Lennarz W.J., Lane M.D. (eds.) Encyclopedia of Biological Chemistry. Four-Volume Set . V. 4
Подождите немного. Документ загружается.

proliferation and cytokine production in response to
their respective ligands expressed on dendritic cells. For
example, mice deficient in the OX40–OX40L inter-
action show impaired T-cell proliferative capabilities
and cytokine production in response to viruses such as
lymphocytic choriomengiitis virus (LCMV) or influenza
virus, and the ability to generate or sustain memory
T cells is diminished. Additionally, some evidence
indicates that several receptors such as CD30, Fas, or
HVEM may play a role in positive and negative selection
in thymocyte differentiation. Thus, TNFR family
members can contribute to immune tolerance.
On B cells, CD40 is the most prominent costimula-
tory receptor where it interacts with its ligand, CD40L,
on T cells to initiate T-cell-dependent B-cell antibody
responses. Mutations in CD40 or its ligand affect a
number of immune functions including immunoglobulin
class switching; in humans, X-linked hyper-IgM syn-
drome is due to defects in CD40L structure and function
manifesting as an inability to switch immunoglobulin
M to IgG classes.
ORGANOGENESIS AND DEVELOPMENT
A number of TNFRs play roles in the organization of
tissues. The lymphotoxin system mediated by LT
b
R,
and its ligand LT
ab
, play a critical role in lymph node
development; mice deficient in either receptor or ligand
completely lack lymph nodes and Peyer’s patches.
RANK and its ligand RANKL mediate osteoclast
differentiation and therefore are important for main-
taining normal bone homeostasis. Osteoporosis, a
condition of bone thinning, can be caused by over-
expression of the soluble RANKL decoy receptor,
OPG, which is induced by estrogen and prevents
normal bone formation. RANK-RANKL signaling also
supports the development of mammary gland alveolar
buds important for lactation, and consequently an
absence of RANK induces accelerated apoptosis of
mammary epithelial precursors. EDAR, XEDAR, and
TROY, and the ligand EDA, regulate the development
of ectodermal tissues including hair follicles. In fact,
mice or humans with deficiencies in EDAR or EDA
lack primary hair follicles and sweat glands. Finally,
NGFR is important for the development of sensory
neurons; acting alone it induces apoptosis, but upon
association with other nerve growth factor receptors it
mediates the differentiation and survival of neurons.
NGFR-deficient mice develop cutaneous sensori-
neuronal defects.
Viral Targeting of TNFR
Since TNFRs and their ligands play a critical role in
mediating immune defenses against invading pathogens,
and control death and survival fates for cells, it is not
surprising that viruses have evolved mechanisms that
directly target the TNF family in order to subvert
immune effector mechanisms. In fact, viruses when
viewed collectively have targeted virtually every step
of the TNFR signaling pathway, from ligand binding
to activation of apoptosis. For example, infection by
some viruses, including cytomegalovirus (CMV),
herpes simplex virus (HSV), adenovirus, and HIV,
induce the expression of FasL and/or TRAIL on the
host cell resulting in the probable killing of recruited
immune effector cells that express the cognate TNFR
death receptor. In contrast, adenovirus down-regulates
the proapoptotic receptors Fas and TRAILR from the
surface of infected cells, thereby preventing the killing
of the host cell harboring the virus. Another
mechanism of immune evasion employed by pox-
viruses is the expression of soluble, secreted orthologs
of TNFR2 or CD30 that inhibit the interaction of
their respective ligands with their cellular receptors.
In some cases, viruses and TNFR signaling have been
adapted to function cooperatively. For instance,
signaling through the LT
b
R or TNFR1 can prevent
the replication of human CMV in fibroblasts without
inducing death of the infected cell thereby allowing
continued existence of CMV within the host. This
molecular example of host– virus coexistence might
provide a mechanism for CMV to persist latently in
individuals with a strong host immune system, and re-
emerge to cause disease in individuals who are
immunosuppressed.
SEE ALSO THE FOLLOWING ARTICLES
Bax and Bcl2 Cell Death Enhancers and Inhibitors †
Cell Death by Apoptosis and Necrosis † Chemokine
Receptors † Mitogen-Activated Protein Kinase Family †
Nuclear Factor kappaB † Toll-Like Receptors
GLOSSARY
apoptosis An orderly process of programmed cell death whereby
cellular machinery undergoes changes leading to death.
autoimmunity An immune response against self-antigens.
cytokine Proteins made by cells that affect the behavior of other cells.
signal transduction Term used to describe the processes inside cells
used to respond to changes in their environment.
transcription factor A protein that binds DNA to control the
transcription of genes involved in a large number of normal
cellular and organismal processes.
FURTHER READING
Benedict, C. A., Banks, T. A., and Ware, C. F. (2003). Death and
survival: Viral regulation of TNF signaling pathways. Curr. Opin.
Immunol. 15, 59–65.
