Lennarz W.J., Lane M.D. (eds.) Encyclopedia of Biological Chemistry. Four-Volume Set . V. 4
Подождите немного. Документ загружается.

that interacted with baits contingent upon the modifi-
cation of the bait by a coexpressed kinase. A second
point of interest was the feasibility of identifying
proteins that interacted not with a single protein, but
with a complex formed by a bait and one or more
simultaneously expressed “co-baits.” A number of
investigators have used a two-hybrid approach to
identify proteins that interact as constituents of ternary
or even quarternary complexes.
Another application of interest has been to use the
two-hybrid system to identify and characterize inter-
actions between proteins and non-protein ligands.
To date, two-hybrid derivative systems have been used
to score interactions between proteins and small
molecules/drugs, and proteins and RNAs. In each case,
the derivative system has been shown to function at the
level of library screening. While screening for RNAs has
become relatively broadly adapted as a research tool,
screening for drugs that regulate protein–protein
interactions remains at an earlier stage of validation.
Detailed description of the derivatization of the two-
hybrid system required for the screening of non-protein
ligands is beyond the scope of this review.
The two-hybrid system has been used by a number of
investigators to identify peptide “aptamers” capable of
binding and regulating the functions of specific bait
proteins. Peptides isolated based on two-hybrid selection
have been found capable of regulating diverse functions
of their cognate baits, and may provide the basis for
design of peptidomimetics or small molecule derivatives
of therapeutic value. This is currently an area of
active investigation.
Finally, a recent effort has been to refine the two-
hybrid system so as to make it a more useful tool for
evaluating the selectivity of protein interactions. In these
approaches, the two-hybrid system is “parallelized,”
using two discrete sets of baits and reporters within a
single strain of yeast. In one example, bait 1 (a fusion to
LexA) directs the expression of the lacZ and LEU2
reporters, while bait 2 (a fusion to cI) directs the
expression of gusA (colorimetric) and LYS2 (auxo-
trophic) reporters. In a situation where a prey initially
interacts with both baits 1 and 2, turning on all four
reporters, mutagenesis of the prey can be coupled with
selective screening for activation of the lacZ and LEU2,
versus the gusA and LYS2, reporters, providing a
convenient means of identifying altered specificity
mutants that can be used to deconvolute signal
transduction pathways.
Alternative Two-Hybrid Systems
Although the yeast two-hybrid system has found many
useful applications, it has not been appropriate in all
cases. Toward the goal of identifying small molecules or
mutations that disrupt, rather than permit, protein –
protein interactions, a “reverse” two-hybrid system has
been developed. In this system, activation of a reporter
gene is toxic, providing selection pressure for clones that
have lost protein–protein interactions. For proteins that
strongly activate transcription in yeast, and hence
cannot be used to create baits, one solution has been
to move the two-hybrid components to an RNA
polymerase III-based reporter system, eliminating tran-
scriptional activity. Other investigators have generated
two-hybrid-like systems in bacteria, where eukaryotic
transcriptional activation sequences are generally
ineffective. For proteins that are membrane associated,
or cannot efficiently be nuclear localized, several
methods for assessing protein interaction at membranes
or in the cytoplasm have been developed. These include
systems in which interaction of the bait and prey
reconstitutes an intracellular signaling pathway (e.g.,
the SOS system), or generate an assayable enzymatic
by-product, as in the ubiquitin-based split-protein
sensor system (USPS). In one recent and promising
development, the USPS system has been further modified
such that interactions of a bait and prey at the cell
membrane triggers the cleavage and release of a
transcription factor that migrates to the nucleus and
activates standard yeast two-hybrid colorimetric and
auxotrophic reporters. This approach combines advan-
tages of membrane-based interaction detection with the
ability to use well-validated screening modalities.
Role in Proteomics
Two hybrid protein –protein interaction systems are an
important element of strategies to analyze the complete
protein–protein interactions of proteomes. To date,
extensive protein interaction maps have been con-
structed for viruses and yeast, and large-scale screening
efforts are underway in Caenorhabditis elegans
(C. elegans), Drosophila, and humans. These efforts
provide a useful complement to simultaneous efforts to
analyze protein interactions by mass spectrometry:
whereas mass spectrometry is of value in identifying
proteins which bind with high affinity to an assembled
complex, albeit potentially weakly to any single inter-
active partner, the yeast two-hybrid system excels at
identifying proteins that interact with high affinity with
single defined partners. Interactions being detected by
these means are being integrated into datasets includ-
ing transcriptional expression profiles, genetic inter-
action data, and protein localization data for the same
proteins, providing a fundamental tool for the emerging
field of systems biology. It is likely that continuing
application of the two-hybrid protein– protein inter-
action will remain a source of scientific insights for many
years to come.
292
TWO-HYBRID PROTEIN–PROTEIN INTERACTIONS
SEE ALSO THE FOLLOWING ARTICLES
LexA Regulatory System † Yeast GAL1– GAL10 System
GLOSSARY
bait A term used to describe a DNA-binding domain–protein “X”
fusion, used as a probe in the yeast two-hybrid system (e.g., to
screen a library).
interaction map A schematic showing a network of protein–protein
interactions involving one or more proteins of interest.
interactome A recently introduced term, proposed to describe the
complete network of protein–protein interactions occurring within
an organism.
prey A term used to describe a transcriptional activation domain–
protein “Y” fusion, which interacts with a bait.
two-hybrid system A system in which the interaction of two “hybrid”
proteins (created so that (1) one is a fusion between a DNA-
binding domain and protein “X,” and the second is a fusion
between a transcriptional-activation domain and protein “Y”;
and (2) X and Y normally interact) in a host organism such as
yeast or bacteria causes the activation of one or more scorable
reporter genes.
FURTHER READING
Bernstein, D. S., Buter, N., Stumpf, C., and Wickens, M. (2002).
Analyzing mRNA–protein complexes using a yeast three-hybrid
system. Methods 26, 123– 141.
