Martin P.M. Handbook of Deposition Technologies for Films and Coatings, Third Edition: Science, Applications and Technology
Подождите немного. Документ загружается.

Thin Film Nucleation, Growth, and Microstructural Evolution 591
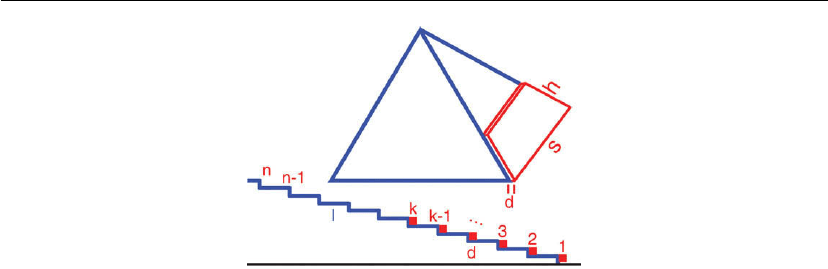
Figure 12.31: Upper figure: schematic illustration of the nucleation of a new {105} facet with
length s and height h on a Ge pyramid grown on Si(001). Lower figure: schematic cross-section of
a facet consisting of individual steps. A partly covered facet is completed up to the kth step.
(Adapted from [79].)
the facet will be completed very rapidly (note also that the step length s(k) decreases with k).
Thus, there is a large activation barrier G*. Estimates of G* as a function of s and ε have
been carried out using kinetic calculations incorporating step formation energy, facet surface
energy, and elastic and dipole step–step interaction energies [83].
The strain energy per unit interface area associated with the deposition of one monolayer of Ge
on Si(001) can be estimated using the classical equation E
elas
=2μ
f
(1 + ν
f
)ε
2
h
f
/(1 − ν
f
) which
assumes growth of a cubic structure material in the [001] direction and that the strain ε is
within the Hooke’s law limit [17]. The terms μ
f
and ν
f
are the shear modulus and Poisson ratio
of the film. Inserting (bulk) Ge values for μ
f
and ν
f
, E
elas
∼ 0.021 eV/atom = 1.4 × 10
13
eV-cm
−2
= 0.16 GPa. Thus, it should not be surprising that relaxation of the large interfacial
strain associated with the growth of Ge/Si(001)2×1 manifests itself long before the
observation of QD formation above the 3 ML-thick wetting layer.
At Ge coverages θ
Ge
< 1 ML, the surface (2×1) reconstruction evolves to (2×N) via the
formation of dimer vacancy lines (DVLs) orthogonal to the 2×1 dimer rows, in which N is the
average number of dimers separating DVLs. In situ STM measurements of MBE Ge layers
grown at 300
◦
C with R = 0.05 ML/s show that the (2×N) periodicity decreases from N ∼ 17 at
θ
Ge
= 0.8 ML toward N = 8, limited by trench–trench repulsion, at 2 ML [21]. The strain is
reduced, at the cost of the trench formation energy, as Ge atoms adjacent to the trenches relax
outwards into the trench. At θ
Ge
greater than ∼ 2 ML, dimer row vacancies (DRVs) form along
the rows to further decrease the strain and the (2×N) reconstruction becomes (M×N)inan
orthogonal quasi-periodic grid pattern. M is the DRV width, the number of vacant dimer rows.
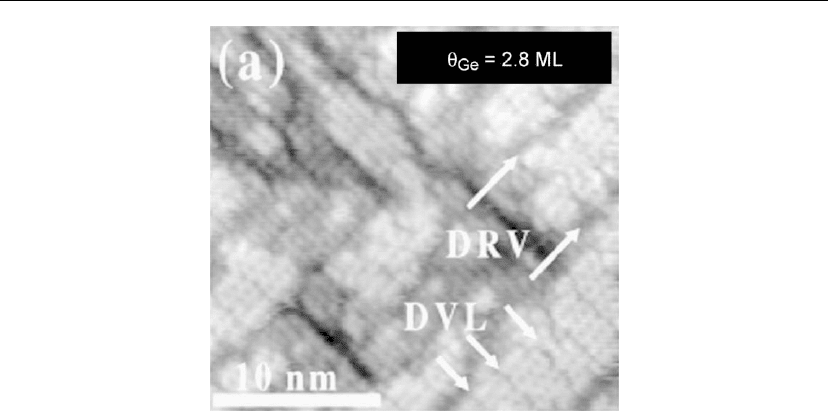
A few representative DVLs and DRVs are labeled in Figure 12.32, an in situ STM image of a
2.8 ML thick Ge/Si(001)2×1 film deposited by GS-MBE from Ge
2
H
2
at T
s
= 650
◦
C (well
above the H
2
desorption temperature) with R = 0.03 ML/s [22]. DVLs and DRVs are also
visible in the wetting layer in Figures 12.28 and 12.30.
