Martin P.M. Handbook of Deposition Technologies for Films and Coatings, Third Edition: Science, Applications and Technology
Подождите немного. Документ загружается.


Thin Film Nucleation, Growth, and Microstructural Evolution 601
With slightly higher oxygen concentration levels (J
O
/J
Al
∼ 10
−2
), coarsening during
coalescence is severely suppressed, resulting in grains with random orientation. The
competitive growth which follows is governed by anisotropic crystallographic effects [118]:
O segregates fastest at 111 surfaces. Oxygen is incorporated into the lattice of 001 and 110
crystal faces, while an oxide layer is formed on the 111 faces [119]. Oxygen tends to
accumulate at step edges on 111 surfaces, blocking step motion and leading to step bunching.
These pinning sites serve to nucleate the oxide phase. Neighboring 111 grains have rounded
edges due to oxygen segregation while 111-oriented grains in contact with 001-facets remain
sharp, as oxygen is incorporated in the latter. The 111 grains eventually develop rounded
surfaces, indicating that local epitaxial Al growth has been interrupted by an oxide layer,
above which renucleation of metal islands takes place. Crystal growth on 001-oriented grains
is unimpeded by oxygen; these grains protrude above the average film surface and eventually
win in competitive growth. They develop the shape of truncated octahedrons bounded by a 001
top face and 111 side faces. The degree of 001 preferred orientation increases with film
thickness and is accompanied by greater surface roughness with increasing oxygen
concentrations (Figure 12.38c).
At still higher oxygen concentrations (J
O
/J
Al
∼ 0.1–1), the oxide layer completely covers
islands of all orientations at an early stage and coarsening during coalescence is blocked.
Thus, film growth proceeds by repeated renucleation. The film is composed of 3D equiaxed
(globular) grains with random orientation and a zone III structure (Figure 12.38d). With
increasing oxygen concentration, the grain size decreases and can reach the nanometer range.
An important byproduct of repeated nucleation and nanograin film formation is that surface
faceting on individual columns, and the related shadowing effects, are eliminated. Thus,
nanophase films are inherently much smoother and, as a result, denser. The presence of oxide
phases also inhibits grain boundary migration in the bulk of the film, preventing grain
coarsening and imparting higher thermal stability. This approach has been systematically
exploited in order to synthesize superhard nanocomposite films based on transition metal (TM)
nitrides and carbides, e.g. nc-TMN/a-Si
3
N
4
[120, 121], nc-TMC/a-C [122],TiN
x
B
y
[123,
124],TiC
x
B
y
[125], and TMN/Cu [126], as well as supertough Y
2
O
3
-stabilized ZrO
2
/Au
layers [127].
As the oxygen concentration is further increased, (J
O
/J
Al
∼ 2–5), the role of the oxide and
metal phases are reversed: the oxide phase nucleates first, while Al segregates to the surface
and forms 3D islands [117, 128]. Resulting films are composed of metallic grains dispersed in
an oxide matrix (Figure 12.38e) [129]. Such composite films, consisting of a low-diffusivity
matrix with higher diffusivity metallic inclusions, are the basis of a class of ceramic–metallic
coatings with diverse applications: resistors [130, 131], sensors [132], solar cell elements
[133], low-friction hard coatings (e.g. TM/a-C) [134], and smart tribological coatings that
adapt to the environment [135, 136].

602 Chapter 12
At very high oxygen fluxes (J
O
/J
Al
1), the films consist entirely of aluminum oxide, which
for room temperature growth is amorphous. T
s
values exceeding 800
◦
C are required for the
synthesis of the chemically and mechanically stable κ and α crystalline phases of alumina.
There has, however, been a concerted effort to achieve hard crystalline alumina using
ion-irradiation during growth at temperatures below 500
◦
C [137].
