Lennarz W.J., Lane M.D. (eds.) Encyclopedia of Biological Chemistry. Four-Volume Set . V. 4
Подождите немного. Документ загружается.


Tubulin and its Isoforms
Eva Nogales
Howard Hughes Medical Institute, University of California, Berkeley, California, USA
Tubulin is an
ab
dimeric protein that self-assembles into
microtubules and is present in all eukaryotes. Tubulin is highly
conserved across species, reflecting the sequence constraints
imposed by microtubule structure and function. Both
a
- and
b
-subunits exist in numerous isotypic forms and undergo a
variety of posttranslational modifications. Tubulin assembly
and disassembly, which are linked to GTP hydrolysis, make the
microtubule network dynamic both in time and space to
accommodate the needs of the cell during the cell cycle. Purified
tubulin retains its self-assembling capabilities, allowing the
biochemical and biophysical characterization of the micro-
tubule polymerization and depolymerization processes.
A variety of proteins interact with tubulin in the cell, affecting
the stability of microtubules and their function. Numerous
ligands bind to tubulin and influence its assembly properties,
among them several drugs that have proven to have anticancer
properties.
g
-tubulin is a rarer tubulin isoform involved in the
nucleation of microtubules at microtubule organizing centers.
Recently, additional tubulin isoforms of yet ill-defined function
have been identified.
ab
-Tubulin
PHYSICAL PROPERTIES
General
Tubulin
a
- and
b
-subunits have molecular weights of
, 50 kDa and are 36–42% identical and 63% homolo-
gous. Both tubulin subunits bind guanine nucleotides.
The binding to
a
-tubulin at the N-site is nonexchange-
able, while the binding to
b
-tubulin at the E-site is
exchangeable. Magnesium increases the affinity of the
b
-subunit for GTP with respect to GDP. Nucleotide in
oligomeric tubulin or in microtubules does not exchange
with the solution, except for terminal subunits at
microtubule ends.
Neuronal cells are particularly rich sources of tubulin
because microtubules are required for axonal transport.
Neural tissue contains sufficient tubulin to allow tubulin
purification by repeated cycles of 378-induced assembly
and 08-induced disassembly, with intervening centrifu-
gation to alternately pellet microtubules or impurities.
The yield of tubulin from 1 kg of brain and yeast is
, 150 and , 5 mg, respectively. The assembly of purified
tubulin can be assayed by light scattering, X-ray
scattering, centrifugation, and electron microscopy.
Isotypes and Posttranslational Modifications
Tubulin exists in different isotypic forms, the biological
significance of which is still a matter of debate. The
number of tubulin isotypes increases with the organism
complexity. While yeast has only two
a
- and one
b
-isotypes, higher eukaryotes have up to seven
b
- and
six
a
-tubulin isotypes. Certain isotypes have been found
to be tissue specific, and differential expression of
b
-tubulin isotypes has been observed during the cell
cycle. Some of these isotypes have been shown in vitro to
have different relative stabilities, and such differences
seem important for the response of the cell to anti-
tubulin, anticancer drugs. The majority of differences
between isotypes localize within the last 15 residues of
the sequences, a region that has been identified as
important in the interaction of microtubules with micro-
tubule associated proteins (MAPs), pointing to a possible
relevance for the functionality of microtubules in the cell.
Both tubulin subunits can be extensively altered by
posttranslational modification, including detyrosina-
tion/tyrosination, acetylation/deacetylation, phos-
phorylation, polyglutamylation, and polyglycylation.
All of these modifications, except for the acetylation of
a
-tubulin at Lys 40, occur at the divergent, highly charged
C-terminal end of
a
-and
b
-tubulin. As for the different
tubulin isotypes, the functionality of the posttransla-
tional modifications is still a matter of debate. Certain
modifications were initially identified as causing micro-
tubule stability, and had been later described as the
effect, not the cause of microtubule stability.
Structure
The structure of tubulin was solved by electron crystal-
lography of zinc-induced two-dimensional tubulin
sheets stabilized with the anticancer drug taxol.
The
a
- and
b
-tubulin have basically the same secondary
structure, each being made of a core of two
b
-sheets
Encyclopedia of Biological Chemistry, Volume 4. q 2004, Elsevier Inc. All Rights Reserved. 272
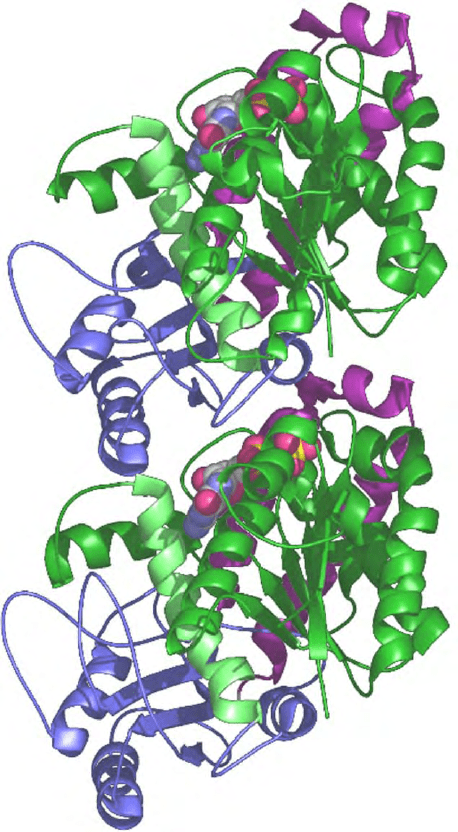
surrounded by helices as shown in Figure 1.