282 TUMOR NECROSIS FACTOR RECEPTORS
Bodmer, J., Schneider, P., and Tschopp, J. (2002). The molecular
architecture of the TNF superfamily. Trends Biochem. Sci. 27,
19–26.
Chung, J. Y., Park, Y. C., Ye, H., and Wu, H. (2002). All TRAFs
are not created equal: Common and distinct molecular mech-
anisms of TRAF-mediated signal transduction. J. Cell Sci. 115,
679–688.
Dejardin, E., Droin, N. M., Dehase, M., Haas, E., Cao, Y., Makris, C.,
Li, Z. W., Karin, M., Ware, C. F., and Green, D. R. (2002). The
lymphotoxin-
b
receptor induces different patterns of gene
expression via two NF
k
B pathways. Immunity 17, 1–11.
Locksley, R. M., Killeen, N., and Lenardo, M. J. (2001). The TNF and
TNF receptor superfamilies: Integrating mammalian biology. Cell
104, 487– 501.
Orlinick, J. R., and Chao, M. V. (1998). TNF-related ligands and their
receptors. Cell. Signal. 10, 543–551.
BIOGRAPHY
Karen G. Potter is an NIH Postdoctoral Fellow in the lab of Dr. Carl F.
Ware at the La Jolla Institute for Allergy and Immunology. She received
her Ph.D. in Cell and Molecular Biology from Duke University in July
2002. Her research interests include the regulation and immunomo-
dulatory functions of the TNFR HVEM.
Carl F. Ware is Head of Molecular Immunology at the La Jolla Institute
for Allergy and Immunology, and a Professor of Biology at UCSD. His
research program investigates the regulation of immunity by cytokines
and viruses. He is an advisor for the National Institutes of Health and is
an editor of scientific journals. Dr. Ware received his Ph.D. in
Molecular Biology and Biochemistry from the University of California
Irvine, and was an NIH Postdoctoral Fellow at the Harvard Medical
School.
TUMOR NECROSIS FACTOR RECEPTORS 283

Two-Dimensional Gel
Electrophoresis
Gerhard Schmid, Denis Hochstrasser and Jean-Charles Sanchez
Biomedical Proteomics Research Group, Geneva University Hospital, Geneva, Switzerland
Two-dimensional polyacrylamide gel electrophoresis (2D
PAGE) is a high-resolution protein separation technique
exploiting two independent physico-chemical properties
(charge and size) of the protein components to be separated.
During the first dimension, under the influence of an electrical
field, charged proteins migrate in a pH gradient until each of
them reaches a distinct pH value, which corresponds to its
isoelectric point (pI). At its pI, a protein has zero net charge.
This separation technique is called isoelectric focusing (IEF). In
the second dimension, the protein mixture previously separ-
ated by IEF is sorted orthogonally by electrophoresis in the
presence of sodium dodecyl sulfate (SDS). Migration of SDS-
coated proteins in a sieving polyacrylamide gel results in a
separation according to their relative molecular mass (M
r
).
Nowadays, 2D PAGE is a powerful and widely used tool for
the analysis of complex soluble protein mixtures extracted
from cells, tissues, or other biological samples.
Some Milestones in the
Development of 2D PAGE
HOW IT ALL BEGAN
In 1975, three papers (Klose, O’Farrell, Scheele)
introduced two-dimensional polyacrylamide gel electro-
phoresis (2D PAGE) using denaturing isoelectric focus-
ing (IEF) and SDS-PAGE. At that time, IEF was carried
out in cylindrical rod IEF gels cast in glass capillary tubes
(1–1.5 mm inner diameter). The gels contained syn-
thetic carrier ampholytes, which formed a continuous
pH gradient under the influence of an electric current.
After IEF the gel rods had to be removed from their tubes
for second dimension separation by SDS-PAGE.
TECHNICAL IMPROVEMENTS
With the introduction of immobilized pH gradients
(IPGs) by Bjellqvist et al. in 1982, problems associated
with carrier ampholyte pH gradients such as gradient
instability, low sample loading capacity, and difficulty to
achieve reproducibility could be eliminated. In an IPG, a
set of acidic and basic buffering groups is covalently
incorporated into a polyacrylamide gel at the time it is
cast. Precast IPG gel strips supported by a plastic film
backing are commercially available in a variety of
narrow and broad, linear and nonlinear pH ranges.
This represents an important development and allowed
2D PAGE to become the tool of choice for high-
resolution protein separation (Figure 1). Besides
improvements in the 2D technique, critical develop-
ments in other fields greatly contributed to the more
widespread use of 2D PAGE. In 1987, Matsudaira
showed that classical N-terminal sequencing by Edman
degradation can be applied to picomole quantities of
proteins electro-transferred onto polyvinylidene difluor-
ide (PVDF) membranes. In 1993, several groups
independently showed that mass spectrometry allows
the identification of proteins previously separated by 1D
or 2D gel electrophoresis. Improvements on the level of
the mass spectrometers as well as rapid access to genome
sequence databases for various species from across the
world nowadays allow the high-throughput identifi-
cation of hundreds of proteins separated by 2D PAGE.