The addition of metallic elements, rather than gas-phase species, for multicomponent
microstructural modification has been shown to both increase and decrease the average grain
size depending on the choice of materials. For example, adding a few at.% Pt [138] or Cu
[139] to Al leads, in contrast to tissue phase formation, to the nucleation of 3D islands of the
minority phase(s) on the surface of the majority phase. Here, all adspecies have high surface
mobilities. For the case of 350
˚
A thick Al + 4 at.%Pt layers co-evaporated onto a-C
substrates at T
s
= 350
◦
C, TEM imaging and diffraction analyses show that the minority
phases are Al
5
Pt and Al
6
Pt. Equiaxed grains of the minority phase decorate grain boundaries
and triple points, thus significantly decreasing grain boundary migration and grain coarsening
[140].
The addition of Sn to Al has quite the opposite effect; rather than decreasing the grain size as
with O and Pt, it acts to enhance grain growth. In a combinatorial materials science
experiment, Barna and Adamik [92] deposited a 1000
˚
A thick Al(Sn) film on a-C at room
temperature by co-evaporation using a geometry and relative deposition rates such that the Sn
concentration in the film varied from near zero at the left edge of the field of view of a
plan-view TEM image to 10 at.% at the right edge. At low Sn concentrations, the film is
continuous with an average grain size of 30–50
˚
A, while at higher concentrations the film is
still in the island growth stage with the grain size enhanced by a factor greater than 5×.Sn
appears to be acting as a surfactant in increasing Al adatom surface mobility [141].
12.6.3 Ion Irradiation Effects
Films deposited at low temperatures with little or no ion irradiation tend, as noted in Section
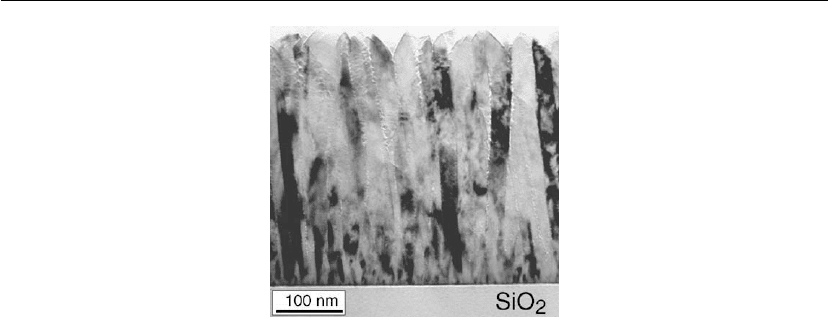
12.6.1, to be columnar and underdense. Figure 12.39 is a typical XTEM image of such a
structure; in this case, a TiN film grown by reactive magnetron sputtering in pure N
2
, with
P
N2
= 20 mtorr, at T
s
= 350
◦
C(T
s
/T
m
= 0.17) and R = 0.75 m/h (2.1
˚
A/s) on amorphous SiO
2
with no applied substrate bias [142]. The film lattice constant was found by XRD to be equal
to that of unstrained bulk TiN. Figure 12.39 shows that the film grain size, initially small,
increases continuously with film thickness while the column boundaries become increasingly
more open. The self-organized zone T (see Figure 12.33) columnar microstructure forms
through random nucleation, limited coarsening during coalescence, and competitive column
growth resulting in a microstructure consistent with that predicted by the kinetic Monte Carlo
simulations and schematic diagram in Figure 12.34. The column tops are faceted owing to
kinetic roughening, which, in combination with atomic shadowing, results in deep cusps
Thin Film Nucleation, Growth, and Microstructural Evolution 603
Figure 12.39: A bright-field XTEM micrograph of a TiN film deposited by magnetron sputtering in
a pure N
2
atmosphere (20 mtorr) on amorphous SiO
2
at 350
C. The ion-to-Ti flux ratio J
i
/J
Ti
incident at the growing film was < 1 with an ion energy E
i
= 20 eV. (From [142].)
between columns and open column boundaries. The individual columns, however, are dense,
indicating sufficient adatom surface mobility to sustain local crystal growth.