The N-terminal domain forms a Rossmann fold with
the nucleotide-binding site at the C-terminal end of six
parallel strands that are surrounded by five helices.
An intermediate domain containing the binding site of
taxol and colchicine is formed by a mixed
b
-sheet of four
strands and five surrounding helices. The C-terminal
domain contains two long helices that overlap the
previous two domains and constitute the outside crest of
the protofilaments in the microtubule to which motor
molecules bind. The nucleotide sits at the interface
between subunits along the protofilament. The N-site in
a
-tubulin is buried within the dimer, while the E-site in
b
-tubulin is partially exposed in the dimer but occluded
in microtubules. FtsZ, a ubiquitous protein in eubacteria
and archebacteria essential for cytokinesis, is the only
known structural homologue of tubulin.
Synthesis and Folding
Tubulin synthesis in cells is regulated by a process in
which an increased subunit concentration leads to
specific degradation of
b
-tubulin mRNA. In animal
cells there is a mechanism to assure equivalent synthesis
of
a
- and
b
-subunits. For its correct folding tubulin
requires the citosolic chaperonin CCT (also referred to
as TriC, TCP1, or Ct-cpn60), a hetero-oligomer formed
by eight different subunits assembled into a hexadeca-
mer of two double rings. Folding by CCT requires cycles
of binding and full release, each cycle consuming one
ATP by the chaperonin. In addition tubulin requires
additional chaperonin cofactors that bind sequentially
to
a
-and
b
-monomers and are necessary for the
formation of the tubulin heterodimer. Folding cofactors
are important also in regulating
a
/
b
tubulin ratios.
TUBULIN POLYMERIZATION
Microtubule Assembly and Structure
The tubulin sequence and structure contains the
information required for its self-assembly into polar,
dynamic microtubules, which in turn interact with a
variety of cellular factors. Tubulin dimers bind head
to tail making linear protofilaments, which associate
in a parallel fashion giving rise to a polar microtubule.
In a cell the so-called minus-end of microtubule, capped
by
a
-subunits, is attached to the centrosome where
g
-tubulin and related proteins nucleate microtubules.
The more dynamic plus-end, capped by
b
-subunits,
binds the kinetochore in mitosis.
The orientation of the tubulin subunits in the
microtubule is such that the C-terminal helices form
the crest of the protofilaments on the outside surface,
making them an essential part of the binding site
for motor proteins (kinesins and dyneins) and MAPs.
The taxol-binding site is on the inside surface of the
microtubule, and close to lateral interactions. The lateral
contact between protofilaments is dominated by the
interaction of the so-called M-loop in the second domain
with loop H1–S2 and helix H3 in the N-terminal,
nucleotide-binding domain.
The plus end of the microtubule is crowned by
b
-tubulin subunits exposing their nucleotide end to the
solution, while the minus end is crowned by
a
-subunits
exposing their catalytic end. When a dimer is added to a
plus end, its catalytic end contacts the E-site nucleotide
of the previous subunit forming the interface that should
bring about hydrolysis.
FIGURE 1 Ribbon diagram for the structure of
ab
-tubulin
corresponding to a view from the inside of the microtubule with the
plus end at the top.
b
-Tubulin (top) is bound to GDP while
a
-tubulin
(bottom) is bound to GTP. The color scheme highlights the three
domains in the structure of each monomer: green for N-terminal,
nucleotide-binding domain (helix H7 or core helix is shown in lime);
second or intermediate domain in blue, C-terminal domain in purple.
TUBULIN AND ITS ISOFORMS 273
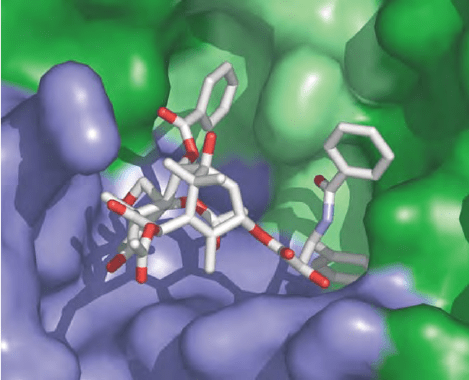
Dynamic Instability and GTP Cap Model
Microtubules are highly dynamic and can switch
stochastically between growing and shrinking phases,
both in vivo and in vitro. This nonequilibrium behavior,
known as dynamic instability, is based on the binding and
hydrolysis of GTP by tubulin subunits. Only dimers with
GTP in their E-site can polymerize, but following
polymerization this nucleotide is hydrolyzed by inter-
actions with the previous tubulin dimer and then becomes
nonexchangeable. In the GTP cap model the unstable
body of the microtubule made of GDP–tubulin is stabil-
ized by a layer of tubulin subunits at the ends that still
retain their GTP. When this cap is stochastically lost, the
microtubule rapidly depolymerizes. Depolymerization
may occur by weakening of lateral contacts at the ends,
and the consequent release of the constrained GDP sub-
units into a curved, lower energy, conformational state.
Microtubule assembly and stability are further
modified in the cell by interaction with cellular factors
that stabilize or destabilize microtubules at different
points in the cell or at different stages in the cell cycle.