More powerful, less expensive computers and appro-
priate software made computer-assisted evaluation of
the highly complex 2D patterns possible.
2D PAGE IN THE FUTURE
After more than 25 years, one can say that 2D PAGE
finally became a routine protein separation method;
however, its further development is a continuous process.
A major interest, especially for the application of 2D
PAGE in the clinical laboratory, lies in the field of
miniaturized and automated 2D protein mapping.
Recently, a fully automated 2D electrophoresis system
(a2DE
TM
) has been presented by NextGen Sciences.
A microchip format that is based on 2D PAGE (digital
ProteomeChip
TM
from Protein Forest Inc.) allows
protein separation, staining, and digital imaging in as
little as 20 min, with a detection sensitivity below 1 pg of
protein.
Encyclopedia of Biological Chemistry, Volume 4. q 2004, Elsevier Inc. All Rights Reserved. 284
The Current Protocol
The method described by O’Farrell back in 1975 already
contained the magic bullets of 2D PAGE, i.e., urea,
dithiothreitol (DTT), a nonionic or zwitterionic deter-
gent for the first and SDS for the second dimension.
The procedure of 2D PAGE using IPG gel strips in the
first dimension is largely based on the work of Go
¨
rg
and co-workers.
SAMPLE SOLUBILIZATION
An ideal procedure results in the complete solubili-
zation, disaggregation, denaturation, and reduction of
all the proteins in the sample. However, dealing with
several thousand different proteins with different
physico-chemical properties makes complete solubili-
zation practically impossible. In general, cells or tissues
are disrupted by various techniques such as grinding in a
liquid nitrogen cooled mortar, sonication, shearing-
based methods, or homogenization. Proteins are
then solubilized in a buffer containing 9 M urea, 4%
3-[(3-cholamidopropyl)dimethylammonio]-1-propane-
sulfonate (CHAPS), 50–100 mM DTT. Various minor
modifications from this basic, IEF compatible solubil-
ization buffer have been described over the years, trying
to improve the solubilization of certain subclasses of
proteins. DNA or RNA molecules, which can interfere
with 2D PAGE, are removed by incubation with
nucleases. Centrifugation is used to separate the
solubilized proteins from nonsolubilized material. Pre-
fractionation of complex samples is a good choice when
only a subset of the proteins in a tissue or a cell is of
interest or when abundant proteins dominate the sample
as in the case of albumin in plasma.
FIRST DIMENSION: IEF
IEF separates proteins according to their isoelectric
point. Proteins diffusing away from their pI immediately
gain charge and migrate back. This focusing effect
allows proteins to be separated on the basis of very small
charge differences. Simplified handling and improved
performance made the commercially available precast
IPG gel strips (from 7 to 24 cm in length) the method of
choice for most applications. IPG strips with a pH range
from 3 to 10 will display a wide range of proteins,
whereas narrower pH ranges allow to zoom into a
particular pH range. With a set of narrow range,
overlapping pH gradients, a wide pH range can be
covered at increased resolution. There are several ways
to load the sample on the IPG gel strip. Efficient entry of
the solubilized proteins, especially hydrophobic ones,
into the gel is always a critical point. IPG dry strips need
to be rehydrated before use in IEF. Traditionally, samples
are applied on a discrete point via sample cups
(Figure 2A). Cups are placed at either the acidic or
basic pH extreme of the strip. Under these conditions,
most of the proteins in the sample will be charged and
immediately start migrating under the influence of an
electric current, thereby minimizing sample loss through
precipitation. Alternatively, the sample can be applied
over the whole IPG gel surface during strip rehydration.
This method is of special interest for more dilute samples
since larger volumes can be loaded. For strip rehydra-
tion, a solution containing 8 M urea, 2% CHAPS,
10 mM DTT, 1% carrier ampholytes, traces of Bromo-
phenol Blue (tracking dye) shows excellent results for
most samples. As for the solubilization solution, various
modifications were described. A general IEF protocol
starts with the sample entry phase at low initial voltage
(200 V). Voltage is then increased in a stepwise manner
up to the desired final focusing voltage (up to 8000 V).
Optimal focusing time depends on the nature of the
sample. In general, overfocusing is not deleterious below
a total of 100 kVh. State-of-the-art IEF systems allow
simultaneous migration of up to 12 strips under
temperature-controlled conditions.