Fashena, S. J., Serebriiskii, I. G., and Golemis, E. A. (2000).
The continued evolution of hybrid screening approaches in
yeast: How to outwit different baits with different preys. Gene
250, 1–14.
Fields, S., and Song, O. (1989). A novel genetic system to detect
protein–protein interaction. Nature 340, 245–246.
Schwikowski, B., Ideker, T., and Uetz, P. (2002). Visualization and
integration of protein– protein interactions. In Protein Interactions
(E. A. Golemis, ed.) Cold Spring Harbor Laboratory Press, Cold
Spring Harbor, NY.
Serebriiskii, I. G., Khazak, V., and Golemis, E. A. (2001). Redefinition
of the yeast two-hybrid system in dialogue with changing priorities
in biological research. BioTechniques 30, 634–655.
Stagljar, I., and Fields, S. (2002). Analysis of membrane protein
interactions using yeast-based technologies. Trends Biochem. Sci.
27, 559–563.
Walhout, A. J., Reboul, J., Shtanko, O., Bertin, N., Vaglio, P., Ge, H.,
Lee, H., Doucette-Stamm, L., Gunsalus, K. C., Schetter, A. J.,
Morton, D. G., Kemphues, K. J., Reinke, V., Kim, S. K., Piano, F.,
and Vidal, M. (2002). Integrating interactome, phenome, and
transcriptome mapping data for the C. elegans Germline. Curr.
Biol. 12, 1952–1958.
Zhu, H., and Snyder, M. (2002). “Omic” approaches for unraveling
signaling networks. Curr. Opin. Cell Biol. 14, 173– 179.
BIOGRAPHY
Ilya Serebriiskii is a Staff Scientist in the Division of Basic Sciences at
the Fox Chase Cancer Center in Philadelphia, PA. His research
interests include bacterial and yeast genetics and proteomics. He has
created and evaluated numerous advanced protein interaction screen-
ing tools based on two-hybrid paradigms. He holds a Ph.D. in Biology
from VNIIGenetika, Moscow, Russia. He received his postdoctoral
training with Prof. Gerard Leblon at The Institute of Genetics
and Microbiology, University of Paris-Sud, France, and then with
Dr. Erica Golemis at Fox Chase Cancer Center.
Erica Golemis is a Member of the Division of Basic Sciences at Fox
Chase Cancer Center. Her interests include the study of protein
interactions, and the intersection of cell attachment and cell cycle
control signaling in mammalian cancer development. She performed
research with Dr. Nancy Hopkins leading to a Ph. D. in Biology from
the Massachusetts Institute of Technology in Cambridge, MA.
Subsequent postdoctoral work with Dr. Roger Brent at Massachusetts
General Hospital in Boston, MA, led to the development of an original
LexA-based two-hybrid system. She has worked extensively in the area
of yeast two-hybrid system reagent development.
TWO-HYBRID PROTEIN–PROTEIN INTERACTIONS 293

Tyrosine Sulfation
Denis Corbeil and Wieland B. Huttner
Max Planck Institute of Molecular Cell Biology and Genetics, Dresden, Germany
The O-sulfation of tyrosine residues of membrane and
secretory proteins that transit through the secretory pathway
of eukaryotic cells is a ubiquitous posttranslational modifi-
cation conserved in all multicellular organisms. Tyrosine
sulfation is catalyzed by tyrosylprotein sulfotransferase
(TPST) isoenzymes, which are integral membrane proteins of
the trans-Golgi network. Tyrosine sulfation has been shown to
be important for protein–protein interactions occurring in
diverse biological processes, ranging from the receptor binding
of regulatory peptides to the interaction of viral envelope
proteins with the cell surface.
Tyrosine-Sulfated Proteins
OCCURRENCE
Sulfation is one of the most abundant posttranslational
modifications of tyrosine, since up to 1% of the tyrosine
residues of total protein in an organism can be sulfated.
Tyrosine is the only amino acid residue in proteins known
to undergo sulfation. Following the first description of a
sulfated-tyrosine residue in a peptide derived from
fibrinogen by Bettelheim in 1954, it has been known
since 1982 that tyrosine-sulfated proteins occur in all
animals, from lower invertebrates up to humans.
Tyrosine-sulfated proteins also exist in the plant king-
dom, for example, in the green alga volvox, one of the
earliest truly multicellular organisms, or in higher plants
such as rice. While occurring throughout metazoan
evolution, tyrosine-sulfated proteins appear to be absent
in unicellular eukaryotes and prokaryotes, implicating
this post translational modification in some aspect of
multicellularity.
In a given animal, tyrosine-sulfated proteins have been
observed in all tissues examined. Each tissue appears to
contain a characteristic set of tyrosine-sulfated proteins,
suggesting that proteins with tissue-specific expression
are major targets for tyrosine sulfation. In cell culture,
tyrosine sulfation of proteins has been detected in all
primary cultures and cell lines investigated, including
various secretory cells, epithelial cells, fibroblasts,
neuronal cells, and cells of the immune system.
Tyrosine-sulfated proteins can be identified by var-
ious methods, including labeling using radioactive
sulfate followed by tyrosine sulfate analysis of a given
protein. In line with the intracellular localization of
tyrosylprotein sulfotransferase (TPST) in the trans-Golgi
network, all known tyrosine-sulfated proteins are either
secretory or plasma membrane proteins. Reviews with
comprehensive lists of tyrosine-sulfated proteins have
been published, and several of these proteins have been
shown to play important biological roles.