Measurement of microtubule dynamics in vitro and
in vivo by DIC or fluorescence microscopy yields the
rate constants for addition of tubulin–GTP and loss
of tubulin-GDP subunits at the two microtubule ends,
as well as the rates of catastrophe (switch from growth
to shrinkage) and rescue (switch from shrinkage
to growth). A variety of drugs bind to tubulin and affect
its polymerization. Microtubule-depolymerizing drugs,
such as colchicine, nocodazole, vinca alkaloids, or
podophyllotoxin, have a much higher affinity for the
dimer than for tubulin in microtubules, so that
disassembly is caused by their mass action effect. At
substoichiometric concentrations tubulin-drug subunits
bind to the microtubule ends and form caps that
dramatically modify microtubule dynamics. Micro-
tubule-stabilizing drugs such as taxoids, epothilones,
or discodermolide act by binding preferentially to the
polymerized form of tubulin.
An alternative microtubule behavior is treadmilling, a
net flow of subunits from the plus to the minus end
without a significant change in microtubule length.
Anti-Mitotic Tubulin Ligands
Recent years have seen the discovery of numerous
tubulin ligands with anti-mitotic properties and anti-
cancer potential. Concerning their binding site these
agents can be classified into three main groups: those that
bind tubulin at the colchicine binding site; those who
bind it at the vinblastine site; and those who bind it at
the taxol site. Functionally these antimitotic ligands
can be separated into two classes: those that inhibit
microtubule assembly (e.g., the colchicine and vinblas-
tine families), and those that promote microtubule
assembly and stabilization (e.g., the taxol family). In
spite of these differences, the main action of all these
agents seems to be to cause mitotic arrest by inhibiting
normal dynamic instability at very low concentrations.
The colchicine-binding site is located at the
monomer–monomer interface within the dimer, in
agreement with a model of colchicine action in which
binding a distortion of the dimer structure that
inhibits its polymerization. Vinblastine-like compounds
are thought to bind at longitudinal polymerization
contacts, resulting in a distorted protofilament
structure. Finally, there is direct information on the
binding site of taxol from the crystal structure of
tubulin. Taxol binds to
b
-tubulin, near the M-loop
and the site of lateral interactions, in a hydrophobic
pocket that in
a
-tubulin is occupied by eight extra
residues (see Figure 2). New microtubule-stabilizing
agents with promise for cancer treatment, such as
epothilones, discodermolide, eleutherobin, and the
sarcodictins, while very different in structure, all seem
to compete with taxol for the binding to a common site.
It has been proposed that taxol stabilizes lateral
contacts, or alternatively, that it acts as a bridge
that holds the N-terminal and second domains in a
relative orientation that favors longitudinal contacts
between subunits.
g
-Tubulin and Microtubule
Nucleation
Crucial to their dynamic behavior and function is the
nucleation of microtubules in the cell at microtubule
FIGURE 2 Anticancer drug taxol at its tubulin-binding site. Tubulin
is shown on a surface representation with the same color scheme as in
Figure 1. Both the N-terminal and second domain are part of the taxol-
binding pocket.
274 TUBULIN AND ITS ISOFORMS
organizing centers (MTOCs), to which they are attached
by their minus ends. An essential role in microtubule
nucleation is played by
g
-tubulin, a protein with high
homology to
a
/
b
-tubulins that localizes at MTOCs.
g
-Tubulin forms ring structures that serve as templates
for microtubule growth. The direct involvement of
g
-tubulin in microtubule nucleation has been demon-
strated in vitro using purified
g
-tubulin-containing ring
complex (
g
TuRC) containing at least seven different
proteins. There are two main models in the literature.
A model based on the shape and size of the
g
-TuRC is
that the ring forms the first helical turn of the growing
microtubule, serving as a template for longitudinal
interaction with the tubulin
ab
dimers. An alternative
model, based on the similarity of rings structures formed
by
ab
-tubulin,
g
-tubulin, and FtsZ, is that
g
-tubulin
forms a protofilament-like structure by longitudinal self-
association that then serves as a template for lateral
interaction with
ab
-tubulin protofilaments.
RARER TUBULIN ISOFORMS:
d
-,
1
-, z-,
AND
h
-TUBULINS
Four new tubulin isoforms have recently been discovered.
The
d
-tubulin was identified in Chlamydomonas mutants
having abnormal basal bodies (these are microtubule
structures at the base of cilia and flagella structurally
similar to the centrioles in the centrosome at MTOCs).
Human
d
-tubulin was subsequently found in the human
genome database, and shown to localize to the centro-
some, where it partially colocalizes with
g
-tubulin.
The 1-tubulin was identified from the human genome
database on the basis of sequence similarity to other
tubulins. Like
d
-tubulin, 1-tubulin localizes to the
centrosome, but in a cell-cycle-dependent manner: in
cells with duplicated centrosomes 1-tubulin localizes
only with the old centrosome.
Even rarer,
z
-tubulin has so far only been found on
kinetoplastid protozoa where it localizes to the basal
body, while
h
-tubulin has been found in paramecium
where it may interact with
g
TuRC.
TUBULINS AND THE CELL
The involvement of the microtubule cytoskeleton in a
large number of essential and diverse functions requires
both reliability and flexibility from the system at the
expense of biochemical and structural complexity.