SECOND DIMENSION: SDS-PAGE
Prior to the second dimension, the IPG gel strips are
equilibrated in a solution containing SDS. This anionic
detergent binds to the majority of proteins in a constant
mass ratio (1.4 g SDS/g protein), such that the net charge
per mass unit becomes approximately constant. This
allows protein separation in a polyacrylamide gel
according to their relative molecular mass. For an ideal
transfer of the proteins separated in the IPG strip onto
the second dimension gel, the equilibration step should
result in the complete resolubilization of all proteins in
the IPG gel. Again, the enormous chemical diversity
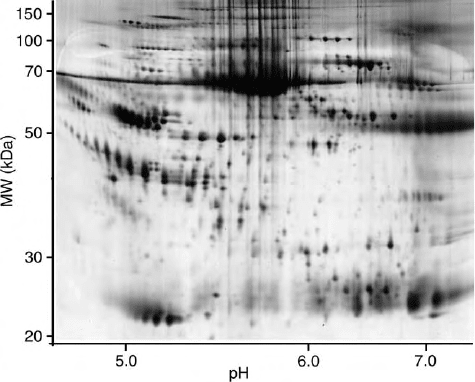
FIGURE 1 High-resolution protein separation of human plasma by
2D PAGE, silver staining.
TWO-DIMENSIONAL GEL ELECTROPHORESIS 285
present within most samples makes it impossible to use
ideal conditions for all its components. Composition of
the equilibration solution and especially duration of the
equilibration steps represent a compromise between
accessing poorly soluble proteins with a tendency to
adsorb to the gel matrix and diluting highly soluble
proteins out of the gel strip. Equilibration in a buffer
containing 6 M urea, 50 mM Tris–HCl buffer (pH 8.4),
2% w/v SDS, 30% w/v glycerol proved to be a good
compromise. For protein reduction, IPG strips are first
incubated in equilibration buffer containing 2% w/v
DTT for 12 min. In a second step, proteins are alkylated
by incubation in equilibration buffer containing 2.5%
w/v iodoacetamide (IAA) and traces of bromophenol
blue (tracking dye) for 5 min. In vertical SDS-PAGE, the
equilibrated strip is placed on the surface of the second
dimension gel and embedded in molten agarose
(Figure 2B). Most commonly, the tris-glycine buffer
system described by Laemmli is used. State-of-the-art 2D
gel electrophoresis systems allow the simultaneous
migration of up to 24 large format gels (20 by 24 cm)
under temperature-controlled conditions.
VISUALIZATION
Most of the staining methods used for conventional 1D
SDS-PAGE can be applied to 2D gels. Because of its very
high sensitivity (down to 1 ng/spot), silver staining is
probably most commonly used. However, the use of
glutaraldehyde in the silver-staining procedure should be
avoided for subsequent protein identification by mass
spectrometry. Recently, MS-compatible, fluorescent
stains with sensitivities comparable to silver became
available. At the time being, application of this stains in
large-scale and high-throughput 2D PAGE is hardly
affordable. In addition, special imaging systems are
required, whereas a simple desktop scanner can be
sufficient for “visible” stains. This still leaves plenty of
room for other MS-compatible, but less sensitive stains
such as coomassie blue and zinc negative staining.
Proteins labeled in vivo by the use of radioactive amino
acids (metabolic labeling) can be visualized by exposing
the dried 2D gel to an X-ray film or a storage phosphor
screen for use with a PhosphorImager system. As for 1D
SDS-PAGE, proteins from 2D gels can be electro-
transferred on membranes, which are subsequently
probed with antibodies. This so-called Western blot
technique allows highly sensitive and specific visualiza-
tion of a protein of interest.
Applications of 2D PAGE
The combination of a high-resolution protein separ-
ation tool (2D PAGE) with a highly sensitive protein
identification tool (MS) offers an enormous potential.
In 1994, on the occasion of the first Siena 2D
Electrophoresis meeting, the expression “proteome”
was proposed by Marc Wilkins for the description of
the proteins expressed by a genome of a species, an
organ, or a cell at a particular moment under
particular conditions. Since then, the field of proteo-
mics, i.e., the analysis of proteomes, is booming. The
global approach taken with proteomics allows the
massively parallel analysis of expressed proteins,
including their isoforms originating from posttransla-
tional modifications (PTMs). Global gene expression
can also be studied at the level of mRNA. However, it
needs to be emphasized that there is not a good
correlation between mRNA abundance and protein
amount in a cell at a given time and that PTMs
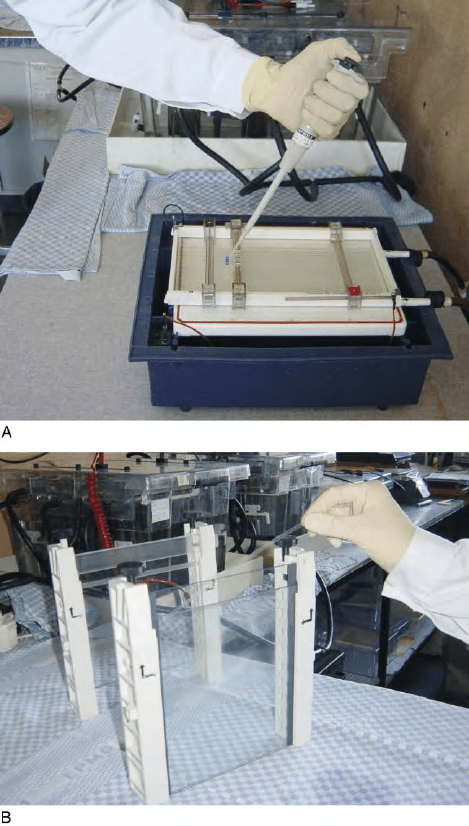
FIGURE 2 (A) Sample application for the first dimension of 2D
PAGE – samples are loaded in cups at the cathodic end of the IPG strip.