Many of the early applications of ion irradiation during film growth were designed to address
issues arising from low-temperature deposition such as those apparent in Figure 12.39. The
motivation was generally to increase film density and modify grain morphology (e.g. columnar
to equiaxed) as well as to manipulate film texture. The experiments were carried out using
plasma-based sputtering techniques by applying a negative bias to the substrate and later by
using ion beam assisted deposition (IBAD) in which a secondary ion source is aimed at the
substrate and growing film deposited by primary ion beam sputtering or evaporation. However,
essentially all experiments employed what is today considered to be high-energy ion
bombardment (E
i
> 100 eV); that is, well above the bulk lattice atom displacement threshold,
giving rise to significant residual ion-induced lattice damage. It was not until the development,
in the late 1980s and early 1990s, of gridless Hall-current ion sources [143] for IBAD
applications and tunable magnetically unbalanced magnetron sources [144] for plasma
deposition, that the ability to independently vary ion currents (or, more importantly,
ion/deposited-atom ratios J
i
/J
Me
) at the growing film over large values while using low-energy
ions (typically ≤ 20 eV), that it became possible to control density, grain morphology,
preferred orientation, surface smoothness, and film stress without the collateral introduction of
detectable residual defects.
12.6.3.1 High Energy, Low Ion Flux
Mattox and Kominiak [145] were among the first to quantitatively demonstrate that the density
of films grown under conditions leading to porous microstructures (e.g. low T
s
and/or high
pressures such as are typical of DC sputtering) can be increased by ion bombardment during
604 Chapter 12
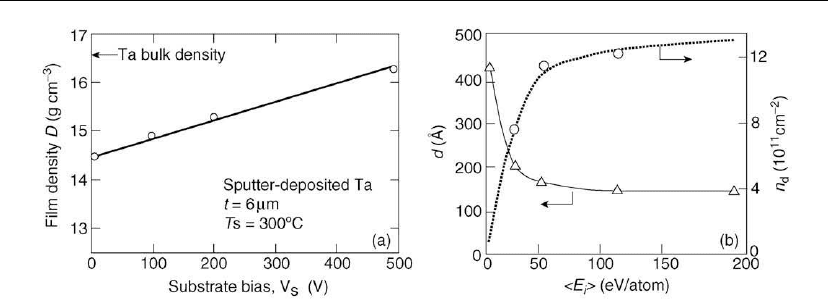
Figure 12.40: (a) Density, as a function of the negative substrate bias V
s
,of6-m-thick Ta films
deposited by dc sputtering in Ar (adapted from [145]). (b) Average grain size d and dislocation
density n
d
in Ag films deposited at room temperature as a function of the average normalized ion
energy <E
i
> per deposited Ag atom. (Plotted from tabulated data in [146].)
deposition. The density of 6-m-thick polycrystalline Ta films deposited at 300
◦
C
(T
s
/T
m
= 0.175) by dc sputtering in Ar increased continuously with the applied negative
substrate bias V
s
from ∼ 14.5 g cm
−3
with V
s
= 0 to 16.3 g cm
−3
(bulk density = 16.6 g cm
−3
)
with V
s
= −500 V, as shown in Figure 12.40(a).
Huang et al. [146] investigated the effect of Ar
+
bombardment during the growth of Ag films
at room temperature using a UHV dual ion-beam apparatus. They observed that the void
density decreased with increasing ion energy, consistent with the Ta dc sputtering result.
However, they also found that the average grain size d decreased, from 420 to 145
˚
A, while the
dislocation number density n
d
increased from 7 × 10
10
to 1.3 × 10
12
cm
−2
, as a function of the
normalized ion irradiation energy <E
i
>, defined as the total Ar
+
ion energy delivered to the film
per deposited Ag atom. <E
i
> was varied from thermal (obtained by evaporation with no ion
bombardment) to 190 eV/atom (see Figure 12.40b). In addition, the degree of 111 preferred
orientation, determined by XRD, decreased and the plane stress reversed from 0.06 GPa tensile
for underdense evaporated films to −0.45 GPa compressive with <E
i
> > 42 eV/atom.