Dynamic instability is an inherent property of micro-
tubules, built into the
ab
-tubulin structure. The spatial
and temporal organization of the microtubule network
in the cell is obtained through the regulation of dynamic
instability by an increasing number of factors that
fine-tune the behavior of the microtubule system
to accommodate the requirements of the cell.
Regulation may happen at many different stages,
via transcription of different tubulin isotypes, the
control of tubulin monomer folding, the formation of
functional dimers, the posttranslational modification
of tubulin subunits, the nucleation of microtubules, or
the interaction of microtubules with numerous stabil-
izers and destabilizers. Tubulin-binding drugs can
dramatically disrupt the finely tuned behavior of
microtubules. Finally, while
g
-tubulin is known to
be essential for microtubule nucleation, additional
tubulin isoforms, only recently discovered, have yet ill-
defined functions.
SEE ALSO THE FOLLOWING ARTICLES
Centrosomes and Microtubule Nucleation † Chapero-
nins † Kinesins as Microtubule Disassembly Enzymes †
Microtubule-Associated Proteins
GLOSSARY
ab
tubulin dimer Essential, highly conserved protein dimer present in
all eukaryotes that self-assembles forming microtubules. It is the
target of antimitotic drugs with anticancer potential.
dynamic instability Nonequilibrium behavior of microtubules by
which they can stochastically switch between phases of growth and
shrinkage. It originates from the hydrolysis of GTP in
b
-tubulin and
can be regulated by the interaction of tubulin/microtubules with
cellular factors and antimitotic agents.
microtubules Cytoskeletal polymers made of
ab
-tubulin essential for
cell transport and cell division. They are polar, dynamic, and
regulated through the cell cycle by their interaction with stabilizers
and depolymerizers.
g
-tubulin Tubulin isoform most abundant at microtubule organizing
centers where it is involved in microtubule nucleation. It forms
higher-order complexes with associated proteins.
FURTHER READING
Desai, A., and Mitchison, T. J. (1997). Microtubule polymerization
dynamics. Ann. Rev. Dev. Biol. 13, 83– 117.
Downing, K. H. (2000). Structural basis for the interaction of tubulin
with proteins and drugs that affect microtubule dynamics. Ann.
Rev. Cell Dev. Biol. 16, 89–111.
Lewis, S. A., Tian, G. and Cowan, N. J. (1997). The
a
- and
b
-tubulin
folding pathways. Trend. Cell Biol. 7, 479– 484.
Lowe, J., Li, H., Downing, K. H., and Nogales, E. (2001). Refined
structure of alpha beta-tubulin at 3.5 A resolution. J. Mol. Biol.
313, 1045– 1057.
Mitchison, T., and Krischner, M. (1984). Dynamic instability of
microtubule growth. Nature 312, 237 –242.
Nogales, E. (2000). Structural insights into microtubule function. Ann.
Rev. Biochem. 69, 277–302.
Nogales, E., Wolf, S. G., and Downing, K. H. (1998). Structure of the
ab
tubulin dimer by electron crystallography. Nature 391,
199–203.
Nogales, E., Whittaker, M., Milligan, R. A., and Downing, K. H.
(1999). High resolution structure of the microtuble. Cell 96,
79–88.
TUBULIN AND ITS ISOFORMS 275
BIOGRAPHY
Eva Nogales is an Assistant Professor in the Department of
Molecular and Cell Biology at UC Berkeley, an Assistant Investigator
at the Howard Hughes Medical Institute, and a Staff Scientist at
Lawrence Berkeley National Laboratory. She was trained as a
physicist in Spain, her country of origin, and obtained a Ph.D. in
biophysics from Keele University for her work at the Synchrotron
Radiation Source at Daresbury, UK. During her postdoctoral
studies with Dr. Kenneth H. Downing at Lawrence Berkeley
National Laboratory she obtained the structure of
ab
-tubulin using
electron crystallography. Her present research centers around the
structural bases of microtubule dynamics and the structural
characterization of protein complexes involved in eukaryotic
transcription.
276 TUBULIN AND ITS ISOFORMS

Tumor Necrosis Factor Receptors
Karen G. Potter and Carl F. Ware
La Jolla Institute for Allergy and Immunology, San Diego, California, USA
Tumor necrosis factor receptors (TNFRs) are a family of
structurally similar cytokine receptors that act as transducers
of cell death and induce the expression of genes involved in
cellular differentiation and survival. Binding of specific ligands
to their cognate TNFR initiates the recruitment of adaptor
proteins, either death domain (DD)-containing or TNFR-
associated factor (TRAF) family of adaptor proteins, to the
cytosolic signaling domain of the receptor to initiate diverse
effector functions. The most well-known function of the TNF
superfamily is in immune regulation and development with
specific roles in host defense, inflammation, cellular homeo-
stasis, and lymphoid organogenesis. A critical role for TNFR in
immunobiology is evidenced by the linkage of naturally
occurring mutations in TNF family genes to human disease,
as well as by the targeting of TNF family members by viruses as
a mechanism of immune evasion. However, some TNF family
members also act outside the immune system by regulating
the development of hair follicles, sensory neurons, or bone-
resorbing osteoclasts.