(B) Strip transfer to the second dimension of 2D PAGE – an
equilibrated IPG strip is placed on the top of a large format
polyacrylamide gel.
286 TWO-DIMENSIONAL GEL ELECTROPHORESIS
cannot be predicted from gene sequences. In general,
applications of 2D PAGE can be divided into
descriptive protein mapping without a need for
protein quantification and differential analysis, where
protein quantification is essential.
PROTEIN MAPPING AND 2D PAGE
P
ROTEIN DATABASES
The classical approach, 2D PAGE coupled with MS,
allows the establishment of 2D reference maps. One aims
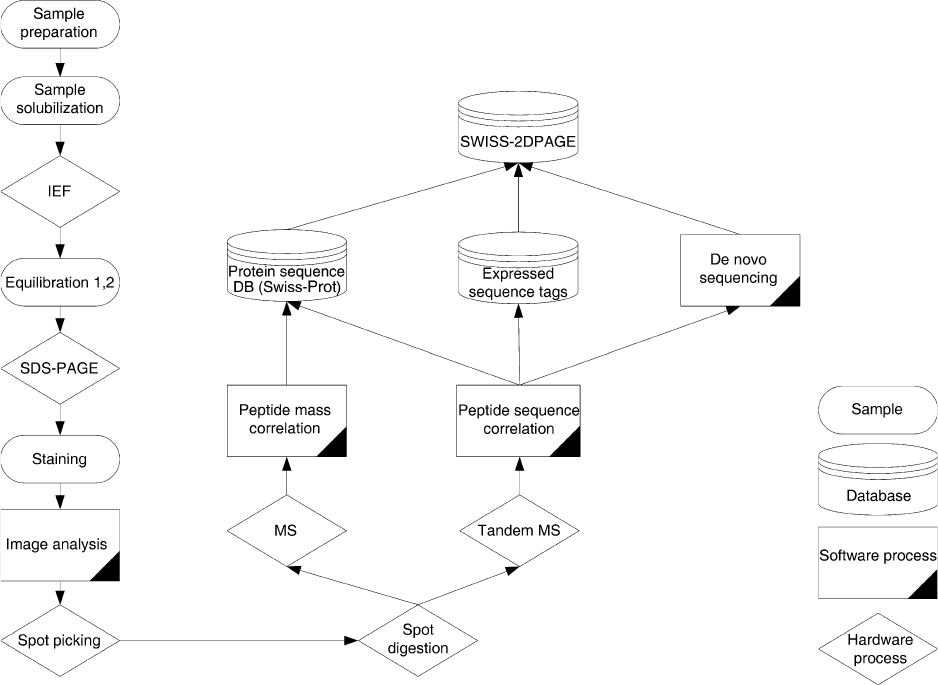
at identifying as many proteins as possible. A typical
workflow is depicted in Figure 3. With the number of
completely sequenced genomes increasing daily, protein
identification became possible for a growing number of
species. Over the last years, many 2D reference maps
have been established using different tissues, body fluids,
or cell lines as sample (for an overview see http://ch.
expasy.org/ch2d/2d-index.html). Due to the enormous
complexity of cells from higher eukaryotes, characteriz-
ation of their complete proteome in a single step is still
impossible. Isolation of subcellular fractions such as
organelles, macromolecular structures, and multiprotein
complexes represents a possibility to reduce the com-
plexity of the entire cell.
DIFFERENTIAL ANALYSIS
Digitized 2D maps can serve more than just descrip-
tive purposes. With specialized software packages,
quantitative image analysis of the complex 2D patterns
can be performed. This allows one to compare protein
expression levels between different conditions for
thousands of proteins in parallel. In general, one
aims at identifying only the proteins showing differ-
ential expression in the samples under comparison.
Since the early 1990s this approach has been used to
tackle various biological questions. Comparison of
proteomes from tissues or cells representing the
healthy and the diseased state, respectively, allows
the detection of putative molecular markers of the
disease. Such a global approach is particularly useful
in polygenic diseases such as cancer and cardiovas-
cular diseases. Analysis of cell differentiation and
monitoring therapy are other fields of application of
differential analysis.
FIGURE 3 Typical proteomic workflow using 2D PAGE as protein separation tool.