Densification via high-energy ion irradiation at the cost of residual defect production is clearly
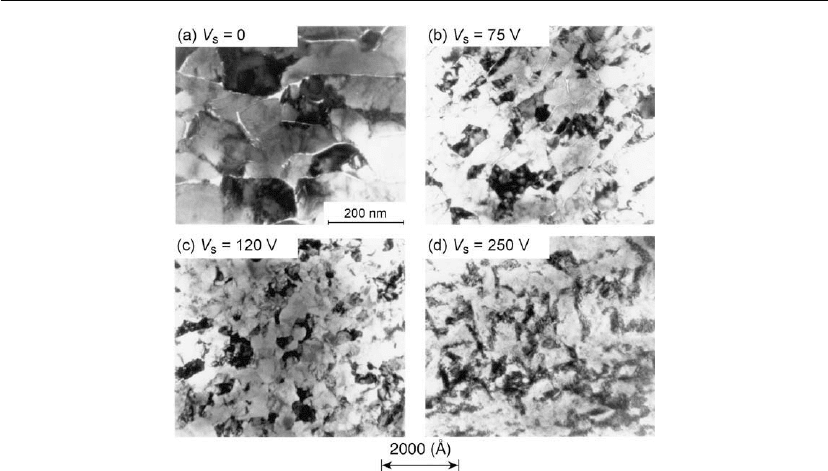
illustrated by the plan-view TEM micrographs in Figure 12.41 of polycrystalline Ti
0.5
Al
0.5
N
films deposited by reactive magnetron sputtering in mixed Ar/N
2
discharges with
P
tot
= 8.2 mtorr (P
N2
= 2.9 mtorr) on stainless-steel substrates at ∼ 450
◦
C(T
s
/T
m
∼ 0.20) with
J
i
/J
Me
∼ 0.9 and R = 0.2 m/minute (33.3
˚
A/s) [147]. The applied negative substrate bias V
s
ranged from 0 to 250 V (corresponding to ion energies of ∼ 6.5 to 255 eV when the plasma
potential is included). XTEM images (not shown) reveal that films grown with V
s
= 0 have a
columnar microstructure with intercolumnar void networks similar to those in Figure 12.39.
Film porosity decreases sharply with V
s
> 100 eV until, with V
s
> 120 V, no voids are observed
Thin Film Nucleation, Growth, and Microstructural Evolution 605
Figure 12.41: Transmission electron micrographs of polycrystalline Ti
0.5
Al
0.5
N films deposited by
reactive magnetron sputter deposition on stainless-steel substrates at T
s
∼ 450
C as a function
of the applied negative substrate bias V
s
with J
i
/J
Me
∼ 0.9. (a) V
s
= 0; (b) 75 eV, (c) 120 eV, and
(d) 250 eV. (From [147].)
using underfocus and overfocus contrast. However, the film compressive stress (owing to a
combination of ion trapping and recoil implantation) increases and high dislocation loop
densities are observed within individual grains in panels (b)–(d). In fact, the defect densities
are large enough to disrupt column growth and force renucleation, as evidenced by the
presence of Moir
´
e fringes, leading to smaller grain sizes.
X-ray and electron diffraction patterns also reveal a very slow change in TiN preferred
orientation from 111 to 002 with increasing E
i
. However, the ion energy required to complete
the transition is > 800 eV, for which the films have unacceptably high stress levels [148]. Thus,
the use of high-energy, low-flux ion irradiation is not a practical approach for controlling film
texture. The formation of 002 texture under such conditions is directly related to collision
cascade effects [149]. Grains with open channel directions, such as 002, have higher survival
probabilities due to the anisotropy in collision cascades; that is, the ion energy is distributed
over larger depths in open channels leading to lower sputtering yields and less lattice distortion.
In summary, densification obtained in the high-energy ion irradiation regime (E
i
> 100 eV)
comes at a steep price, leading to high defect densities [150], high compressive stresses
[151–153], and inert gas incorporation [148]. Ar concentrations C
Ar
in TiN layers deposited on
amorphous SiO
2
at 350
◦
C as a function of E
i
, with J
i
/J
Ti
≤ 1, are below 0.5 at.% with

606 Chapter 12
E
i
< 100 eV, while at higher ion energies, C
Ar
increases approximately linearly from 1 at.% at
200 eV to > 3 at.% at 800 eV.