Features of Tumor Necrosis
Factor Receptors
STRUCTURE
Tumor necrosis factor receptors (TNFRs) are identified
by a highly conserved, cysteine-rich domain (CRD) in
the extracellular portion of the protein that binds ligand
(Figure 1). The CRD generally contains six cysteines that
form three disulfide bonds typically recognized by the
signature sequence motif CxxCxxC. Currently, there are
29 members of the cellular TNFR family in mammals,
and several variants of TNFRs are found in herpes-
viruses and poxviruses. The cellular receptors are
primarily type I transmembrane proteins (extracellular
N terminus). Some receptors in this family lack
transmembrane and cytoplasmic domains and are
secreted, functioning as decoy receptors for the ligand.
The TNFR family can be divided into two general
groups – those that contain a death domain (DD) and
those with a peptide motif that binds TNFR-associated
factors (TRAF) adaptors – based on the structure of
their cytoplasmic tails and the signaling adaptors they
recruit to propagate signals to the cells.
Given the predominant role of the TNFR family in
regulating immunity, this suggests that the evolution of
the receptors in this family arose coincident with the
evolution of adaptive immunity, also found exclusively
in vertebrates. The size of the TNFR superfamily
appears to have grown in a large part by gene
duplication as many of the TNFR genes are linked to
discrete loci reflecting their evolutionary derivation.
Perhaps most obvious are those TNFRs found on
chromosome (Ch) 12p13 (TNFR1, LT
b
R, and CD27),
which likely underwent duplication and translocation
events giving rise to the larger locus of TNFR on Ch
1p36 (TNFR2, HVEM, Ox40, CD30, AITR, 4-1BB, and
DR3) (Figure 2A). Strikingly, NGFR is the only receptor
that binds ligands structurally unrelated to TNF,
representing a clear functional demarcation from the
typical TNFR (Figure 2B). Another subtle branch in the
TNFR family tree are those receptors that engage BAFF,
the B cell survival factor, and related ligands APRIL and
TWEAK (Figure 2C). These receptors have a single CRD
and in the case of BAFFR only two disulfide bonds.
Functional divergence is evident in the role some
TNFRs play in bone (Osteoprotegerin-RANK-RANKL/
TRANCE) and ectodermal (Ectodermal dysplasin EDA-
EDAR), and angiogenesis (TWEAK-Fn14).
EXPRESSION
Expression patterns of the receptors are complex.
Several members of the TNFR superfamily are expressed
on cells of the immune system; for example, BAFFR
expression is exclusive to B lymphocytes. Other recep-
tors are found in hair follicles (e.g., EDAR and TROY)
or the nervous system (e.g., NGFR), yet others have
very broad tissue expression patterns, such as TNFR1.
The expression of some TNFR is inducible and regulated
and others are constitutive. For example, TNFR2 is
absent on naı
¨
ve T cells but is rapidly upregulated upon
activation of T cells. In contrast, HVEM is highly
expressedonnaı
¨
ve T cells, but shows diminished
expression upon T-cell activation. Regulation of recep-
tor expression can also be achieved through the
Encyclopedia of Biological Chemistry, Volume 4. q 2004, Elsevier Inc. All Rights Reserved. 277
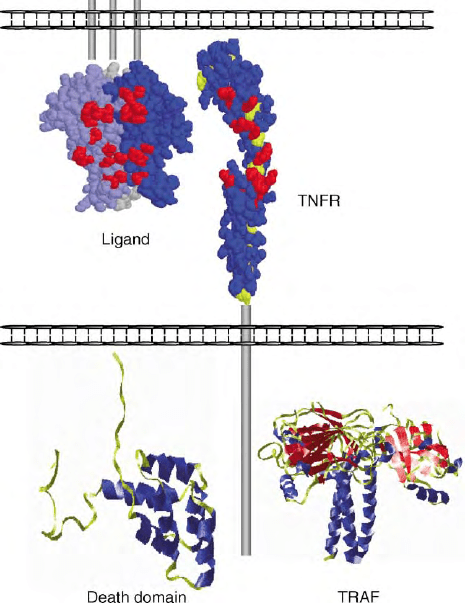
generation of soluble receptors; soluble TNFRs can be
generated by proteolytic processing (CD27, CD30,
CD40, TNFR1, and TNFR2), alternative splicing of
membrane forms (Fas, 4-1BB), or encoded in the
genome without a transmembrane region (OPG,
DcR3, TRAIL-R3).
LIGAND BINDING
TNF ligands are characterized as type II (intracellular N
terminus) transmembrane proteins containing a “TNF
homology domain” (THD) in their C terminus
(Figure 1). The THDs of each ligand associate to form
trimeric proteins; in most cases, the ligands form
homotrimers, but LT
a
and LT
b
form heterotrimers,
and so do BAFF and APRIL, as has been shown recently.
The TNF ligands are active in their membrane forms
where cell-to-cell contact is required to initiate signaling;
however, some ligands can be proteolytically cleaved
from the surface creating soluble cytokines that affect
cells distally.
The TNF ligand family currently includes 20 proteins
that are able to pair off with one or more receptors.
In fact, some ligands display overlapping receptor
recognition; for example, LIGHT and LT
a
1
b
2 bind
LT
b
R, and LT
a
and LIGHT bind HVEM. The ligand-
receptor pairing seems promiscuous, although compara-
tive studies using mice deficient in a specific TNF ligand
or receptor suggest that each cytokine-receptor system
has a unique role in immune physiology.
TNFR Signaling
Each TNF-related ligand has three receptor binding sites
that can cluster together two or three cell surface
receptors, juxtapositioning the cytoplasmic tails of the
receptors to initiate signal transduction. Recruitment of
specialized signaling molecules (adaptors) to the cyto-
plasmic domain occurs following receptor clustering.