STRUCTURAL DETERMINANTS
OF
TYROSINE SULFATION
The recognition, by TPST, of the tyrosine residue to be
sulfated in a secretory protein or the extracellular domain
of a membrane protein requires the presence of certain
structural features. These have been deduced from the
comparison of identified tyrosine sulfation sites and the
in vitro tyrosine sulfation of synthetic peptides. Although
no strict consensus sequence for tyrosine sulfation exists,
all sequences exhibit the presence of acidic amino acid
residues in the vicinity, i.e., positions 2 5 (N-terminal) to
þ5 (C-terminal), of the sulfated tyrosine residue. A
particularly critical position appears to be amino-
terminal (2 1) to the tyrosine. Turn-inducing amino
acids (P, G) are also frequently present. The few examples
of tyrosine sulfation sites lacking proline and glycine are
located near the N- or C-terminus of the protein and/or
contain several of the three other amino acid residues (D,
S, N) with significant turn-conformational potential.
Finally, another characteristic feature common to most
identified tyrosine sulfation sites is the absence of cysteine
residues or potential N-glycosylation sites (NXS or NXT)
in the vicinity (positions 2 7toþ7). In either case, the
presence of a disulfide-bridge or N-linked oligosacchar-
ides is likely to prevent sulfation of nearby tyrosine
residues due to steric hindrance.
Tyrosylprotein Sulfotransferase
(EC 2.8.2.20)
THE TYROSINE SULFATION REACTION
The sulfate transfer reaction to tyrosine residues is
catalyzed by TPST, first described in 1983, and used as a
Encyclopedia of Biological Chemistry, Volume 4. q 2004, Elsevier Inc. All Rights Reserved. 294
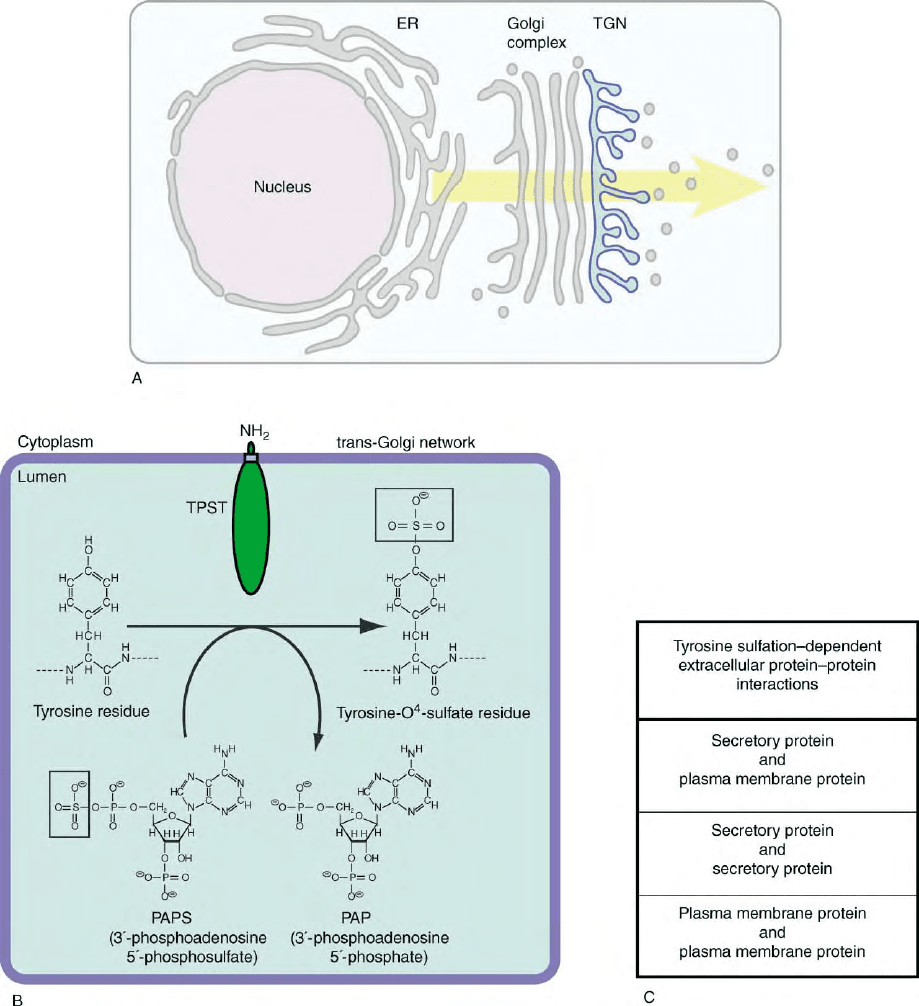
sulfate donor, the cosubstrate 3
0
-phosphoadenosine
5
0
-phosphosulfate (PAPS) (Figure 1B). The transfer is
thought to occur in an ordered reaction mechanism that
involves the following sequential steps: (1) cosubstrate
PAPS binding; (2) substrate binding; (3) sulfate transfer;
(4) tyrosine-sulfated product release and, (5) PAP
release. TPST activity can be detected by incubation of
appropriate membrane preparations with [
35
S]PAPS,
using either endogenous or exogenous protein substrate.
Biologically, tyrosine sulfation appears to be an irre-
versible event in vivo due to the lack of a sulfatase
capable of catalyzing the desulfation of tyrosine-sulfated
proteins under physiological conditions.
PROPERTIES OF TPST
TPST is an integral membrane protein residing in the
trans-Golgi network (Figure 1A). The TPST protein can
FIGURE 1 (A) Tyrosine sulfation occurs in the trans-Golgi network (TGN). (B) TPST, a type II transmembrane protein, catalyzes the transfer of
the sulfate group (boxes) from the co-substrate PAPS to tyrosine residues of secretory proteins and ectodomains of membrane proteins passing
through the lumen of the trans-Golgi network. (C) The three principal types of tyrosine sulfation-dependent protein–protein interactions.
TYROSINE SULFATION 295
be identified in membrane preparations by MSC
(modification after substrate cross-linking) labeling,
which is based on the cross-linking of a substrate
peptide to TPST followed by intramolecular [
35
S]sulfate
transfer from the cosubstrate PAPS. Various nonionic
detergentscanbeusedtosolubilizeTPSTfrom
membranes.