592 Chapter 12
Figure 12.32: In situ STM image of a Ge(001)M×N layer grown on Si(001)2×1 by GS-MBE
from Ge
2
H
6
at T
s
= 650
C with R = 0.03 ML/s to a coverage θ
Ge
= 2.8 ML. A few representative
dimer vacancy lines (DVLs) and dimer row vacancies (DRVs) are highlighted by arrows. (Adapted
from [22].)
12.6 Structural Evolution of Polycrystalline Films
at the Nanoscale and Microscale
Polycrystalline thin films have found diverse applications ranging from metallization and
dielectric layers to optical, magnetic, and tribological coatings, to diffusion and thermal
barriers, to catalytic and bioactive layers. The films exhibit a wide variety of microstructures
characterized in terms of grain size and crystallographic orientation, lattice defects, phase
composition, and surface morphology.
Atomic scale control of thin film microstructure during kinetically-limited low-temperature
deposition, crucial for a broad range of industrial applications, has been a leading goal of
materials science during the past few decades. Industrial demands for ever lower processing
temperatures in device and product manufacturing mean that films are often deposited at
T
s
/T
m
≤ 0.2–0.3. Thus, film synthesis takes place far from thermodynamic equilibrium. As a
consequence, grain shape and orientation often evolve in a competitive fashion and the kinetic
limitations induced by low-temperature growth allow for the controlled synthesis of
metastable phases and artificial structures such as multilayers and self-organized
nanocomposites.
Among the determinant atomic processes controlling structure evolution during film growth
are surface and bulk diffusion. In addition to T
s
, energetic particle bombardment can be used to
enhance adatom mobilities and manipulate nucleation rates. The presence of alloying or

Thin Film Nucleation, Growth, and Microstructural Evolution 593
impurity elements and their segregation to surfaces and grain boundaries also strongly
influences the final result.
Extensive studies of the correlation between polycrystalline film structure and deposition
parameters have been carried out over the past five decades. From an understanding of film
formation follows the possibility for structural engineering at the microscale and nanoscale in
order to design materials for specific technological applications. This spawned the
development and refinement of structure zone models (SZMs) which systematically categorize
self-organized structural evolution during film growth as a function of film growth parameters
[84–90]. The history of SZMs has been reviewed by Thornton [91], Barna and Adamik [92],
and Mahieu et al. [93]. In 1969, Movchan and Demchishin [84] observed that the
microstructural evolution of evaporated Ti, Ni, W, ZrO
2
, and Al
2
O
3
coatings can be
systematically represented by a single SZM diagram plotted as a function of film thickness h
and the homologous growth temperature T
s
/T
m
.
The first SZMs were based on relatively low-resolution optical and scanning electron
microscopy observations. Later, cross-sectional transmission electron microscopy (XTEM),
STM, and AFM were employed to provide more detailed structural characterization, and in
situ analyses began to reveal the rich dynamics of film growth. This, together with
computational materials science, has provided atomistic insights into microstructural evolution
during polycrystalline film growth. The use of amorphous substrates is beneficial for isolating
the effects of individual deposition variables on texture development. Polycrystalline
substrates bias texture through local pseudomorphic epitaxy. Nevertheless, the overall
microstructure will still evolve toward a final state driven by the extant deposition conditions.