12.6.3.2 Low Energy, High Ion Flux
Magnetron sputtering is a primary technique for the growth of polycrystalline thin films. It
provides high deposition rates due to the Lorentz force, arising from the cross-product of the
applied electric field used to accelerate the ions to the target and the permanent magnetic field,
which traps the plasma immediately adjacent to the target and efficiently utilizes secondary
electrons in the discharge to produce new ions. However, this very advantage becomes a
disadvantage when attempting to use ion bombardment at the substrate for microstructural and
compositional modification during film growth. The strong electromagnetic trap near the target
means that the substrate and growing film are essentially outside the plasma; thus, a high
substrate potential is required to obtain significant ion bombardment and, even then, the ion
flux at the substrate is small. For typical magnetron discharges, depending on the target
material, gas, pressure, and system geometry, ion/deposited-atom ratios at the substrate with
V
s
= −100 V are of the order J
i
/J
Me
∼ 0.1–0.5. This dramatically changed with the
development of the tunable unbalanced magnetron which incorporates a Helmholtz coil
external to the permanent magnets of the magnetron to unbalance the magnetic circuit and
controllably open a leak in the plasma [144]. High ion/deposited-atom ratios, J
i
/J
Me
> 50, can
be achieved using very low ion energies (E
i
< 10–20 eV) with no significant change in film
deposition rates. Moreover, the ion energy and ion flux incident at the substrate are
independently controlled by V
s
and the external magnetic field B
ext
, respectively.
E
i
≤ 20 eV is below the threshold for bulk lattice atom displacement in NaCl-structure TM
nitrides. The residual stresses remain low in as-deposited films, yet the effects on texture and
microstructure are dramatic. In some of the earliest experiments using unbalanced magnetrons
[144, 154, 155], it was clearly demonstrated that the average ion bombardment energy per
deposited atom <E
i
> is not a universal parameter for microstructural and texture modification
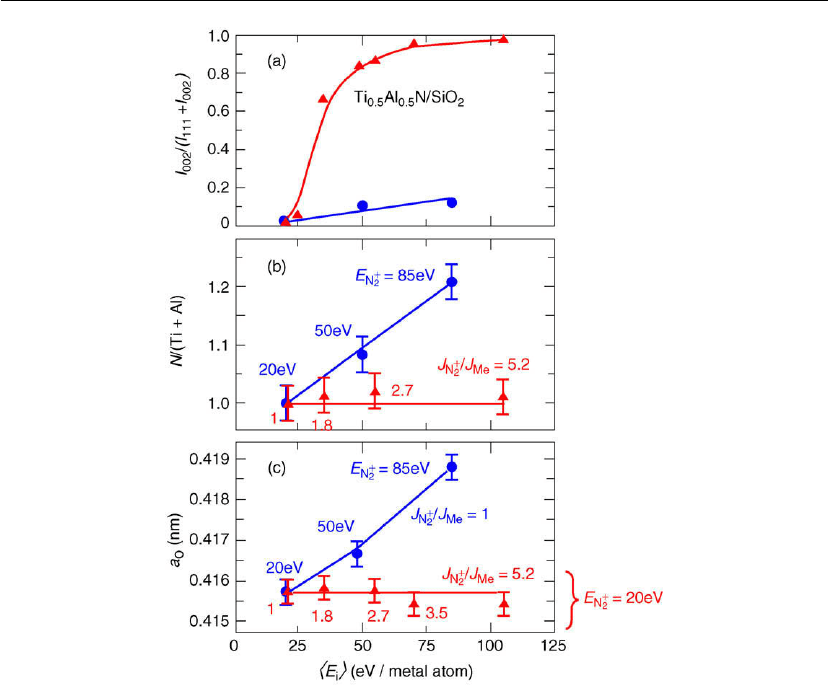
as had been proposed by many authors. Figure 12.41 conclusively shows, for example, that
changes in the stress state, stoichiometry, and texture of NaCl-structure polycrystalline
Ti
0.5
Al
0.5
N films deposited on a-SiO
2
follow completely different reaction paths depending on
whether <E
i
> is varied by changing E
i
(with a constant value of J
i
/J
Me
) or varying J
i
/J
Me
(with
constant E
i
) [154, 155]. Similar results have been reported for TiN [142],TaN[113], ScN
[156], and many other materials.