TPST purified from bovine adrenal medulla is a
50–54 kDa sialoglycoprotein with an apparent S-value
of 6. Its pH optimum is between 6.0 and 6.5, which is in
line with the slightly acidic pH of the trans-Golgi
network. The catalytic activity of the enzyme, towards
endogenously as well as exogenously added proteins, is
stimulated by divalent cations (Mg
2þ
,Mn
2þ
), and
fluoride ions are required for maximal activity. The K
m
for the cosubstrate PAPS is 1.4 mM. Like most sulfo-
transferases, TPST is inhibited by PAP. In vitro, the
activity of the enzyme is also inhibited by certain lipids
such as sphingosine, but the physiological relevance of
this observation is unknown. Synthetic inhibitors of
TPST (with IC
50
values of 30 –40 mM) have been
generated by a combinatorial target-guided ligand
assembly technique.
The K
m
for most peptides (8–13 amino acid residues)
with a single tyrosine sulfation site is in the range of
10–100 mM. It is likely that similar values hold true for
individual tyrosine sulfation sites in proteins. Interest-
ingly, K
m
values for synthetic substrates containing
multiple tyrosine sulfation sites are considerably lower.
Given that several proteins exist with multiple adjacent
sulfation sites, e.g., cionin, heparin cofactor, and prepro-
cholecystokinin, the increased affinity of TPST for such
substrates might be of physiological significance, e.g., by
promoting stoichiometric sulfation. A remarkable
example of the sequential sulfation of four tyrosine
residues within a short stretch of amino acids is the
N-terminal domain of the CC-chemokine receptor 5
(CCR5), which is a coreceptor for HIV.
MOLECULAR CLONING AND MEMBRANE
TOPOLOGY OF TPST
Distinct TPST isoenzymes, anticipated from the differ-
ential substrate specificities of various TPST prep-
arations, exist in the human and mouse genome. Each
contains two TPSTs, TPST-1 (see Swiss-Prot Database,
Accession No. 060507) and TPST-2 (see Swiss-Prot
Database, Accession No. 060704), which were first
characterized at the molecular level in 1998. The human
TPST-1 (7q11) and TPST-2 (22q12.1) genes encode for
370- and 377-amino acid proteins, respectively, that
share an overall 65% identity. The murine tpst genes are
located on chromosome 5, and the corresponding
proteins show , 95% identity to their human counter-
part. Both TPST isoenzymes are predicted to have a type
II membrane topology, with a very short NH
2
-terminal
cytoplasmic domain (8 residues) and the bulk of the
polypeptide, which is responsible for the catalytic
activity, being located in the Golgi lumen (see Figures
1A and 1B). Although overall, TPST isoenzymes display
a low degree of amino acid identity to other cytosolic
and Golgi-associated sulfotransferases, most of the
residues involved in PAPS binding, as deduced from
comparison with the crystal structure of estrogen
sulfotransferase, are conserved. The luminal domain of
either isoenzyme also contains two potential N-glyco-
sylation sites, consistent with presence of N-linked
glycans in the TPST protein.
In agreement with the occurrence of tyrosine sulfa-
tion in all metazoan species, cDNAs that predict
proteins obviously related to mammalian TPSTs are
found in various vertebrates and invertebrates, including
fish (GenBank Accession No. BI846282), frog (GenBank
Accession No. BG815875), chicken (GenBank Acces-
sion Nos. BU142429, BU217098), fly (GenBank Acces-
sion No. AY124548), and worm.
TPST-1 VERSUS TPST-2
TPST-1 and TPST-2 transcripts are found in all tissues
examined, e.g., brain, heart, skeletal muscle, gut, kidney,
liver, lung, and leukocytes. Although ubiquitously
expressed, the tissue distribution of TPST-1 and TPST-
2 are not identical, recombinant TPST-1 and TPST-2
show similar, but not identical, activities toward certain
small peptide substrates, consistent with some func-
tional redundancy as well as a certain degree of
differential substrate specificity.
TPST-1- AND TPST-2-DEFICIENT MICE
TPST-1- and TPST-2-deficient mice, generated by
targeted disruption of the tpst-1 and tpst-2 genes, also
point to functional redundancy between the two
isoenzymes, as either line of knock-out mice is viable
(K.L. Moore, personal communication). Although
TPST-1
2/2
animals appear normal, their body weight
is reduced about 5% and an increase in postimplanta-
tion fetal death is observed, suggesting that unidentified
proteins involved in regulation of body weight and
reproductive physiology require tyrosine sulfation for
optimal function. TPST-2 deficient mice show a transi-
ent delay in growth during postnatal development. In
addition, the males, but not females, appear to be
infertile. Together, these observations are consistent with
the idea that TPST-1 and TPST-2 have distinct, but
partially overlapping, physiological roles.
296
TYROSINE SULFATION
Physiological Function
and Medical Relevance
For most tyrosine-sulfated proteins, the physiological
function of this posttranslational modification is pres-
ently unknown. With regard to the cases in which the
biological role of tyrosine sulfation of a particular
protein has been elucidated, the common denominator
has emerged that tyrosine sulfation promotes extra-
cellular protein–protein interactions. In line with the
identification of numerous secretory and plasma mem-
brane proteins that are tyrosine-sulfated, paradigmatic
examples exist showing that tyrosine sulfation promotes
the interaction between (1) a secretory and a plasma
membrane protein, (2) two secretory proteins, or (3) two
plasma membrane proteins (Figure 1C).
The regulatory peptide cholecystokinin is a classical
example where tyrosine sulfation of a secretory protein
dramatically promotes its interaction with a plasma
membrane protein, i.e., its cell surface receptor. Thus,
sulfated cholecystokinin is 260 times more potent than
its unsulfated form. Of the several examples of tyrosine
sulfation promoting the interaction between two
secretory proteins, the case of the binding of the
tyrosine-sulfated blood coagulation factor VIII to von-
Willebrand-factor is particularly intriguing, as it also
documents the medical relevance of this posttransla-
tional modification. Humans with a mutation in the
critical tyrosine residue of factor VIII that is sulfated and
involved in its binding to von-Willebrand-factor are
afflicted with hemophilia A.