It is important to note, however, that if surface diffusion rates are significant, once the substrate
is covered, film growth will still proceed via local epitaxy on individual grains, even with
amorphous substrates.
12.6.1 Elemental Polycrystalline Films and Structure-Zone Models
The nucleation barrier for low-temperature deposition on amorphous substrates is generally
quite small, leading to randomly oriented islands [94, 95]. This has been demonstrated in a
wide variety of materials. Early in situ TEM investigations included Au/SiO
2
[96, 97] and
In/a-C [55, 98]. During island coalescence, there is a strong driving force for coarsening
through surface atom diffusion and grain boundary motion. Islands with lower energy per
atom E
b
consume their neighbor(s) during coalescence and this process can result in large
single-crystal islands as the system attempts to minimize the overall surface and interface
energy. Thus, coarsening during coalescence is the first and most active phenomenon leading
to selection of preferred orientation [99–101]. Absenting other kinetic constraints (see
following sections), islands with the densest atomic planes are typically selected: (111) for fcc,
(0002) for hcp, and (110) for bcc.
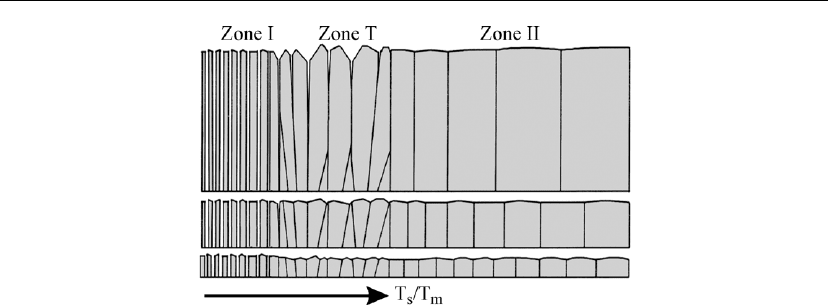
594 Chapter 12
Figure 12.33: SZM schematically representing microstructural evolution of pure elemental films as
a function of the reduced temperature T
s
/T
m
, where T
s
is the deposition temperature and T
m
is
the melting point of the material, both expressed in K. (From [8].)
Depending on T
s
/T
m
and island size (due to melting point depression for nanoscale clusters)
[56], coarsening can be very fast, often termed liquid-like coalescence, occurring either by
rapid surface diffusion or by melting upon contact followed by crystallization. The driving
force is the release of edge and surface energy. Rapid coalescence also results in new open
substrate area for secondary nucleation, as shown in the in situ TEM image sequence
(Figure 12.15) obtained during coalescence of In/a-C. At lower temperatures or larger island
sizes, coarsening is slower and proceeds through grain boundary migration. Grain coarsening
during coalescence of the contacting crystals is repeated until the local grain size becomes
sufficiently large that grain boundary mobility is low on the timescale of coalescence.
The SZM in Figure 12.33, characterizing microstructure evolution in pure elemental films,
consists of three regions [8]: zone I corresponds to very low deposition temperatures for which
adatom diffusion is negligible; surface diffusion becomes significant in the transition zone T;
and zone II represents film growth at deposition temperatures for which both surface and bulk
diffusion are operative. The boundaries between zones are diffuse and transitions occur
gradually over wide ranges in T
s
/T
m
.
During film growth in the low-T
s
zone I regime (Figure 12.33), an underdense structure with a
fine fiber texture is formed. Initial in-plane grain sizes are set by the saturation nucleation
density N
sat
. Adatom mobilities are low and columns preserve the random orientation of the
nuclei as predicted by ballistic deposition models [102, 103]. The columns are generally not
single grains, but composed of smaller more equiaxed grains, or completely amorphous.
Surface roughness, which develops owing to atomic shadowing and limited surface diffusion,
leads to extensive porosity. The wide angular distribution of the incident flux during sputter
deposition, especially at pressures corresponding to non-ballistic transport, exacerbates these
effects.
Thin Film Nucleation, Growth, and Microstructural Evolution 595
At higher film growth temperatures (zone T), grain coarsening occurs during coalescence of
small islands with large surface to volume ratios, while grain boundaries are immobile in
continuous films. Orientation selection during coarsening is incomplete, thus crystallites are
nearly random or only weakly textured, and there is a wide distribution of initial grain sizes.