TWO-DIMENSIONAL GEL ELECTROPHORESIS 287
Limitations and Perspectives
of 2D PAGE
In spite of all benefits, 2D PAGE is not without
limitations. On the one hand, there are intrinsic
limitations of 2D PAGE and, on the other hand,
there are general limitations encountered when dealing
with complex protein mixtures.
INTRINSIC LIMITATIONS OF 2D PAGE
Hydrophobic Proteins
Very hydrophobic proteins such as integral membrane
proteins and nuclear proteins were shown to be under-
represented in 2D gels. They have a tendency to stick to
IPG gel matrix, and re-solubilization for the second
dimension becomes a problem. Incorporation of
thiourea, sulfobetaine surfactants, and organic solvents
in the solubilization buffer showed some improvements.
However, very hydrophobic proteins will probably
remain a major limitation of IEF and therefore also of
2D PAGE.
Basic Proteins
Horizontal streaking on the 2D gel is usually observed in
the region with pH . 7. This phenomenon is particularly
prominent when basic narrow range pH gradients are
used. The streaking is due to the disappearance of the
reducing agent from the basic part of the strip.
Oxidation of the protein thiol groups results in the
formation of inter- and intra-chain disulfide bridges.
Recently, oxidation of the protein thiol groups to mixed
disulfides using hydroxyethyl disulfide (DeStreak
TM
) has
been introduced, showing massively improved focusing
for the very basic proteins.
Automation and Miniaturization
For many years, the low degree in automation and
miniaturization of 2D PAGE has hindered this labor-
intense technique from becoming routinely used in
clinical laboratories and in industry, where high
throughput counts and sample amount is limited.
Promising developments toward both automation and
miniaturization can be observed and their availability
seems to be very close.
GENERAL LIMITATIONS
A biological sample usually represents a complex
protein mixture containing thousands of proteins with
different physico-chemical properties and widely vary-
ing expression levels. In human cells the dynamic range
exceeds 6 orders of magnitude and in human plasma it
reaches more than 12 orders of magnitude. In contrast,
2D gels and silver staining offer a maximum of 4 orders
of magnitude. Consequently, application of unfractio-
nated samples allows one to see only the tip of the
iceberg. In order to access the low abundance proteins,
samples need to be prefractionated, using traditional
biochemical separation techniques, for example.
PERSPECTIVES OF 2D PAGE
For many years, 2D PAGE has been the core technology
for protein separation in proteomics research. This
dominant role is now being challenged by various
non-gel-based approaches such as protein chips, serial
liquid chromatography, or capillary electrophoresis.
They can be very demanding in terms of data manage-
ment andbioinformatic resources, which mightbe limited
for most standard academic research institutions. In fact,
these liquid-based techniques should be considered as
complementary approaches to 2D PAGE, depending on
the biological question one wants to answer. If one aims
at identifying as many proteins as possible in a given
sample, 2D PAGE coupled to MS no longer represents the
method of choice. However, if one aims at differential
analysis of protein expression, 2D gels remain very
powerful. This is mainly due to the possibility to look at
PTMs offered by 2D PAGE. PTMs such as phosphoryl-
ations, glycosylations, or proteolytic processing can be
very important for the function of a protein.
SEE ALSO THE FOLLOWING ARTICLES
Glycation † Protein Data Resources † Proteinase-
Activated Receptors
GLOSSARY
immobilized pH gradient (IPG) In an IPG gradient, a set of acidic and
basic buffering groups is covalently incorporated into a polyacryl-
amide gel at the time it is cast. Precast IPG gel strips supported by a
plastic film backing are commercially available in a variety of
narrow and broad pH ranges.
isoelectric point (pI) The pH value at which the net electric charge of
an elementary entity, here a protein, is zero. At a pH value below
the pI of a protein, it is charged positively, and at a pH value above
the pI it is charged negatively.
proteome Ensemble of proteins expressed by a genome of a species,
an organ, or a cell at a particular moment under particular
conditions.
proteomics Qualitative and quantitative comparison of proteomes
under different conditions to further unravel biological processes.
FURTHER READING
Chambers, G., Lawrie, L., Cash, P., and Murray, G. I. (2000).
Proteomics: A new approach to the study of disease. J. Pathol. 192,
280–288.
288 TWO-DIMENSIONAL GEL ELECTROPHORESIS
Go
¨
rg, A., Obermaier, C., Boguth, G., Harder, A., Scheibe, B.,
Wildgruber, R., and Weiss, W. (2000). The current state of two-
dimensional electrophoresis with immobilized pH gradients.
Electrophoresis 21, 1037–1053.
Jung, E., Heller, M., Sanchez, J.-C., and Hochstrasser, D. F. (2000).
Proteomics meets cell biology: The establishment of subcellular
proteomes. Electrophoresis 21, 3369–3377.
Rabilloud, T. (ed.) (2000). Proteome Research: Two-Dimensional Gel
Electrophoresis and Identification Methods. Springer, Berlin,
Heidelberg.