An example of tyrosine sulfation promoting the
interaction between two plasma membrane proteins is
the important role of this posttranslational modification
for the high-affinity binding of leukocyte-associated P-
selectin glycoprotein ligand (PSGL)-1 to P-selectin on
activated endothelial cells. This crucial interaction
initiates adhesion of leukocytes to the vascular wall
during inflammation. Tyrosine sulfation also occurs in
seven-transmembrane-segment chemokine receptors,
e.g., CCR5. Under physiological conditions, these
plasma membrane proteins play a central role in
chemokine signalling pathway through G proteins.
Remarkably, human and simian immunodeficiency
viruses use CCR5 as a co-receptor, together with CD4,
to mediate their attachment to the host cell membrane.
Specifically, sulfation of tyrosine residues in the CCR5
N-terminal domain has been shown to be critical for the
interaction of this protein with HIV envelope glyco-
protein gp120, leading to HIV infection. Thus, the
design of tyrosine-sulfated peptide competitors –
mimicking HIV gp120-binding sites – could turn out
to be the basis for new therapeutic compounds that will
block HIV cellular entry. These examples highlight the
medical relevance of protein tyrosine sulfation.
SEE ALSO THE FOLLOWING ARTICLES
Golgi Complex † Oligosaccharide Chains: Free,
N-Linked, O-Linked † Secretory Pathway
GLOSSARY
PAPS 3
0
-phosphoadenosine 5
0
-phosphosulfate, sulfate donor in the
sulfate transfer reaction. PAPS has been known to be the activated
form of sulfate and acts as cosubstrate for the sulfation of a wide
variety of substances, including proteins.
trans-Golgi network The last station of the Golgi complex. This site
is a major branching point of vesicular transport and the origin of
two principal pathways of protein secretion: the regulated and
constitutive pathways.
FURTHER READING
Bettelheim, F. R. (1954). Tyrosine-O-sulfate in a peptide from
fibrinogen. J. Am. Chem. Soc. 76, 2838–2839.
Huttner, W. B. (1982). Sulphation of tyrosine residues – a widespread
modification of proteins. Nature (London) 299, 273– 276.
Huttner, W. B. (1984). Determination and occurrence of tyrosine
O-sulfate in proteins. Meth. Enzymol. 107, 200–223.
Huttner, W. B., and Baeuerle, P. A. (1988). Protein sulfation on
tyrosine. Mod. Cell Biol. 6, 97– 140.
Huttner, W. B., Niehrs, C., and Vannier, C. (1991). Bind or bleed. Curr.
Biol. 1, 309 –310.
Kehoe, J. W., and Bertozzi, C. R. (2000). Tyrosine sulfation: A
modulator of extracellular protein–protein interactions. Chem.
Biol. 7, R57 –R61.
Kehoe, J. W., Maly, D. J., Verdugo, D. E., Armstrong, J. I., Cook, B. N.,
Ouyang, Y. B., Moore, K. L., Ellman, J. A., and Bertozzi, C. R.
(2002). Tyrosylprotein sulfotransferase inhibitors generated by
combinatorial target-guided ligand assembly. Bioorg. Med. Chem.
Lett. 12, 329–332.
Moore, K. L. (2003). The biology and enzymology of protein tyrosine
O-sulfation. J. Biol. Chem. 278, 24243–24246.
Niehrs, C., Beisswanger, R., and Huttner, W. B. (1994). Protein tyrosine
sulfation, 1993 – an update. Chem. Biol. Interact. 92, 257–271.
Ouyang, Y.-B., Crawley, J. T. B., Aston, C. E., and Moore, K. L.
(2002). Reduced body weight and increased postimplantation fetal
death in tyrosylprotein sulfotransferase-1-deficient mice. J. Biol.
Chem. 277, 23781–23787.
BIOGRAPHY
Denis Corbeil is a Group Leader at the University of Dresden. His
research interests are in the cell biology of stem cells, with a focus on
prominin/CD133. He holds a Ph.D. from the University of Montreal
and received postdoctoral training in the laboratory of W.B. Huttner,
where he participated in the molecular cloning of TPST.
Wieland B. Huttner is a Professor of Neurobiology and Director at
MPI-CBG in Dresden. His group made seminal contributions on
protein tyrosine sulfation, including the identification, characteriz-
ation, purification, and cloning of TPST. He holds an M.D. from the
University of Hamburg, received postdoctoral training with Nobel
Laureate Paul Greengard at Yale University, and has been pursuing
research on neurosecretory vesicle biogenesis and neurogenesis in the
mammalian central nervous system.
TYROSINE SULFATION 297

Ubiquitin System
Aaron Ciechanover and Michael H. Glickman
Technion – Israel Institute of Technology, Haifa, Israel
In the ubiquitin system, a target substrate is modified by
ubiquitin or a ubiquitin-like protein. In most cases, proteins are
modified by multiple moieties of ubiquitin, generating a
polyubiquitin chain. This modification leads to their degra-
dation by the 26S proteasome complex. Modification by a
single moiety of ubiquitin can target proteins for degradation in
the lysosome/vacuole. Modification by ubiquitin-like proteins
serves nonproteolytic functions. Conjugation of ubiquitin is
carried out by a modular cascade of enzymes, specific to each
substrate. Ubiquitination of cellular proteins is a highly com-
plex, temporally controlled, and tightly regulated process that
targets in a specific manner thousands of cellular proteins and
plays major roles in a variety of basic pathways, such as cell
division, differentiation, and quality control. Not surprisingly,
aberrations in the system underlie the pathogenesis of many
diseases, certain malignancies, and neurodegenerative dis-
orders. Mechanism-based drugs are currently being developed.