Propagation of TNFR signals occurs through two
distinct classes of cytoplasmic adaptor proteins:
TRAFs or DD molecules.
TRAF SIGNALING
TRAF adaptor proteins are a small family of RING
finger proteins that play a critical role in propagating
signal transduction leading to the activation of latent
transcription factors (Figure 1). There are six numeri-
cally named TRAF proteins (TRAF1–6) that function
predominantly in TNFR-induced signaling, although
TRAF6 is also a key player in signal transduc-
tion initiated by the interleukin-1 receptor and the
Toll-like receptor (TLR) superfamily. Several TRAFs
can bind directly to the cytoplasmic tails of most of
the TNFR. The binding site found in CD40 or the
LT
b
R is a short peptide sequence, PXQXT/S or
IPEEGD, respectively. The binding site in TRAF for
receptor binding is flexible, thus accommodating a
variety of motifs. TNFRs with a DD bind TRAFs
indirectly via other adaptor proteins, such as TRADD
(TNFR-associated DD). Each TRAF interacts with
several different receptors such that TNFRs display
distinct interaction patterns with multiple TRAFs.
Since each TRAF is believed to have distinct biological
effects, the variation in TRAF binding by the TNFR
may be able to direct the signaling pathway to distinct
biological outcomes.
Functionally, binding of TRAFs to TNFR culminates
in the activation of transcription factors that act to
regulate gene expression. For example, nuclear factor
k
B (NF
k
B) is a small family of latent transcription
factors that induces the expression of a large variety of
genes involved in inflammatory and immune responses
(Figure 3). Two distinct forms of NF
k
B are recognized:
NF
k
B1 (RelA, p65) and NF
k
B2 (p100/p52), and each
pair with itself or other proteins (e.g., p50, RelB, and
cRel) that form active transcription factors that bind
FIGURE 1 Structural features of TNFR family. Space filling model of
a trimeric TNFR1 ligand, LT
a
, as it would exist as a membrane
anchored ligand, and a single TNFR1 rotated 1808 to reveal contact
residues. Also shown are crystal structures of the cytoplasmic DD
found in some TNFR and the TRAF that interacts with TNFR-
containing TRAF binding sites.
278 TUMOR NECROSIS FACTOR RECEPTORS
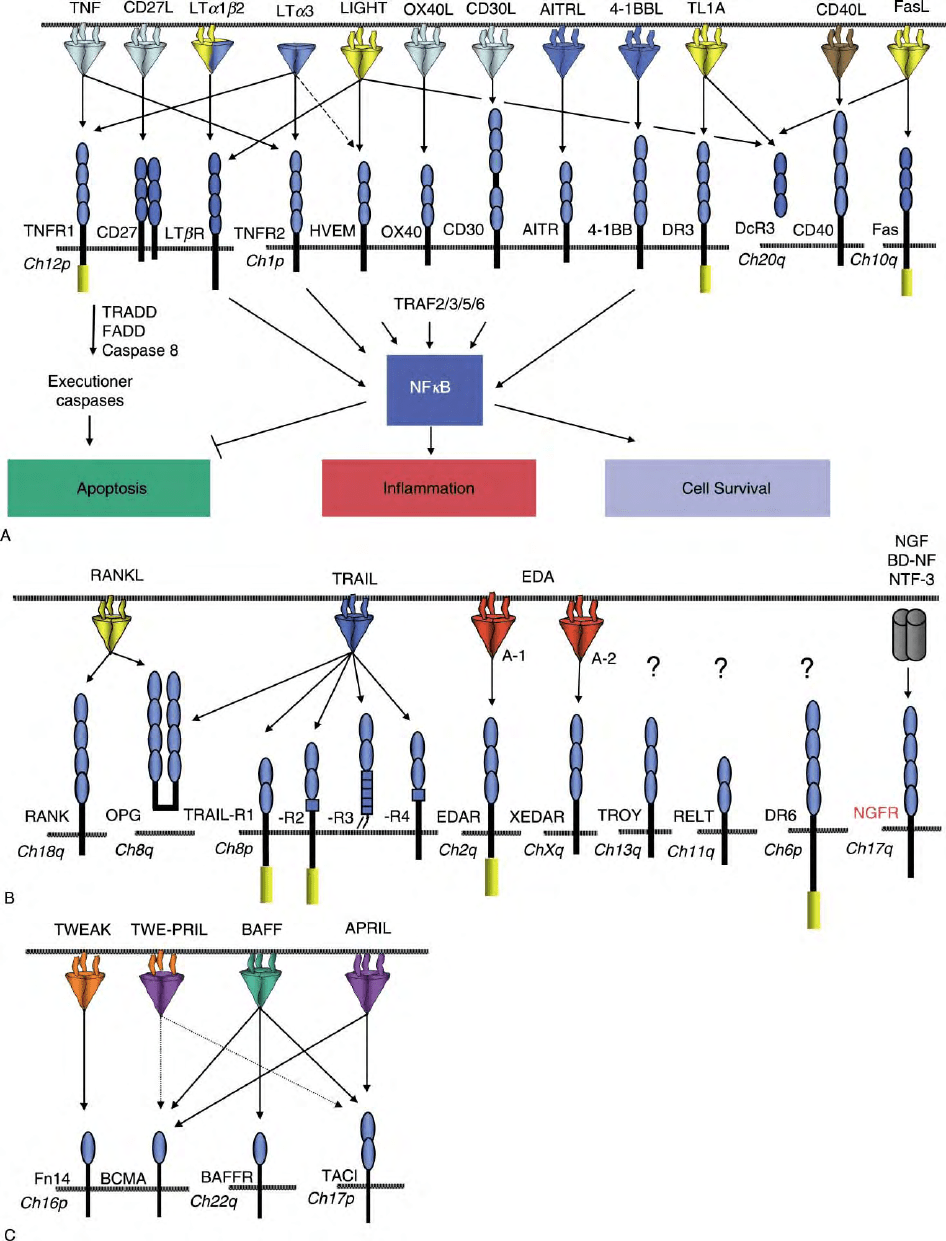
FIGURE 2 The TNF–TNFR superfamily grouped on the basis of chromosome (Ch) localization of TNFR. (A) TNFR and TNF ligands are shown
with arrows connecting ligand– receptor pairs. CRDs are shown as small ovals. DD is denoted as a rectangular box in the cytoplasmic tail of
appropriate receptors. The signaling and effector functions induced by either DD-containing or TRAF-binding TNFR is shown. (B and C) Same as
(A), with remaining TNFR grouped on the basis of chromosome localization and ligand binding.
