1
and 250
C, respectively.
(Adapted from [25].)
although some ‘secondary’ nucleation is clearly visible. At intermediate deposition times,
nucleation, cluster growth, and coalescence are competing for adatoms. This is summarized in
Figure 12.8, a plot of 3D cluster density N vs t [25]. The positive slope dN/dt of the N vs t
curve (the nucleation rate) at t → 0 begins to bend over as the average island separation
becomes of the order of the adatom mean free path and adatom capture by established clusters
(cluster growth) starts to overcome adatom loss by additional nucleation. At the maximum
island density, dN/dt = 0, the rate at which existing clusters are lost by coalescence becomes
equal to the time-dependent (i.e. coverage dependent) nucleation rate. On the far side of the
curve at long times, the slope is negative and coalescence dominates.
Since the invention of the STM in 1983 [36], and the continuous development of UHV
high-resolution in situ TEM over the past decades, it has been only natural that thin film
scientists have studied nucleation on highly perfect single-crystal surfaces in pristine
environments. Clusters as small as single adatoms and dimers can be imaged with STM under
favorable conditions allowing the possibility of obtaining complete statistical cluster size
distributions as a function of deposition conditions and layer coverage. Figure 12.9 shows
STM results for 2D Fe cluster size distributions, in which j is the number of atoms per cluster,
on Fe(001) as a function of the growth temperature T
s
= 20–356
◦
C [29]. Deposition was
carried out by MBE using J
Fe
= 1.4 × 10
13
cm
−2
s
−1
,asforFigure 12.5, to provide constant
total coverages θ
Fe
= 0.07 ML. Nucleation and growth are competing processes and at a given
coverage, cluster growth kinetics are favored over nucleation with increasing T
s
due to longer
adatom mean free paths leading to a tendency toward larger average island sizes with lower
maximum island densities. Thus, cluster size distributions vary dramatically with T
s
in
Figure 12.9.
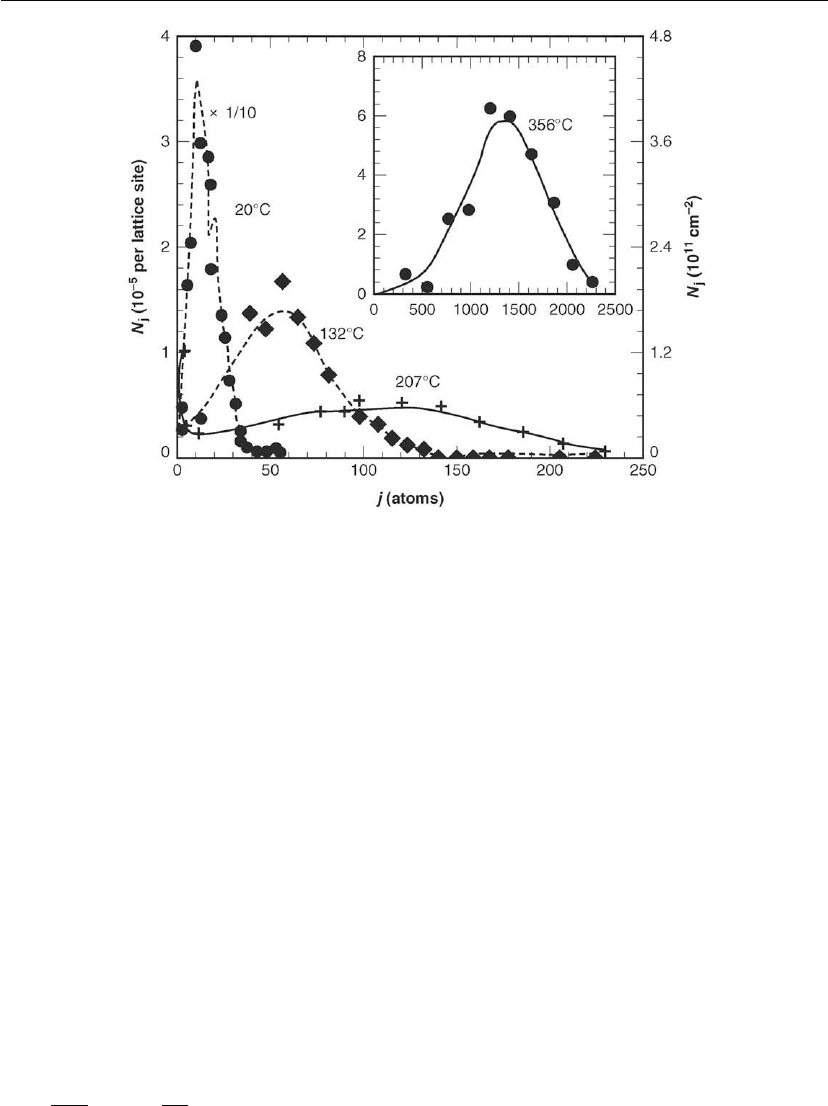
Thin Film Nucleation, Growth, and Microstructural Evolution 565
Figure 12.9: STM results for 2D Fe cluster size distributions, in which j is the number of atoms
per cluster and N
j
is the number of clusters of size j, on Fe(001) as a function of the growth
temperature T
s
= 20–356
C. Deposition was carried out by MBE using J
Fe
=1.4× 10
13
cm
−2
s
−1
,
as for Figure 12.5, with deposition times adjusted to provide constant total coverages
θ
Fe
= 0.07 ML. (Adapted from [29].)