The polycrystalline 1-m-thick NaCl-structure Ti
0.5
Al
0.5
N films used to obtain the
experimental results summarized in Figure 12.42 were deposited by reactive magnetron
sputtering from a TiAl alloy target on a-SiO
2
at 250
◦
C in pure N
2
discharges at 20 mtorr
[155]. The relatively high pressures were chosen in order to thermalize the sputtered metal
atoms and neutralized ions reflected from the target such that the only energetic species
incident at the growing film were accelerated nitrogen ions (∼ 96.5% N
2
+
and 3.5% N
+
) [67]
Thin Film Nucleation, Growth, and Microstructural Evolution 607
Figure 12.42: (a) Normalized X-ray diffraction peak intensities I; (b) composition ratios
N/(Ti + Al); and (c) out-of-plane lattice parameters a
o
from Ti
0.5
Al
0.5
N alloys deposited on
amorphous SiO
2
at 250
C under N
2
+
ion irradiation as a function of the normalized energy <E
i
>
per deposited metal atom varied through changes in either the ion energy E
i
or the ion/metal flux
ratio J
i
/J
Me
. (Adapted from [155].)
whose flux was controlled by varying B
ext
. The results in Figure 12.42 show that raising <E
i
>
through increases in E
i
, with J
i
/J
Me
maintained constant at 1, increases the out-of-plane lattice
constant a
o
(panel c) and leads to trapping of excess N in the film (panel b). Both of these
effects are signatures of a rapidly increasing compressive stress. TEM and XTEM analyses
show that films grown with E
i
=20eV(∼ 10 eV per N following collisionally induced
dissociation) and J
i
/J
Me
= 1 are underdense with both intercolumnar and intracolumnar
porosity and an average column size d = 300 ± 150
˚
A. Increasing E
i
leads to densification and
a reduction in column size to ∼ 250
˚
A at 85 eV with a small increase in the volume fraction of
002 grains to ∼ 15%. However, at E
i
= 100 eV, the films are no longer single phase and instead

608 Chapter 12
consist of a TiN-rich columnar structure, with d ∼ 150
˚
A, and wurtzite-structure AlN-rich
precipitates of diameter ∼ 30
˚
A. Thus, increasing the ion energy in order to achieve
densification in low-temperature Ti
0.5
Al
0.5
N films results in residual lattice defects which
create compressive stress and, at E
i
> 85 eV, phase separation.
In sharp contrast, maintaining E
i
constant at 20 eV and increasing <E
i
> through increases in
J
i
/J
Me
still leads to densification, but with no significant ion-induced stress, and the layers
remain stoichiometric and singe phase. The column size distribution becomes more uniform
with no measurable change in column size. An additional major benefit of this approach to
coupling kinetic energy to the growing film during low-temperature deposition is shown in the
upper panel of Figure 12.42. That is, the use of low-energy, variable ion flux irradiation allows
film preferred orientation to be set at any value between 111 and essentially complete 002.
This is important in many applications due to the anisotropic properties of TM nitrides. The
mechanism for the change in texture, which involves a combination of ion-irradiation-induced
surface chemistry, anisotropic adatom diffusion dynamics, and momentum transfer, is
discussed in the following sub-section.