Mechanisms of Ubiquitination
and Degradation
UBIQUITINATION
Degradation of a protein via the ubiquitin–proteasome
pathway involves two discrete and successive steps:
(1) tagging of the substrate by covalent attachment of
multiple ubiquitin molecules, and (2) degradation of the
tagged protein by the 26S proteasome complex with
release of free and reusable ubiquitin. This last process is
mediated by ubiquitin-recycling isopeptidases (deubi-
quitinating enzymes). Conjugation of ubiquitin, a highly
evolutionarily conserved 76 residue polypeptide, to the
protein substrate proceeds via a three-step cascade
mechanism. Initially, the ubiquitin-activating enzyme,
E1, activates ubiquitin in an ATP-requiring reaction
to generate a high-energy thiol ester intermediate,
E1-S, ubiquitin. One of several E2 enzymes (ubiquitin-
carrier proteins or ubiquitin-conjugating enzymes
(UBCs)) transfers the activated ubiquitin from E1, via
an additional high-energy thiol ester intermediate,
E2-S , ubiquitin, to the substrate that is specifically
bound to a member of the ubiquitin-protein ligase
family, E3. There are a number of different classes of E3
enzymes. For the homologous to the E6-AP C-terminus
(HECT) domain E3s, the ubiquitin is transferred once
again from the E2 enzyme to an active site Cys residue
on the E3, to generate a third high-energy thiol ester
intermediate, ubiquitin-S , E3, prior to its transfer to
the ligase-bound substrate. RING finger-containing E3s
catalyze direct transfer of the activated ubiquitin moiety
to the E3-bound substrate.
E3s catalyze the last step in the conjugation process:
covalent attachment of ubiquitin to the substrate.
The ubiquitin molecule is generally transferred to an
1-NH
2
group of an internal lysine residue in the substrate
to generate a covalent isopeptide bond. In some cases
however, ubiquitin is conjugated to the N-terminal amino
group of the substrate. By successively adding activated
ubiquitin moieties to internal lysine residues on the
previously conjugated ubiquitin molecule, a polyubi-
quitin chain is synthesized. The chain is recognized by
the downstream 26S proteasome complex. Thus, E3s
play a key role in the ubiquitin-mediated proteolytic
cascade since they serve as the specific recognition factors
of the system. In certain cases the first ubiquitin moiety is
conjugated to the substrate by one E3, while chain
elongation is catalyzed by a different ligase often termed
E4. Modification by a single moiety of ubiquitin is
catalyzed via an identical mechanism and set of enzymes.
The specific enzymes that catalyze modification by
ubiquitin-like proteins are somewhat different, though
they utilize a similar mechanism.
DEGRADATION
Degradation of polyubiquitinated substrates is carried
out by a large protease complex, the 26S proteasome
that does not recognize nonmodified substrates. In one
established case, that of the polyamine synthesizing
enzyme ornithine decarboxylase (ODC), the protea-
some recognizes and degrades the substrate without
prior ubiquitination. The proteasome is a multicatalytic
protease that degrades polyubiquitinated proteins to
short peptides. It is composed of two subcomplexes: a
20S core particle (CP) that carries the catalytic activity,
and a regulatory 19S regulatory particle (RP). The 20S
CP is a barrel-shaped structure composed of four
u
Encyclopedia of Biological Chemistry, Volume 4. q 2004, Elsevier Inc. All Rights Reserved. 299
stacked rings, two identical outer
a
-rings and two
identical inner
b
-rings. The eukaryotic
a
- and
b
-rings
are composed each of seven distinct subunits, giving
the 20S complex the general structure of
a
1–7
b
1–7
b
1–
7
a
1–7
. The catalytic sites are localized to some of the
b
-subunits. Each extremity of the 20S barrel can be
capped by a 19S RP. One important function of the
19S RP is to recognize ubiquitinated proteins and other
potential substrates of the proteasome. A ubiquitin-
binding subunit of the 19S RP has indeed been
identified; however, its importance and mode of action
have not been discerned. A second function of the 19S
RP is to open an orifice in the
a
-ring that will allow
entry of the substrate into the proteolytic chamber.
Also, since a folded protein would not be able to fit
through the narrow proteasomal channel, it is assumed
that the 19S particle unfolds substrates and inserts
them into the 20S CP. Both the channel opening
function and the unfolding of the substrate require
metabolic energy, and indeed, the 19S RP contains six
different ATPase subunits. Following degradation of
the substrate, short peptides derived from the substrate
are released, as well as reusable ubiquitin. Proteasomal
degradation is not always complete. In some cases, the
proteasome, rather than completely destroying its
target, processes the ubiquitinated substrate precisely,
releasing a truncated product. In the case of the NF-
k
B
transcriptional regulator, an active subunit (p50 or p52)
is thus released from a longer inactive precursor
(p105 or p100). For a general scheme of the ubiquitin
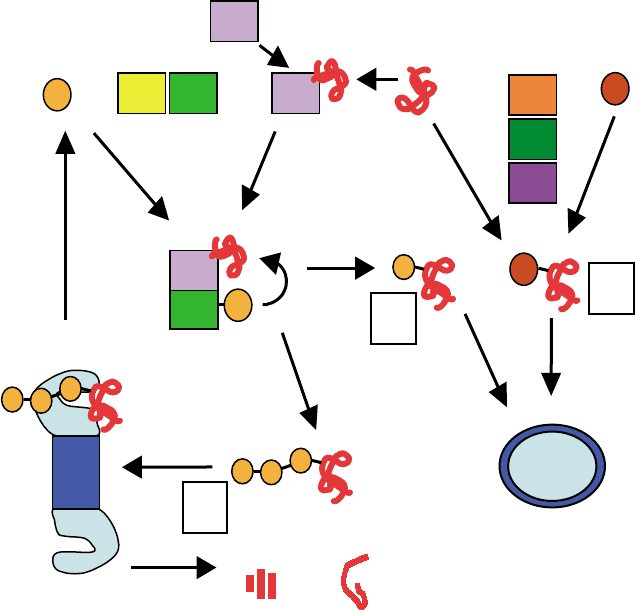
system and its multiple functions, see Figure 1.