The orientation and size of individual crystallites determine their behavior during subsequent
growth processes characterized by competition among neighboring grains. In this T
s
/T
m
range,
adatom surface diffusion is significant, resulting in local epitaxial growth taking place on
individual grains. Pronounced columnar structure develops in which the columns are actually
elongated grains.
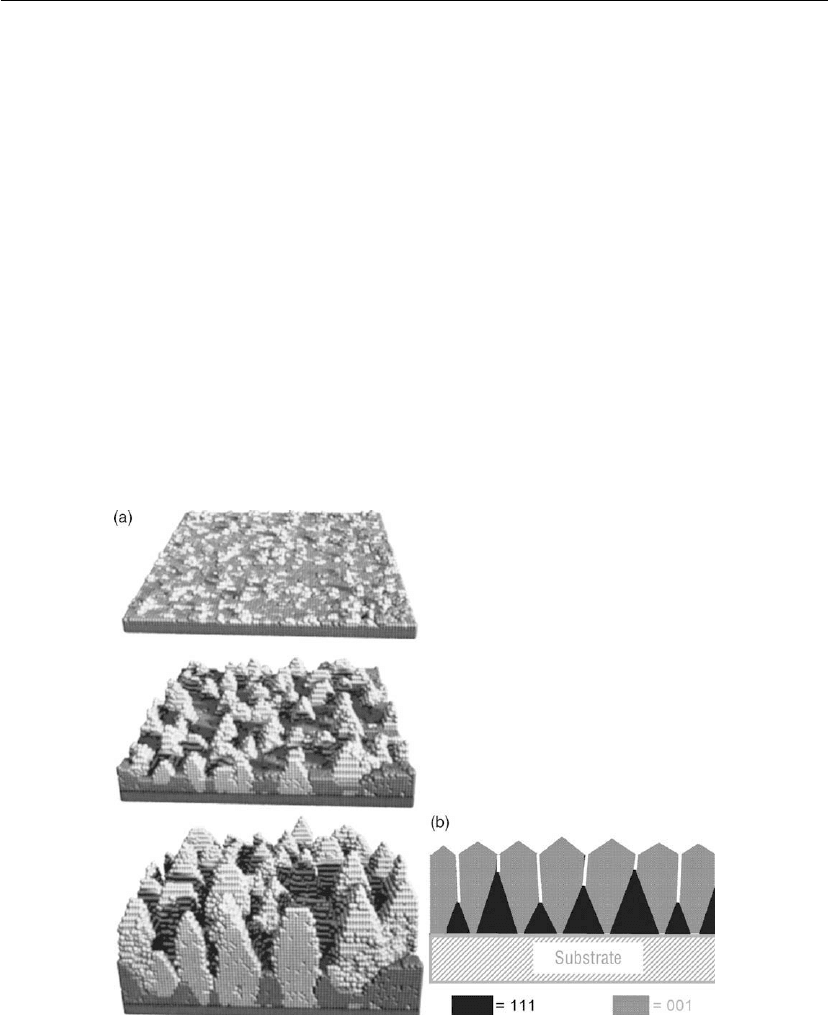
The primary features of zone T competitive grain growth are illustrated by the kinetic Monte
Carlo simulations of Gilmer et al. [104, 105] for sputter-deposited Al growth as shown in
Figure 12.34(a). While there are initially equal distributions of 111 and 001 islands, the latter
orientation eventually dominates, even though 111 is the low-energy surface, owing to
anisotropies in surface diffusivities and adatom potential energies. That is, the average adatom
residence time is significantly higher at lattice sites on low diffusivity (low potential energy)
001 surfaces versus high diffusivity (high potential energy) 111 surfaces. Therefore, during the
Figure 12.34: (a) Kinetic Monte Carlo simulation of competitive texture evolution during
low-temperature sputter deposition of an Al film. Islands (and later columns) with lighter contrast
are 001 oriented, while darker islands/columns are 111 oriented (from [104, 105]).