TUMOR NECROSIS FACTOR RECEPTORS 279
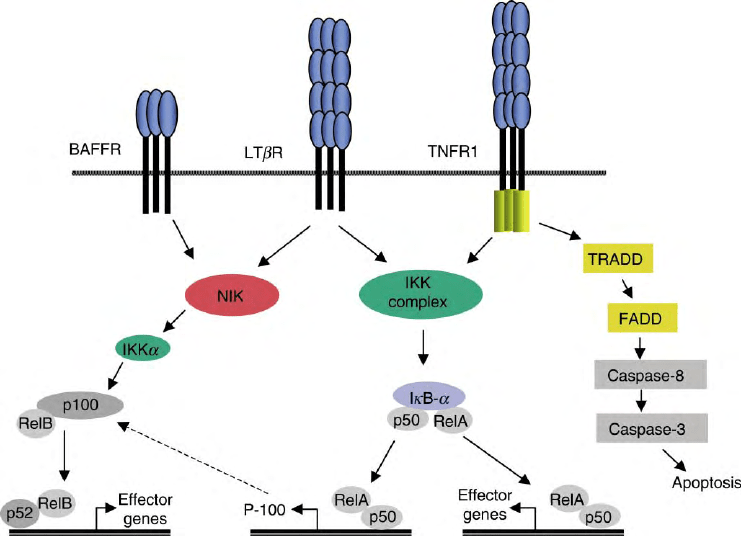
DNA. NF
k
B1 is held in the cytosol by an inhibitor
protein (I
k
B) while an inhibitory domain controls the
cytosolic localization of NF
k
B2. The inhibitory pro-
teins are phosphorylated and degraded in response to
activation signals coming from diverse sources that all
target the inhibitor
k
B protein kinase complex (IKK).
Degradation of the inhibitor allows NF
k
B to localize
to the nucleus and activate transcription. The NF
k
B1
and B2 pathways induce distinct sets of genes: NF
k
B1
regulates the expression of proinflammatory chemo-
kines and adhesion molecules, while the NF
k
B2
pathway controls the expression of distinct chemokines
and cytokines involved in lymphoid organogenesis.
BAFFR is only able to activate the NF
k
B2 pathway,
suggesting that this pathway is crucial for the
expression of survival genes specifically important for
B lymphocytes. By contrast, TNFR1 is unable to
activate NF
k
B2, which may account for its strong
proinflammatory action.
TRAF2 propagates signals to mitogen-activated
protein (MAP) kinases, including JNKs/SAPKs, ERKs,
and p38s, to activate the transcription factor AP-1 that
plays roles in stress responses and cellular homeostasis.
Like TRAF2, TRAF5 cooperates to activate NF
k
Band
AP-1 transcription factors, while TRAF3 negatively
regulates NF
k
B activation and thus may play a role
in promoting cell death. TRAF6 also activates NF
k
B
and AP1.
DEATH RECEPTOR SIGNALING
A number of TNFR, including TNFR1 and Fas, are
also termed “death” receptors because they regulate
apoptotic cell death. Their cytoplasmic tails contain a
region of , 80 amino acids that fold into six
a
-helices,
termed the DD. The DD serves as a protein interaction
motif to recruit signaling molecules to the inert
cytoplasmic domain of TNFRs. As such, the DD of
TNFR can self-associate or associate with other DD-
containing adaptor proteins such as FADD, TRADD,
and RIP.
In the simplest scheme known to activate the cell
death machinery, the adaptor FADD is recruited to Fas
initiating formation of the death-inducing signaling
complex (DISC). Procaspase 8 is recruited to the DISC
through a second interaction motif contained in FADD,
termed the death effector domain (DED), activating
downstream effector caspases (e.g., caspase 3) resulting
in the cleavage of critical cellular substrates and the
eventual collapse of the cells and death (apoptosis).