12.2.2 Kinetic Descriptions of Experimental Results
Owing to the limitations of the thermodynamic models discussed above, STM and TEM data
are usually fitted using mean-field rate theory models, of the type originally developed by
Zinmeister [37–41], to obtain critical cluster sizes i*, surface diffusion energies E
s
, jump
attempt frequencies ν
s
, and pairwise cluster-atom binding energies E
b
. Reviews and more
detailed discussions of the development of this approach may be found in [15] and [42].
The essence of atomistic kinetics approaches is to simultaneously solve parallel rate equations
for the change in number density N
j
of clusters of size j. Neglecting coalescence for the
moment, one can write a series of ordinary differential equations to describe the time rate of
change in the concentration of monomers, dimers, trimers, and higher order clusters on a
substrate surface in response to an incident flux J of atoms. For monomers N
1
,
dN
1
dt
= J −
N
1
τ
d
− K
1
N
2
1
− N
1
n
j=2
(K
j
N
j
) (12.12)
566 Chapter 12
where n is the largest cluster size in the experiment being analyzed. The first term on the right
side of Eq. (12.12) is the impingement rate, the second is the desorption rate, the third is the
dimer formation rate in which K
1
is the rate constant, and the last term is the rate of monomer
loss to higher order clusters. The adatom desorption lifetime τ
d
= (1/ν
d
)exp(E
d
/kT
s
), in which
ν
d
is the desorption attempt frequency and E
d
is the desorption energy. For deposition at low
temperatures (the complete condensation regime), the adatom desorption rate is insignificant
and the second term can be ignored. At higher temperatures, τ
d
can often be calculated since
ν
d
and E
d
are known for many materials systems, especially for relatively high vapor pressure
metal condensates such as P on Si(001), from thermally programmed desorption (TPD)
experiments [43]. The rate constant K
1
corresponds to bimolecular recombination and is
directly related to the adatom diffusivity; K
1
= σ
1
D
s
= σ
1
D
o
exp(E
s
/kT
s
), where σ
1
is the adatom
capture probability. Cluster loss terms due to coalescence can also be added to Eq. (12.12) [42].
For clusters of size j > 1, parallel equations are written in the general form,
dN
j
dt
= K
j−1
N
1
N
j−1
− K
j
N
1
N
j
. (12.13)
The first term on the right side of Eq. (12.13) is the formation rate of clusters of size j by
attachment of monomers to clusters of size ( j − 1). The second term is the loss rate due to
clusters of size j forming larger clusters of size ( j + 1). In the simplest expression of this
model, it is assumed that clusters of size j > 1 do not desorb or diffuse. In addition, under
conditions of local equilibrium,
N
j
N
j−1
= N
1
C
j
exp
E
b
kT
s
(12.14)
E
b
in Eq. (12.14), the pairwise binding energy difference between clusters of size j and
( j − 1), is obtained either by fitting data as a function of T
s
(see below) or by calculation using
density functional theory. C
j
is a statistical weighting factor which is a constant for a particular
cluster size and configuration [44].