(b) Schematic cross-section.

596 Chapter 12
early stages, 111 islands tend to expand more two-dimensionally and thus fill more surface
area (see upper panel in Figure 12.34a), while 001 grains favor more 3D growth. Following
coalescence, however, adatoms which are stochastically deposited near grain boundaries and,
through surface diffusion, can sample sites on both sides of the boundary, have a higher
probability of becoming incorporated at the low-diffusivity surface which provides more
stable, lower potential energy sites. Conversely, adatoms on high diffusivity planes have longer
mean free paths with correspondingly higher probabilities to move off the plane and become
trapped on adjacent grains. Thus, 001 grains become favored. Atomic shadowing enhances the
difference, as protruding surfaces capture more off-normal flux. Thus, the low-diffusivity 001
grains slowly expand, overgrow the high-diffusivity grains, and become bounded by 111 facets.
This leads to considerable surface roughness which scales with the average in-plane grain size.
The consequence of competitive growth is a continuous change in morphology, texture, and
surface topography (hence, film properties) as a function of film thickness. Near the substrate,
the microstructure consists of randomly oriented small grains, out of which V-shaped columns
(Figure 12.34b) with favored orientations slowly emerge and overgrow kinetically
disadvantaged columns. This gives rise to preferred orientation. The faceted column tops
result, as noted above, in surface roughness which increases with thickness resulting in open
column boundaries due to atomic shadowing.
At still higher T
s
/T
m
(zone II), bulk diffusion becomes significant. Grain boundary migration
takes place not only during coalescence, but throughout the film growth process. Orientation
selection during the coalescence stage is more pronounced and is driven by a decrease in the
total grain boundary area as well as minimization of interface and surface energy [99]. Large
grains with low surface and interface energy grow at the expense of smaller or unfavorably
oriented grains. Normal grain growth is impeded if the grains have a strong texture, i.e. if the
orientation selection was completed during coalescence, or the mean in-plane grain diameter
reaches several times the film thickness [106]. Secondary recrystallization, also called
abnormal grain growth, may follow in which the grain size distribution is transformed from
monomodal, through bimodal, to a new monomodal distribution (unless grain growth is halted
due to solute drag and/or grain boundary grooving) [106, 107] with much larger in-plane grain
size. During secondary recrystallization, the degree of texture is further enhanced.
An example of grain boundary migration is shown in Figure 12.35, a series of STM topographs
from a 300
˚
A thick polycrystalline Au film deposited by MBE on SiO
2
(0001) at room
temperature with R = 0.0028
˚
A/s and then annealed using a very slow linear temperature ramp
dT
a
/dt
a
=20
◦
C/h [107]. X-ray diffraction (XRD) measurements of as-deposited samples reveal
a 111 texture: approximately 84% of the grains are 111, ∼ 16% 001, with no detectable 011
grains. The degree of 111 preferred orientation increases rapidly with annealing temperature.
The as-deposited polycrystalline Au film in the upper panel in Figure 12.35 has a highly
irregular surface in which the roughness is controlled by grains with high-energy boundaries.