Rabilloud, T. (2002). Two-dimensional gel electrophoresis in proteo-
mics: Old, old fashioned, but it still climbs up the mountains.
Proteomics 2, 3–10.
Swiss-2DPAGE on the Expasy Molecular Biology Server – http://ch.
expasy.org/ch2d/.
Wilkins, M. R., Williams, K. L., Appel, R. D., and Hochstrasser, D. F.
(eds.) (1997). Proteome Research: New Frontiers in Functional
Genomics. Springer, Berlin, Heidelberg.
BIOGRAPHY
Gerhard Schmid is a Ph.D. student at the Biomedical Proteomics
Research Group (BPRG) of the Clinical Pathology Department,
Geneva University Hospital.
DenisHochstrasseris the Director of the Clinical Pathology Department.
At the academic level, he is a full Professor both to the Department of
Pathology of the Medicine faculty and to the School of Pharmacy of
the Science faculty. His innovations in the methodology of 2D gel
electrophoresis have contributed decisively to the technique’s becom-
ing one of the main protein separation methods used in proteomics.
Jean-Charles Sanchez is the Head of the BPRG since 1995. Working in
the field of proteomics since 1989, he contributed to the development
of 2D gel electrophoresis and its application in biomedical research. He
obtained Ph.D. in Biochemistry at the University of Buckingham (UK)
in the field of proteomics and diabetes.
TWO-DIMENSIONAL GEL ELECTROPHORESIS 289

Two-Hybrid Protein–Protein
Interactions
Ilya Serebriiskii and Erica A. Golemis
Fox Chase Cancer Center, Philadelphia, Pennsylvania, USA
The two-hybrid system is an artificially constructed genetic
system intended to facilitate the detection and assessment of
protein–protein interactions. In the two-hybrid system a host
organism, typically yeast or bacteria, is so engineered as to
contain three components. These are a first protein fused to a
DNA-binding domain of known specificity (hybrid 1); a
second protein fused to a transcriptional activation domain
(hybrid 2), that can interact with the first protein, constituting
a functional, albeit composite, transcription factor; and one or
more reporter genes transcribed based on the binding of the
composite transcription factor. Many permutations of the two-
hybrid paradigm have been developed, and two-hybrid systems
have become a mainstay of proteomic investigations.
The Yeast Two-Hybrid System
The purpose of the two-hybrid system is to provide a
genetic system that is capable of scoring the degree of
physical interaction between two proteins of interest.
To this end, a simple organism such as yeast
(S. cerevisiae) or bacteria (E. coli) is engineered such
that the interaction of two proteins generates a
scorable signal, that can be used either to gauge the
interaction affinity of two defined proteins, or to
identify interacting partners for one defined protein
by screening an expression library.
The first working example of a two-hybrid system
was described in 1989, by Fields and Song. A schematic
showing the system paradigm is shown in Figure 1.
There are three minimal components for a functioning
two-hybrid system. The first component is an expression
construct, which synthesizes a first protein of interest as
a fusion with a DNA-binding domain (DBD) that binds
a short DNA sequence of defined specificity (hybrid 1:
frequently termed the “bait”). The second component is
an expression construct that is used either to express a
second defined protein, or random clones from a cDNA
or genomic library, as a fusion with a transcriptional
activation domain (AD) (hybrid 2: frequently termed the
“prey”). The third component is a reporter cassette,
which consists of the DNA-binding site for the first
hybrid protein in the context of a minimal promoter,
upstream of the coding sequence for an easily scored
reporter gene.
For the yeast two-hybrid system to work, there are
two preconditions. First, the bait must not be indepen-
dently functional as a transcriptional activator, such that
yeast containing only the bait and the reporter produce
no reporter signal. Second, the bait and the prey must be
capable of localizing to the cell nucleus; hence, proteins
containing signal sequences that efficiently target
them to the plasma membrane, or other non-nuclear
compartments, are not generally acceptable as baits.
However, for proteins that meet these preconditions,
the interaction between the bait and the prey brings the
transcriptional activation domain encoded in the prey to
the promoter sequence bound by the bait, and activates
the transcription of the reporter gene.