Consider a simple 2D nucleation example in which deposition occurs at sufficiently low
temperature such that the desorption term in Eq. (12.12) can be neglected, stable clusters are
immobile, the 2D sticking probability of adatoms to clusters of size j ≥ i* is unity, and the
coverage is small enough that coalescence is insignificant. In this case, the equation set
reduces to:
dN
1
dt
= J − D
s
N
1
N
j
(12.15)
dN
j
dt
= D
s
N
1
N
i
∗
(12.16)
Thin Film Nucleation, Growth, and Microstructural Evolution 567
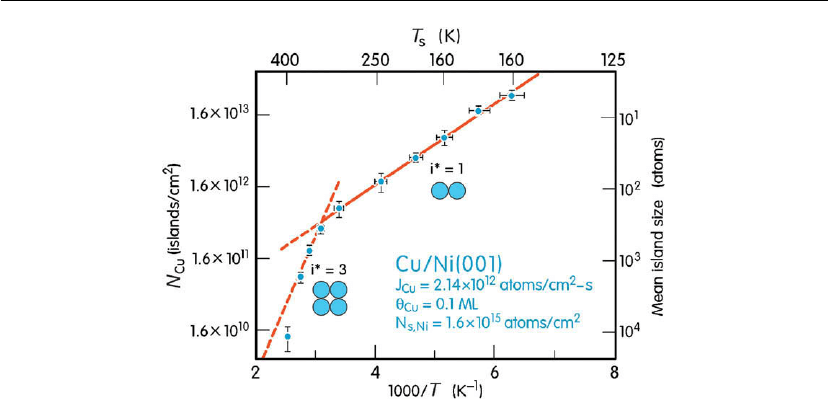
Figure 12.10: STM data from nucleation and growth experiments in which Cu is deposited onto
Ni(001) surfaces to coverages θ
Cu
= 0.1 ML at temperatures ranging from 160 to 400 K (−113 to
127
C) using MBE with J
Cu
=2.14× 10
12
cm
−2
s
−1
. Island density N
Cu
and mean island size are
plotted vs T
s
. N
s, Ni
is the Ni(001) surface site density. (Adapted from [48].)
in which j ≥ i*. Initially, the cluster creation rate in Eq. (12.15) is ∼ D
s
N
1
N
i*
.However,asan
appreciable cluster density is developed such that N
j
N
1
, adatom attachment to existing
stable clusters becomes the dominant adatom loss term (∼ D
s
N
1
N
j
>
i*
) and the total island
density N reaches saturation N
sat
(θ) until the island coalescence rate becomes significant and N
decreases. The steady state solution to Eqs (12.15) and (12.16) yields a power-law scaling
relationship N ∝ (J/D
s
)
p
where for 2D nucleation p = (i* + 1)/(i* + 3) when N
1
∼ N and
p = i*/(i* + 2) when N = N
sat
N
1
[45–47]. The surface diffusivity D
s
= {1/4}(ν
s
/N
s
)
exp(−E
s
/kT
s
).
Figure 12.10 shows STM data from nucleation and growth experiments in which Cu is
deposited onto Ni(001) surfaces to coverages θ
Cu
= 0.1 ML at temperatures ranging from 160 K
(−113
◦
C) to 400 K (127
◦
C) using MBE with J
Cu
= 2.14 × 10
12
cm
−2
s
−1
[48]. γ
Cu
, the surface
tension of (bulk) Cu, is approximately 10% lower than that of Ni [27]; thus, Cu spreads easily
leading, initially, to 2D growth. The first thing to note in the experimental results is that over
this relatively limited T
s
range, the number density N
Cu
of Cu islands decreases by > 10
3
. The
authors report that there was no evidence for the migration of small islands during these
experiments.