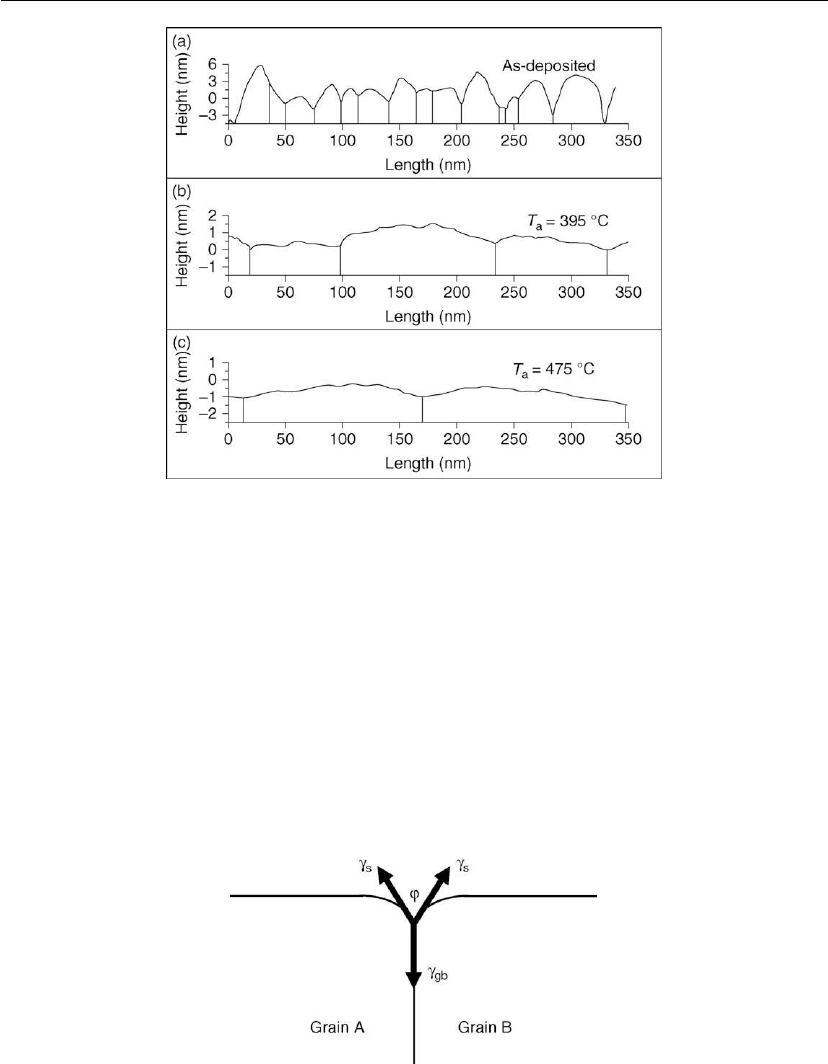
Thin Film Nucleation, Growth, and Microstructural Evolution 597
Figure 12.35: Typical STM topographical profiles from a 300
˚
A thick Au film deposited by MBE
with R = 0.0028
˚
A/s on SiO
2
(0001) and subsequently annealed at 20
C/h: (a) immediately after
deposition, (b) at T
a
= 395
C, and (c) at T
a
= 475
C. Each dome is an individual grain. Vertical
lines indicate the grain boundary locations. Note the different height scales. Individual steps and
terraces are visible in panels (b) and (c). The topographs are from a video file obtained during
annealing. (Adapted from [107].)
The grain boundary line tension γ
gb
is related to the surface tension γ
s
through the relationship
γ
gb
= 2γ
s
cos(ϕ/2) (12.29)
where ϕ is the dihedral angle as defined in Figure 12.36. Grains with high-energy boundaries
have small dihedral angles, resulting in tall islands, and thus a rough surface. However, the
Figure 12.36: Schematic illustration of grain boundary grooving which occurs in order to minimize
the total system energy. γ
gb
is the grain boundary line tension, γ
s
is the surface tension, and ϕ is
the dihedral angle.
598 Chapter 12
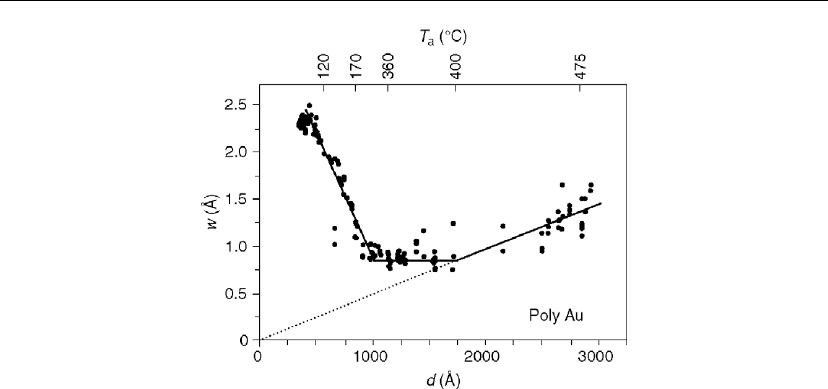
Figure 12.37: Surface roughness w of the polycrystalline Au film, corresponding to Figure 12.35,
as a function of the average grain size d during annealing from room temperature to 475
Cat
20
C/h. (Adapted from [107].)