A number of groups have built systems that exploit
this two-hybrid paradigm in yeast, with several of
these systems in common use. The most common DBDs
used in these yeast two-hybrid systems are derived
from the yeast transcription factor Gal4p, or from the
bacterial repressor protein LexA. A variety of different
AD sequences have been used, and include the
activation domain from Gal4p; the activation domain
from the viral protein VP16; or “artificial” activation
sequences encoded from selected fragments of the
E. coli genome.
Two categories of reporter genes have been used. One
category is colorimetric, exemplified by the bacterial
lacZ gene. Activation of a lacZ reporter causes yeast
colonies to turn blue when plated on medium containing
an appropriate substrate, such as X-gal, or can be
assessed quantitatively over a dynamic range in excess of
1000-fold in liquid assays using a spectrophotometer or
luminometer. The second category of reporter provides
an active selection for yeast growth based on the
strength of interaction between two proteins. These
reporters include genes encoding critical components of
yeast metabolic pathways for amino acid synthesis, such
that expression of the gene is required for yeast to grow
on medium lacking the relevant amino acid (e.g., show
Encyclopedia of Biological Chemistry, Volume 4. q 2004, Elsevier Inc. All Rights Reserved. 290
an auxotrophic requirement). HIS3, required for growth
on medium lacking histidine, and LEU2, required for
growth on medium lacking leucine, have been most
commonly used. In general, two hybrid systems now
generally incorporate one colorimetric and one auxo-
trophic selection, both responsive to the same bait. The
use of two different reporter genes greatly minimizes the
background of false positive and false negative inter-
actions in the system, and is essential for library
screening applications, where in excess of 10
6
clones
must commonly be analyzed.
Because of the ease of manipulation provided by a
genetic system, a yeast two-hybrid system provides a
facile means of exploring the interaction of two known
partner proteins. This can be done in a predetermined
manner, by inserting targeted mutations or deletions into
the AD-fused partner, or based on blind selection, for
example, by using PCR mutagenesis or DNA sonication
to develop a randomized library derived from the AD
partner. Each mutant derivative can be readily screened
for interaction phenotype. However, the most important
use of the yeast two-hybrid system throughout the 1990s
has clearly been for the purpose of screening libraries to
identify novel interacting partners for proteins of
interest. In contrast to other means of identifying
interacting proteins, such as biochemical copurification,
two-hybrid screening is extremely inexpensive, requiring
only engineered strains of yeast and standard micro-
biological media. It is rapid, providing the potential of
completing a screen from start to finish in less than a
month. It is effective at yielding returns for a significant
percentage of bait proteins (estimated at . 50%).
Finally, following completion of a successful library
screen, plasmids encoding the novel interacting preys
can easily be isolated from yeast, avoiding the need for
protein sequencing or mass spectrophotometric analysis
required by biochemical methodologies. It is not an
exaggeration to note that recent broad adaptation of
two hybrid screening methodologies across the scientific
community was a major contributing source of elucida-
tion for key cell signaling pathways.
Advanced Applications
Following the validation of the basic two-hybrid system
as a useful and reliable tool for study of protein–protein
interactions, a number of investigators have explored
the potential of the system to study interactions between
proteins and partner proteins under additional selective
constraints. A first step in this direction was the
demonstration that it was possible to identify preys
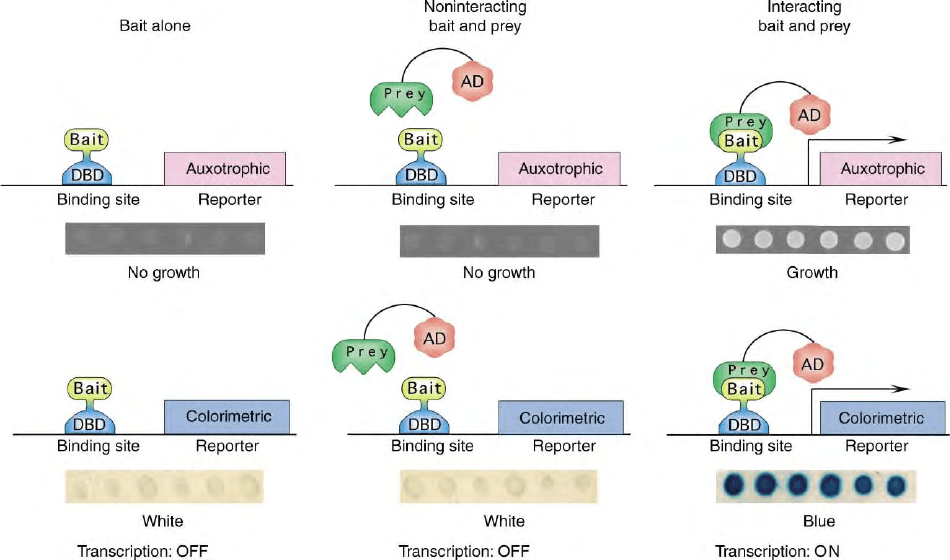
FIGURE 1 Schematic of yeast two-hybrid components, and profile of reporter activation. Details of system components are described in the text.
Three situations are shown: (1) yeast containing only a bait and two reporters, left; (2) yeast containing bait, prey that does not interact with bait, and
reporters, center; and (3) interacting bait and prey, and reporters, right. For each of these three situations, with a typical auxotrophic and colorimetric
reporter, six independent colonies of yeast are dotted on plates with selective medium: expected results (growth/no growth, or color/no color) are
shown as panel inserts below each schematic.
TWO-HYBRID PROTEIN–PROTEIN INTERACTIONS 291