Ion-irradiation-induced surface smoothening
There are many polycrystalline thin film applications in which surface and interface
smoothness is crucial to device operation. Examples include metal multilayer X-ray mirrors
for semiconductor device lithography and giant magnetoresistance (GMR) devices such as
non-volatile magnetic access memory and read head sensors in hard disk drives. The GMR
effect derives from changes in electron-spin-dependent resistance across multilayer stacks,
consisting of high-conductivity films sandwiched between ferromagnetic layers, owing to
orientation switching of an external magnetic field. If the ferromagnetic layers have a
roughness comparable in amplitude and wavelength to the spacer layer thickness, typically
∼ 10–20
˚
A, it is difficult to reverse the magnetic field of the ferromagnetic layers because of
N
´
eel coupling. In addition, layer intermixing increases spin-independent scattering and loss of
local magnetic alignment.
GMR multilayers are generally composed of material pairs with large miscibility gaps, and
deposition is carried out at low temperatures, in order to minimize intermixing by thermal
diffusion during deposition. The best GMR layers are synthesized using low-energy ion
irradiation for reasons illustrated in Figure 12.43 showing molecular dynamics simulations,
using embedded atom potentials, of two three-layer Cu/Co/Cu stacks. The experiments were
designed to probe kinetic effects during the asymmetric growth of Cu/Co versus Co/Cu
interfaces [157]. Both trilayer stacks were grown with thermal atoms (evaporation), but
E
i
=6eVAr
+
ion irradiation with J
i
/J
Me
= 5 was added in panel (b). (Note that E
i
is of the same
order as the average energy of ballistic sputtered atoms, 5–10 eV, incident at the film growth
surface during magnetron sputter deposition at low pressures, ∼ 1 mtorr, with a
target-to-substrate separation of a few cm.) The bottom Cu layer consists of eight planes of
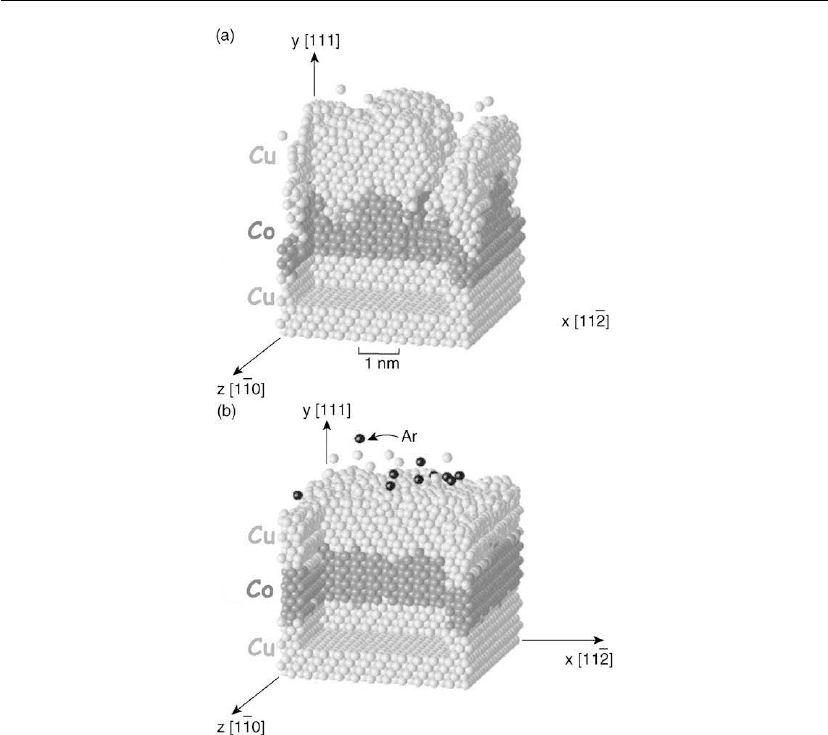
Thin Film Nucleation, Growth, and Microstructural Evolution 609
Figure 12.43: Molecular dynamics simulations of the growth of Cu/Co/Cu trilayers by sequential
deposition of Co and Cu overlayers at room temperature on a Cu(111) substrate consisting of
eight layers. The metal beam fluxes are thermal in both (a) and (b), but E
i
=6eV Ar
+
ion
irradiation, with J
i
/J
Me
= 5, is added in (b). (Adapted from [157].)