high-energy grain boundaries tend to anneal out as T
a
is increased and the remaining lower
energy boundaries have larger ϕ values, yielding a smoother surface. This is illustrated in the
in situ STM topographs Figures 12.36(b) (T
a
= 395
◦
C) and 12.36(c) (T
a
= 475
◦
C). Over the
entire range of temperatures, surface adatom diffusion is sufficiently fast to maintain the
surface shape at (or very close to) equilibrium with the evolving grain boundary configuration,
as demonstrated by the fact that only very small shape changes are observed if the thermal
ramp is halted and T
a
maintained constant.
Figure 12.37 shows that the surface roughness w decreases by a factor of ∼ 3× over the
temperature range from 20
◦
Cto∼ 325
◦
C, remains approximately constant with T
a
until
∼ 400
◦
C, and then increases again at higher T
a
. Over the full annealing range, 20–475
◦
C, the
average grain size d increases by an order of magnitude and XRD results show that the film
texture transforms to essentially complete 111. In the first region, over which w decreases
rapidly and d increases, the high-energy grain boundaries become mobile as T
a
is increased
and quickly reorient themselves in lower energy configurations (corresponding to cusps in the
orientation vs grain boundary energy diagram). Thus, the average grain boundary energy
decreases giving rise to an increase in the average dihedral angle ϕ (Eq. 12.29), and therefore a
corresponding decrease in w from ∼ 23
˚
Ato8
˚
A while d increases from ∼ 300
˚
A to 1000
˚
A.
As T
a
is raised further, the lower-energy grain boundaries also become mobile resulting in a
continued gradual coarsening of the grain boundary network. This mechanism, by itself, has
no significant effect on the average dihedral angle ϕ since the average grain boundary energy
γ
gb
remains constant. However, as a given grain diameter increases, the height variation across
the grain also increases due to a self-similar scaling behavior. Over the intermediate regime in

Thin Film Nucleation, Growth, and Microstructural Evolution 599
Figure 12.37,asd increases from ∼ 1000
˚
A to 1750
˚
A, the surface roughness w remains
essentially constant owing to the competition between grain boundary reorientation (and
texture development) which decreases w, and the growth of grains with low-angle boundaries
which increases w. The third regime (T
a
∼ 400–475
◦
C) is dominated by the continued growth
of highly textured grains with low-energy boundaries as d increases from ∼ 1750
˚
A to 3000
˚
A
causing the average surface roughness w to increase from ∼ 8
˚
Ato15
˚
A. The maximum grain
size at the highest temperature, T
a
= 475
◦
C, is ∼ 3000
˚
A, a factor of 10× larger than the Au
film thickness. Prolonged annealing at 475
◦
C had no further significant effect on d.
The grain growth history just described occurred through a combination of ‘normal’ and
‘abnormal’ mechanisms. During primary or normal grain growth, the grain size distribution
remains monomodal as individual grain boundaries move toward their centers of curvature in
order to reduce boundary curvature, total boundary length, and, thus, the total grain boundary
energy. That is, grains larger than the average size grow, while smaller grains shrink. In
columnar grain systems, grains with five or fewer sides tend to shrink, while those with seven
or more sides grow [108]. Secondary (or abnormal) grain growth results initially in a bimodal
grain size distribution which, if allowed to proceed to completion, leads again to a monomodal
distribution, but with a lower density of larger grains. Driving forces for abnormal grain growth
include local epitaxy [109], anisotropic surface and/or interfacial energies [110], anisotropic
strain [111], and/or kinetic competition [112–114]. For example, since surface energy is
strongly dependent on crystallographic orientation, those grains with orientations that lead to
low surface energies have an energetic advantage during growth. In the polycrystalline Au film
experiments, the entire grain distribution moved toward a complete 111 texture.