The critical cluster size i* in the low (160–320 K) and high (320–400 K) T
s
ranges were
obtained by noting from the above discussion that the deposition rate dependence of N
sat
(T
s
)
for 2D nucleation and growth at constant T
s
(i.e. constant D
s
) in the saturation island density
568 Chapter 12
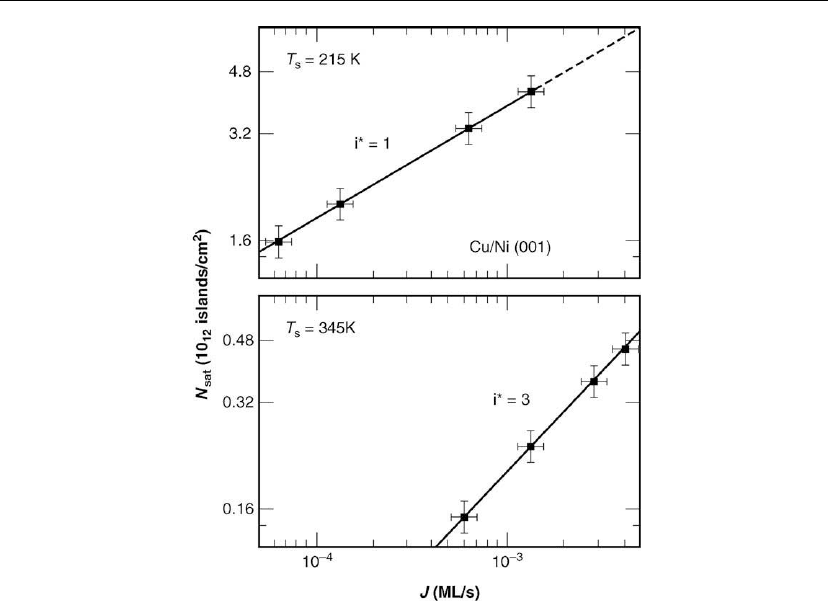
Figure 12.11: Saturation island densities N
sat
, measured by in situ STM, on Ni(001) vs incident
MBE Cu flux J
Cu
at T
s
= 215 and 345 K (−58 and 72
C). i* is the critical island size in units of
atoms. (Adapted from [48].)
limit (N >> N
1
) follows the power law relationship N
sat
∝ J
p
with p = i*/(1* + 2). Figure 12.11
shows measured N
sat
(J) values for temperatures T
s
= 215 K (−58
◦
C) and 345 K (72
◦
C), near
the middle of the two nucleation regions in Figure 12.10. At 215 K, the exponent
p = 0.32 ± 0.01 indicating that i* = 1 and the smallest stable cluster is a Cu dimer. At 345 K,
p = 0.58 ± 0.02 yielding i* = 3 and the Cu tetramer is now the smallest stable cluster. A similar
scaling law approach was used by Zuo et al. [47] to determine minimum stable cluster sizes for
Cu/Cu(001) as a function of deposition conditions.
(i* + 1) = 2 and 4 are ‘magic’ cluster sizes for a square surface lattice. (i*+1)=3isnot
observed on a square net since the rate-limiting step for dissolution back into the 2D gas would
be only one broken bond, just as the case for (i* + 1) = 2. However, dissolving a stable cluster
of size 4 atoms requires two broken bonds. Above (i* + 1) = 4, there is no well-defined
behavior on square lattices since all clusters are characterized by single or double bond
breaking. Magic cluster sizes for a hexagonal surface net are 2, 3, and 7 atoms.

Thin Film Nucleation, Growth, and Microstructural Evolution 569
More general atomistic nucleation kinetics solutions are also available [42]. For example, in
the complete condensation regime (no significant adatom loss by desorption), the cascade of
Eqs (12.12)–(12.14) can be solved (with each term for j < i* being zero) to yield power law
expressions for the saturation island density N
sat
of the form:
N
sat
∝ (J/ν
s
)
p
exp(E
p
/kT
s
) (12.17)
where p and E
p
depend on the experimental conditions. For 3D nucleation, p = i*/(i* + 2.5) and
E
p
=(E
i*
+ E
s
), while for 2D nucleation p = i*/(i* + 2) with E
P
=(E
i*
+ i*E
s
)/(i* + 2). E
i*
is the
binding energy of the critical nucleus; that is, E
0
= E
1
=0,E
2
= E
b
, and E
3
∼ 2E
b
. The latter
value is based on a bond-counting argument in which E
i*
is given by the number of nearest
neighbor adatom bonds in the critical nucleus i* times the pairwise bonding energy per
bond E
b
.
Consistent with the discussion on the thermodynamics of nucleation earlier in Section 12.2.1,
the results in Figure 12.10 show that i* increases with T
s
and that at higher temperatures, the
island density decreases while the average size increases. Equation (12.17) for 2D nucleation
was used to fit the data and obtain the energetics. From the slope of the i* = 1 region, the
surface diffusion energy for Cu on Ni(001) was determined to be 0.35 eV, very close to the
previously reported value for Cu/Cu(001), E
s
= 0.36 eV [49], and the intercept of the i*=1
region provides an adatom jump attempt frequency ν =4× 10
11
Hz. Knowing E
s
, the cluster
binding energy E
b
= 0.46 eV was obtained from the slope of the i* = 3 region.