SUBSTRATE RECOGNITION
A major unresolved question is how does the system
achieve its high specificity and selectivity. Why are
certain proteins extremely stable in the cell, while
others are extremely short-lived? Why are certain
proteins degraded only at a particular time point during
the cell cycle or only following specific extracellular
stimuli, yet they are stable under most other conditions?
It appears that specificity of the ubiquitin system is
determined by two distinct and unrelated groups of
proteins: E3s and ancillary proteins. First, within
Targeting, protection,
or activation
C
A
Substrate
recognition
Ub
+
Ubiquitin ligation
(multiple cycles)
Peptides
E1 E2 E3
E3
+
E2
Ub
E3
Proteolysis
26S
Ub
Ub
Ub
Ub
recycling
Ub
Ubiquitin ligation
(single cycle)
Targeting
(e.g., lysosome)
Ubl
Ubl
E1
E2
E3
Ub activation
Processed product
+
Conjugation
or
Ub
Ub
Ub
B
FIGURE 1 The ubiquitin system for protein modification. Ubiquitin (Ub) is activated by the three enzymes E1, E2, and E3, and the active
ubiquitin moiety is transferred to the target substrate (red). Substrates can be either modified by a polyubiquitin chain (A) and targeted to the 26S
proteasome complex for degradation (or processing), or modified by a single ubiquitin moiety, monoubiquitinated (B) and targeted to other
organelles such as the lysosome (wherein it is degraded). Ubiquitin-like proteins (Ubl) are activated by E1, E2, and E3, which are similar but not
identical to the enzymes that activate ubiquitin. Modification by Ubl (C) serves a variety of nonproteolytic functions such as routing of proteins to
subcellular organelles (e.g., nucleus), protecting them from ubiquitination, or activating them.
300 UBIQUITIN SYSTEM
the ubiquitin system, substrates must be specifically
recognized by an appropriate E3 as a prerequisite to
their ubiquitination. In most cases however, substrates
are not recognized in a constitutive manner, and in
some cases they are not recognized directly by the E3.
In some instances, the E3 must “be switched on” by
undergoing posttranslational modification in order to
yield an active form that recognizes the substrate. In
many other cases, it is the substrate that must undergo
a certain change that renders it susceptible for
recognition. The stability of additional proteins
depends on association with ancillary proteins such as
molecular chaperones that act as recognition elements
in trans and serve as a link to the appropriate ligase.
Others, such as certain transcription factors, have to
dissociate from the specific DNA sequence to which
they bind in order to be recognized by the system.
Stability of yet other proteins depends on oligomeriza-
tion. Thus, in addition to the E3s themselves, modifying
enzymes (such as kinases), ancillary proteins, or DNA
sequences to which substrates bind also play an
important role in the recognition process.
Functions and Substrates
of the Ubiquitin System
Ubiquitin-mediated proteolysis of a variety of cellular
proteins plays an important role in many basic cellular
processes. Among these are regulation of cell cycle and
division, differentiation and development, involvement
in the cellular response to stress and extracellular
effectors, morphogenesis of neuronal networks, modu-
lation of cell surface receptors, ion channels and the
secretory pathway, DNA repair, transcriptional regu-
lation, transcriptional silencing, long-term memory,
circadian rhythms, regulation of the immune and
inflammatory responses, and biogenesis of organelles.
The list of cellular proteins that are targeted by ubiquitin
is growing rapidly. Among them are cell-cycle regulators
such as cyclins, cyclin-dependent kinase inhibitors, and
proteins involved in sister chromatid separation, tumor
suppressors, transcriptional activators, and their inhibi-
tors. Cell surface receptors and endoplasmic reticulum
(ER) proteins are also targeted by the system. Finally,
mutated and denatured/misfolded proteins are recog-
nized specifically and removed efficiently. In this
capacity, the system is a key player in the cellular quality
control and defense mechanisms. The products of the
proteasome can play an important role in the immune
response. In the case of degradation of foreign proteins –
such as those of viral origin – the resulting short
peptides are presented by MHC class I molecules to the
cytotoxic T cell that lyse the presenting cell.
Regulation of the Ubiquitin System
The ubiquitin–proteasome pathway can be regulated at
the level of ubiquitination or at the level of proteasome
activity. Since conjugation and proteasomal degradation
is required for multitude of cellular functions, regulation
must be delicately and specifically tuned. In a few cases,
general rather than specific, components of the pathway
can be modulated by physiological signals. For example,
up-regulation of the pathway is observed during massive
degradation of skeletal muscle proteins that occurs
under normal fasting, but also under pathological
conditions such as cancer cachexia, severe sepsis,
metabolic acidosis, or following denervation. In most
cases however, regulation is specific and the target
substrates are recognized by specific ligases that bind to
defined motifs. The targeting motif can be a single amino
acid residue (e.g., the N-terminal residue) or a sequence
(the “destruction” box in cyclins) or a domain (such as a
hydrophobic patch) that is not normally exposed. In
other cases the motif is a posttranslational modification
such as phosphorylation that is generated in response to
cell needs or external signals. Phosphorylation can occur
either on the substrate or on the ligase.
Ubiquitin-Like Proteins
Both enzymes and substrates of the ubiquitin system
have been found to be modified by ubiquitin-like
proteins. Modification by ubiquitin-like proteins occurs
only once. In the case of enzymes, modification affects
their activity. For example, the modification by the
ubiquitin-like protein NEDD8 enhances the activity of a
certain class of E3 ligases. Conjugation of NEDD8 to
one of the components of the ligase complex (Cullin)
increases its affinity to other components of the
conjugation machinery. In the case of substrates,
modification can affect their availability to the ubiqui-
tination/degradation machinery and consequently cellu-
lar stability. For example, in the case of I
k
B
a
, the
inhibitor of the transcriptional regulator NF-
k
B, modi-
fication by SUMO-1 was shown to protect the substrate
from ubiquitination. In a completely different case,
SUMOylation of RanGAP1 targets the protein to its
final subcellular destination in the nuclear pore complex.