12.6.2 Multicomponent and Multiphase Film Growth
Polycrystalline thin films synthesized by reactive deposition provide additional pathways for
microstructure control while yielding enhanced thermal and process stability. Here, the term
reactive deposition encompasses the purposeful incorporation of dopants as well as
unintentional atmospheric contaminants such as water vapor, oxygen, and hydrocarbons since
even low concentrations of reactive elements (sometimes below the detection limits of modern
analytical techniques) can have strong effects on microstructural development [92, 115, 116].
Consider the case of O-containing polycrystalline Al films deposited at room temperature,
T
s
/T
m
= 0.32, corresponding to zone II in the pure Al SZM [117]. Changes in film structure and
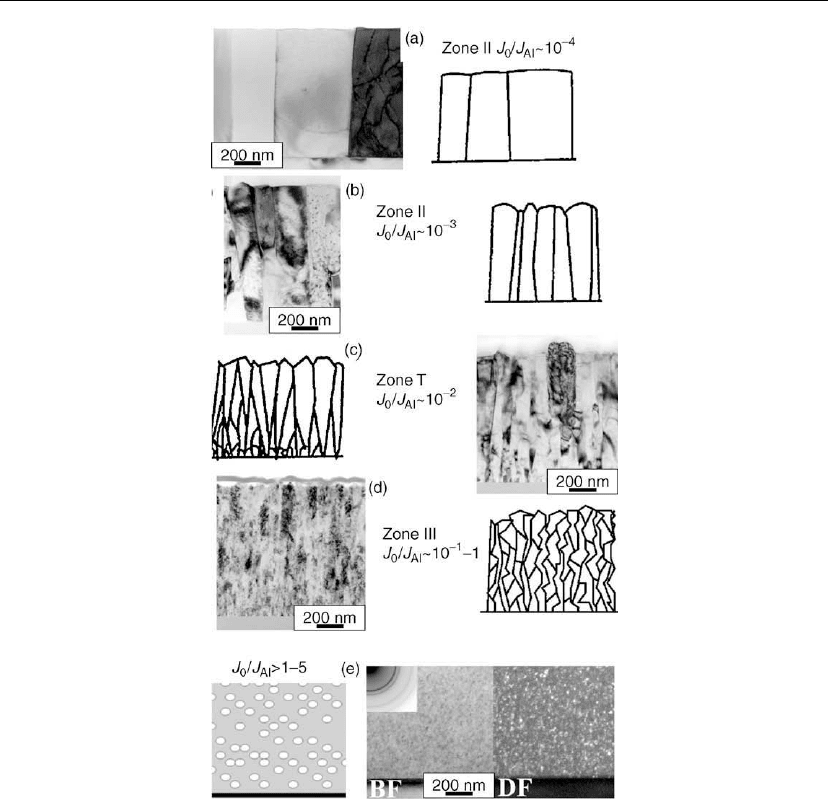
orientation as a function of increasing oxygen concentration, observed via in situ TEM
investigations, are summarized in Figure 12.38 [8, 92]. Oxygen has low solubility in Al and
segregates to surfaces and grain boundaries where it forms 2D oxide layers (oxide tissue
phases) which greatly reduce Al adatom surface and grain-boundary mobilities. These layers
modify all film formation processes, limiting grain coarsening during coalescence and film
growth. They also periodically interrupt the local epitaxial growth of individual crystallites and
600 Chapter 12
Figure 12.38: XTEM images, with corresponding schematic diagrams, showing the microstructure
of Al films deposited by thermal evaporation on amorphous SiO
2
at room temperature as a
function of the incident O to Al flux ratio J
O
/J
Al
. (From [8, 92].)
cause renucleation [55, 98]. By exploiting such phenomena, new microstructures and
nanostructures can be controllably synthesized.
At low O/Al arrival rate ratios, J
O
/J
Al
∼ 10
−3
, oxygen is incorporated into the grain boundaries
and further accumulates during boundary migration, eventually inhibiting grain coarsening
though solute drag. The resulting texture remains zone II with columns extending through the
film, but with a lesser degree of preferred orientation and a smaller grain size, as shown in
Figure 12.38(b).