Cu(111); the Co and Cu overlayers were deposited at room temperature. In the thermal
deposition case, panel (a), the Co diffusivity is low leading to a rough Co surface and Co/Cu
interface. Thus, as the upper Cu layer buries the irregular Co surface, an even rougher upper
surface is formed.
Panel (b) corresponds to the growth of the same trilayer system, but this time with very low
energy ion irradiation simulating ion-assisted evaporation. The experiment is also a crude
simulation of ballistic sputter deposition since ∼ 83% of the arriving particles are energetic
and the mass mismatch among Ar, Cu, and Co is not large. Ion-irradiation-enhanced adatom
610 Chapter 12
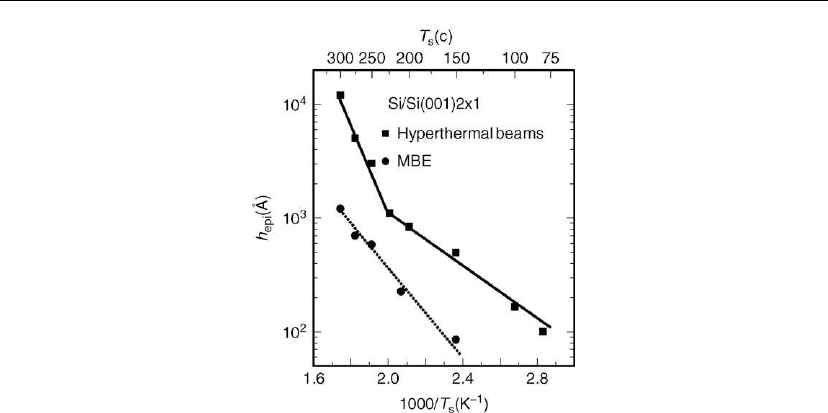
Figure 12.44: Epitaxial thickness h
epi
of Si/Si(001) layers grown with hyperthermal E
Si
=11eV
beams () and with MBE (䊉)atR ∼ 1
˚
A/s as a function of T
s
. (Hyperthermal beams from [160];
MBE from [161].)
mobilities greatly decreases the roughness of both interfaces, and the surface, without adding
to interfacial mixing. Clearly, the driving effect here is momentum transfer from the fast
incident particles to surface atoms. Thus, the ion mass, ion energy, ion-to-thermal flux ratio,
and ion angle of incidence can all be tuned to affect adatom mobilities and interfacial mixing
in slightly different ways [10, 158, 159].
Experimentally, Lee et al. [160] have shown (Figure 12.44) that the epitaxial thickness h
epi
of
low-temperature (T
s
= 80–300
◦
C, T
s
/T
m
= 0.21–0.34) homoepitaxial Si/Si(001) layers grown
from hyperthermal Si beams is increased by up to an order of magnitude above that recorded
for MBE Si(001) epitaxy [161] at approximately the same deposition rate, R ∼ 1
˚
A/s (Figure
12.44). The hyperthermal Si atoms were incident at the growing film with an orthogonal
energy component of E
Si
= 11 eV. In both cases, continued growth at film thicknesses h > h
epi
leads to increased kinetic surface roughening, growth front breakdown, and loss of epitaxy
with a non-reversible transition to amorphous layer deposition. h
epi
ranges from ∼ 100
˚
Aat
T
s
= 100
◦
C, to 500
˚
Aat150
◦
C, to > 1 mat300
◦
C for Si(001) layers grown from
hyperthermal species. For comparison, h
epi
for MBE Si(001) varies from 70
˚
AatT
s
= 150
◦
Cto
1200
˚
AatT
s
= 300
◦
C for MBE Si(001).
The slope change in the h
epi
versus T
s
curve for hyperthermal species in Figure 12.44 is
explained as follows. While the energy of the hyperthermal growth species is too low to give
rise to detectable residual bulk defect densities, they collisionally dissociate surface dimers
and small clusters, thus resulting in higher adatom densities on all exposed surface levels. At