Ubiquitination and Pathogenesis of
Human Diseases
DISEASES
While inactivation of a major enzyme such as E1 is
obviously lethal, mutations or acquired changes in
UBIQUITIN SYSTEM 301
enzymes or in recognition motifs in substrates that do
not affect vital pathways or that affect the involved
process only partially, may result in a broad array of
diseases. The pathological states associated with the
ubiquitin system can be classified into two groups: those
that result from (1) loss of function – mutation in a
ubiquitin system enzyme or target substrate that result
in stabilization of certain proteins, and (2) gain of
function – abnormal or accelerated degradation of the
protein target.
Alterations in ubiquitination and deubiquitination
reactions have been directly implicated in the etiology of
many malignancies. In general, cancers can result from
“stabilization” of oncoproteins, or “destabilization” of
tumor suppressor gene products. Some of the natural
substrates for degradation by the proteasome are
oncoproteins that, if not properly removed from the
cell, can promote cancer. For instance, ubiquitin targets
N-myc, c-myc, c-fos, c-jun, Src, and the adenovirus E1A
proteins. Destabilization of tumor suppressor proteins
such as p53 and p27 has also been implicated in the
pathogenesis of malignancies.
In one fascinating case, that of uterine cervical
carcinoma, the level of the tumor suppressor protein
p53 is extremely low. Most of these malignancies are
caused by high-risk strains of the human papilloma-
virus (HPV). Detailed studies have shown that the
suppressor is targeted for ubiquitin-mediated degra-
dation by the virally encoded oncoprotein E6. Degra-
dation is mediated by the native HECT domain E3
enzyme E6-AP, where E6 serves as an ancillary protein
that allows recognition of p53 in trans. E6-AP will not
recognize p53 in the absence of E6. E6 associates with
both the ubiquitin-ligase and the target substrate and
brings them to the necessary proximity that is assumed
to allow catalysis of conjugation to occur. Removal of
the suppressor by the oncoprotein is probably
an important mechanism used by the virus to trans-
form cells.
Accumulation of ubiquitin conjugates and/or
inclusion bodies associated with ubiquitin, proteasome,
and certain disease-characteristic proteins have been
reported in a broad array of chronic neurodegenerative
diseases, such as the neurofibrillary tangles of Alzhei-
mer’s disease (AD), brainstem Lewy bodies (LBs) – the
neuropathological hallmark in Parkinson’s disease
(PD), and nuclear inclusions in CAG repeat expansion
(poly-glutamine expansion) disorders such as occurring
Huntington’s disease. However, in all these cases, a
direct pathogenetic linkage to aberrations in the
ubiquitin system has not been established. One factor
that complicates the establishment of such linkage is
the realization that many of these diseases, such as
Alzheimer’s and Parkinson’s, are not defined clinical
entities, but rather syndromes with different etiologies.
Accumulation of ubiquitin conjugates in Lewy
inclusion bodies in many of these cases may be
secondary, and reflects unsuccessful attempts by the
ubiquitin and proteasomal machineries to remove
damaged/abnormal proteins. While the initial hypoth-
esis was that inclusion bodies are generated because of
the inherent tendency of the abnormal proteins to
associate with one another and aggregate, it is now
thought that the process may be more complex and
involves active cellular machineries, including inhi-
bition of the ubiquitin system by the aggregated
proteins. This aggregation of brain proteins into defined
lesions is emerging as a common, but poorly under-
stood mechanistic theme in many sporadic and
hereditary neurodegenerative disorders.
The case of Parkinson’s disease highlights the com-
plexity of the involvement of the ubiquitin system in
the pathogenesis of neurodegeneration. Aberrations in
several proteins such as mutations in
a
-synuclein,
an important neuronal protein, or in the deubiquitinating
enzyme UCH-L1, have been described that link the
ubiquitin system to the pathogenesis of the disease. One
important player in the pathogenesis of Parkinson’s
disease is Parkin which is a RING-finger E3. Mutations
in the gene appear to be responsible for the pathogenesis
of autosomal recessive juvenile parkinsonism (AR-JP),
one of the most common familial forms of Parkinson’s
disease. Parkin ubiquitinates and promotes the degra-
dation of several substrates. It is possible that aberration
in the degradation of one of these substrates that leads
to its accumulation is neurotoxic and underlies the
pathogenesis of AR-JP.
The cystic fibrosis gene encodes the CF transmem-
brane regulator (CFTR) that is a chloride channel. Only
a small fraction of the protein matures to the cell surface,
whereas most of it is degraded from the ER by the
ubiquitin system prior to its maturation. One frequent
mutation in the channel is DF508. The mutation leads to
an autosomal recessive inherited multisystem disorder
characterized by chronic obstruction of airways and
severe maldigestion due to exocrine pancreatic dysfunc-
tion. Despite normal ion channel function, CFTR
DF508
does not reach the cell surface at all, and is retained in
the ER from which it is degraded. It is possible that the
rapid and efficient degradation results in complete lack
of cell-surface expression of the DF508 protein, and
therefore contributes to the pathogenesis of the disease.
DRUG DEVELOPMENT
Because of the central role the ubiquitin system plays in
such a broad array of basic cellular processes, develop-
ment of drugs that modulate the activity of the system
may be difficult. Inhibition of enzymes common to the
entire pathway, such as the proteasome, may affect many
processes nonspecifically, although a narrow window
between beneficial effects and toxicity can be identified
302
UBIQUITIN SYSTEM