12.3 Three-Dimensional Nucleation and Growth
It is clear from Eq. (12.6) that 3D film growth (see schematic diagram in the upper right of
Figure 12.3) is favored when a
2
r
2
γ
s−v
< a
1
r
2
γ
f−v
+ a
2
r
2
γ
s−f
; that is, the net surface free energy
associated with the formation of a cluster is positive. Classic examples are depositing metals
on gas–metal compounds such as SiO
2
, NaCl, and TiO
2
. All gas–metal compounds have
relatively low surface energies, with saturated surface bonds, while metal surface energies are
much higher. (Among metals, those with higher melting points have higher γ values; T
m
is a
measure of the cohesive energy and, hence, the bond strength). The relative inertness of
gas–metal compound surfaces also means that, in addition to γ
f−v
> γ
s−v
for metal deposits,
the interfacial energy per unit area γ
s−f
is large (a ‘weakly interacting’ interface), resulting in a
high contact angle as observed in catalytic systems such as Pd/TiO
2
(011) [50].
12.3.1 Nucleation and Early Growth
During the early stages of 3D growth, adatom supply to the islands is primarily by deposition
onto open substrate area followed by surface diffusion, as illustrated in the upper diagram of
Figure 12.12. Direct deposition onto the islands is minimal owing to small average island
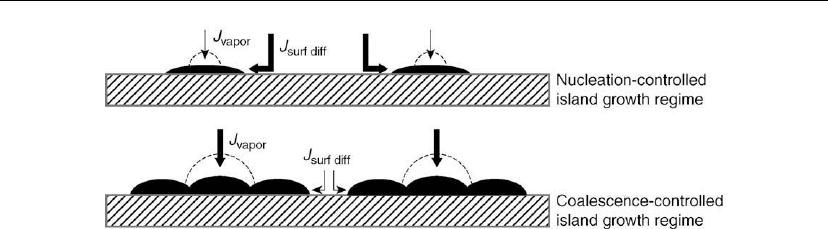
570 Chapter 12
Figure 12.12: Schematic illustration of island growth in the nucleation-controlled (rate
determined primarily by deposition onto the open terrace followed by surface diffusion) and
coalescence-controlled (rate determined primarily by direct deposition) regimes. The dashed lines
indicate equilibrium island shapes with a larger wetting angle ϕ. The widths of the arrows
symbolize the magnitude of adatom supply. (Adapted from [51].)
sizes. Thus, because of the kinetics of adatom supply, the islands at this stage grow primarily
laterally even if the equilibrium film/substrate contact angle is much larger, as illustrated
schematically by the dashed lines. The contact angle can be obtained experimentally via
annealing experiments. After significant island coalescence, however, adatom supply is
primarily through direct deposition (lower diagram in Figure 12.12). At this stage, only a small
amount of material is deposited into the relatively narrow trenches between islands.
Figure 12.13 provides a summary overview of a detailed STM study of the 3D growth of Ag
islands during UHV evaporation, with a deposition rate R = 0.08
˚
A/s, on smooth (rms
roughness ∼ 2
˚
A) amorphous Si (a-Si) substrates at room temperature (T
s
/T
m
= 0.24) [51].
Each panel shows a representative image and line scan corresponding to a different deposition
thickness. At h =3
˚
A(Figure 12.13a), essentially all islands are 3D single crystals. The first
stage of island coalescence is clearly visible by h =10
˚
A(Figure 12.13b) leading primarily to
larger compact single crystal islands (a few islands have grain boundaries) separated by
trenches. Figure 12.13(c), h =54
˚
A, shows that in the second stage of island coalescence
(incomplete coalescence), polycrystalline islands exhibit irregular in-plane shapes separated
by deep trenches; several 111 facets are visible in the region outlined by a rectangle, 111 and
001 facets in the circle. The continuous film regime, with few or no trenches and large 111
faceted grains, is reached by h = 300
˚
A(Figure 12.13d).
12.3.2 Three-Dimensional Island Coalescence
A series of TEM micrographs from a video file (Figure 12.14) illustrate the nature of
morphological changes which occur during coalescence of 3D Au islands on hexagonal
MoS
2
(0002) at 400
◦
C [52, 53]. In the 0.06 s between images (a) and (b), two pseudomorphic
hexagonal Au islands have coalesced and are connected by a neck approximately 500
˚
A wide.