Similarly, TNFR1 activates apoptosis although it
requires the adaptor protein TRADD to facilitate
FIGURE 3 Mechanisms of signaling by TNFR. Upon ligation of LT
b
R, two NF
k
b pathways are induced. The first leads to induction of the
NF
k
B1 pathway and the activation of IKK
b
and RelA, which control expression of inflammatory genes. The second pathway results in the
activation of NF
k
B2 and the processing of p100 to p52 following the activation of NIK and IKK
a
, leading to the transcription of genes implicated in
secondary lymphoid organogenesis and homeostasis. BAFFR also activates the NF
k
B2 pathway, whereas TNFR1 only activates the NF
k
B1
pathway. TNFR1 also contains a DD to signal caspase activation and apoptosis.
280 TUMOR NECROSIS FACTOR RECEPTORS
FADD recruitment, and is also able to recruit another
adaptor protein RIP that also plays a role in procaspase
8 activation. The DISC is regulated by two forms of
another protein known as FLIP, which can switch the
DISC between activating caspases or NF
k
B, which in
turn regulates genes that block apoptosis, such as the
inhibitors of apoptosis (IAP) that promote cell survival.
Effector Functions of
the TNFR Family
Despite similarities in structure and signaling mechani-
sms, TNFRs function in diverse, and often opposing,
roles in immune physiology. As discussed above, several
reasons that explain their functional diversity include
differences in ligand binding, tissue and cellular
expression, regulation of expression, and association
with signaling adaptor proteins.
INFLAMMATION
A role for TNF family members in host defense and
inflammation was first appreciated when it was realized
that TNF is identical to a factor termed cachectin, a
protein known to cause fever and wasting. Inflammatory
cells, such as macrophages, induce TNF when special-
ized innate immune receptors called TLRs recognize
nonself patterns on microbial pathogens. Binding of
TNF to its receptors, TNFR1 or TNFR2, induces a
variety of molecules that are crucial for initiating the
acute inflammatory response. For example, TNF signal-
ing induces increased expression of adhesion molecules
on endothelial cells and secretion of chemokines to
promote inflammatory cell migration to the site of
infection. TNF can also activate macrophages and
neutrophils to increase their phagocytic capacity and
their ability to secrete tissue-degrading enzymes into
infected tissues. Although inflammation is crucial to
prevent infection, it must also be minimized after the
pathogenic challenge subsides in order to prevent
uncontrolled damage to fragile host tissues. As such,
uncontrolled TNF signaling can cause chronic inflam-
mation and wasting, or acutely released, septic shock.
Drugs that block TNF signaling, such as soluble TNFR
decoy receptors, have been developed to prevent
inflammatory diseases such as rheumatoid arthritis and
inflammatory bowel disease.
APOPTOSIS
Cell death by apoptosis is one of the major functions of
DD-containing TNFR. Fas, for example, mediates cell
death for varied purposes. First, Fas, and its ligand
FasL, cooperate as major effectors, along with the
perforin/granzyme pathway, to trigger killing of patho-
gen-infected cells by cytotoxic T-lymphoctyes (CTLs)
or by natural killer (NK) cells. In a second context, Fas is
required to eliminate excessive CTLs and other effector
cells. Fas is induced on long-term antigen-activated
lymphocytes to elicit their removal and minimize the
excessive expansion of T lymphocytes that can occur in
response to T-cell activation in the space-restricted
lymphoid environment. In fact, two naturally occurring
mutations in mice encoding the gene lymphoprolifera-
tive disorder (lpr), lpr,orlpr
cg
that affect the expres-
sion or the biological activity of the Fas receptor,
respectively, demonstrate the important role of Fas in
lymphocyte homeostasis. Both of these mutant mice
develop an autoimmune phenotype characterized
by massive lymphadenopathy, splenomegaly, and auto-
antibody production.
CELL SURVIVAL
BAFFR, BCMA, and TACI are some of the most
recently discovered members of the TNFR family that
are predominantly expressed on B cells and represent a
good example of TNFRs that play a role in cell
survival. All three receptors bind the TNF-related
ligand BAFF, produced by macrophages and dendritic
cells, while BCMA and TACI can also bind the related
ligand APRIL. BAFF is required in the maintenance and
differentiation of mature B cells in peripheral tissues by
supporting B-cell survival and maturation presumably
through activation of anti-apoptotic genes. The mech-
anism for enhanced B-cell survival by BAFF may be
partly due to the stimulation of the pro-survival protein
bcl-2 and a decrease in the expression of the pro-
apoptotic factors Bak and Blk. A crucial role for
BAFFR in BAFF-mediated B-cell survival is evidenced
by the fact that neither BCMA- nor TACI-deficient mice
have defects in B-cell maturation, whereas A/WySnJ
mice, containing a naturally occurring mutation in
BAFFR, do show defects in B-cell maturation. Over-
expression of BAFF in mice can result in autoimmune
symptoms, and similarly patients with systemic lupus
erythematosus (SLE), rheumatoid arthritis, and Sjo-
gren’s syndrome show elevated levels of BAFF in the
blood. Other TNFRs also play roles in the survival and
development of various tissues including the TRANCE
system in bone, EDAR in ectodermal tissues, and
NGFR in neurons.
COSTIMULATION
T lymphocyte activation is also regulated by TNFRs
where they act as costimulatory signals to positively or
negatively influence the proliferation of antigen-stimu-
lated T cells. OX40, CD27, 4-1BB, and HVEM,
expressed on CD4
þ
and CD8
þ
T cells, promote T-cell
TUMOR NECROSIS FACTOR RECEPTORS 281
