Kutz M. Handbook of materials selection
Подождите немного. Документ загружается.


1046 COMPOSITES FABRICATION PROCESSES
Table 2 Implications of Choice of Reinforcement Format for E-glass / Epoxy Resin
Reinforcement Format
V
ƒ
max C
0
R
E
max
(GPa)
G
max
(GPa) Applicable Processes
Uniaxial tow 0⬚ 0.80 1.00 0.80 56 5.0 Tow placement, filament
wind, pultrusion
UD prepreg 0
⬚ 0.65 1.00 0.65 47 5.0 Autoclave, RFI
Biaxial prepreg 0
⬚,90⬚ 0.60 0.50 0.30 22 5.0 Autoclave, RFI
Quasi-isotropic prepeg 0
⬚, Ⳳ45⬚,90⬚ 0.60 0.33 0.18 13 8.0 Autoclave, RFI
Balanced woven 0
⬚,90⬚ 0.50 0.50 0.25 18 4.1 RFI, RTM, contact mold
Isotropic woven 0
⬚,90⬚; Ⳳ45⬚ 0.50 0.33 0.16 12 7.0 RFI, RTM, contact mold
Noncrimp fabric 0
⬚,90⬚; Ⳳ45⬚ 0.55 0.33 0.18 13 7.5 RFI, RTM, contact mold
Orthogonal 3D x, y, z 0.40 0.33 0.13 9.4 4.0 RTM
CSM Random 2D 0.30 0.33 0.10 7.2 3.5 RFI, RTM, contact mold
CRM Random 2D 0.20 0.33 0.07 5.0 3.0 RFI, RTM, contact mold
Short fiber Random 3D 0.15 0.12 0.02 3.4 3.0 Injection mold
Unfilled epoxy resin 0.3 2.5
feedstock and may also involve selection of the basic material system, e.g.,
E-glass/polyester or carbon/epoxy.
2.2 Common Features of Composite Processing Routes
Infiltration
The reinforcing fibers are initially produced as spools of continuous roving. This
may be treated with a size and/or binder but is otherwise dry. During the proc-
essing operations, it must be fully infiltrated with the matrix resin. This may be
accomplished either by some precompounding operation or during the final
molding process. Infiltration of a porous medium by a liquid has been exten-
sively studied and a simple relationship proposed by Darcy is widely used:
S
⌬p
q ⫽ (1)
⌬L
The rate of infiltration, q ,is proportional to the permeability, S, and the driving
pressure gradient,
⌬p/⌬L, and inversely proportional to the viscosity of the me-
dium,
. Thus, increasing the applied pressure or the permeability will assist
infiltration while increasing viscosity will hinder. Viscosity may usually be de-
creased by raising the temperature, but this leads to faster cure in thermosets
and ultimately to degradation in thermoplastics. It is necessary to establish a
processing window where the temperature and pressure are controlled to allow
complete infiltration to be effected before gelation or degradation of the matrix
resin. The length L over which infiltration must occur is basically determined
by the geometry of the part. However, the effective infiltration path can be altered
by multiple gating and also in rather more subtle ways by design of the rein-
forcement.
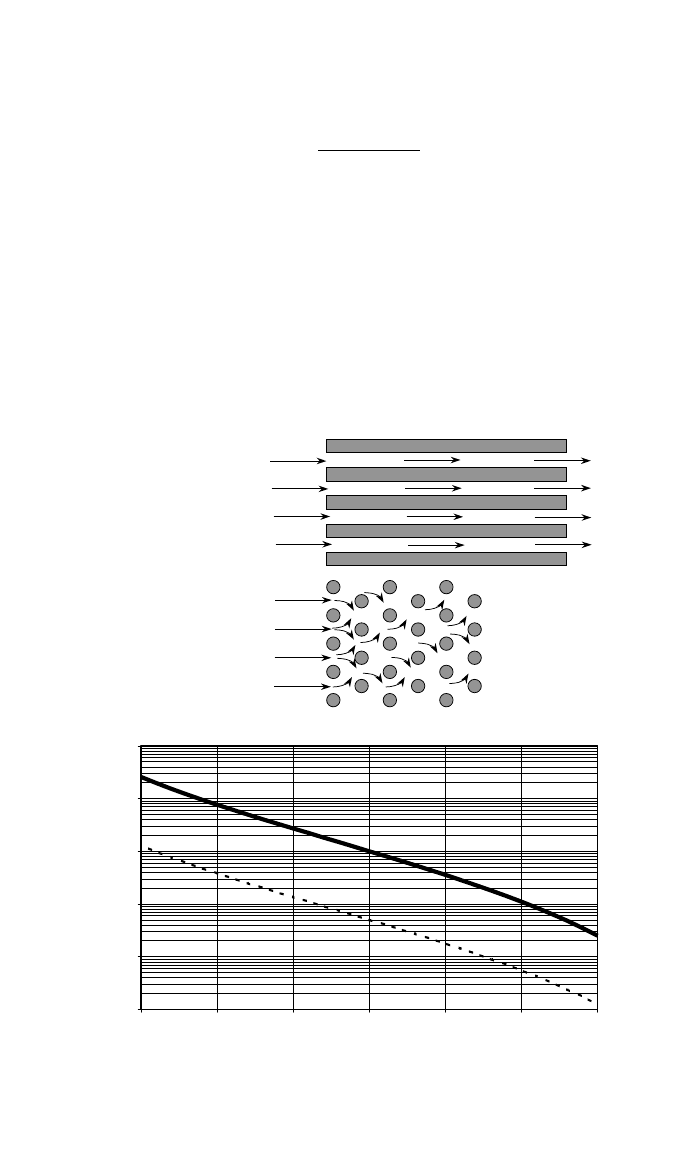
2 BASIC PRINCIPLES FOR PROCESSING 1047
PARALLEL FLOW:
Fluid flows smoothly between
the fibres. Rate proportional
to square of diameter.
TRANSVERSE FLOW:
Path is tortuous - Rate
only
≈
1/20 of that for
parallel flow.
(a)
0.001
0.01
0.1
1
10
100
0.2 0.3 0.4 0.5 0.6 0.7 0.8
FIBER VOLUME FRACTION
RELATIVE PERMEABILITY
AXIAL FLOW
TRANSVERSE FLOW
(b)
Fig. 5 (a) This depicts flow parallel and normal to a uniaxial array of fibers. The tortuous flow
in the normal direction results in a flowrate only ⬇ of that in the parallel direction under simi-
1
––
10
lar conditions. (b) Relative permeabilities in the axial (parallel) and transverse (normal) directions
of uniaxial fiber arrays is plotted against fiber fraction, V
ƒ
. Note that the transverse flowrate is
only of that in the axial direction. There is a reduction of permeability by a factor of approxi-
1
––
20
mately 100 for an increase in V
ƒ
from 0.3 to 0.7.
The permeability, S, is determined by the reinforcement architecture and may
be modeled using the Carman Kozeny relationship:
23
r (1 ⫺ V )
ƒƒ
S ⫽ (2)
2
4kV
ƒ
Here the permeability of a uniform array (of fibers) is given by the square of
the fiber radius, a function of V
ƒ
, and the Kozeny constant, k. It can be seen that
the permeability will be lower when the fibers are of smaller radius. The effect
of V
ƒ
is very severe, and the permeability decreases by a factor of about 6 for
a V
ƒ
increase from 0.6 to 0.7. The Kozeny constant is a measure of tortuosity:
In a uniform uniaxial array of cylindrical fibers (Fig 5a), k for flow parallel to
the fibers is some 20 times lower than that for flow normal to the fibers. This

1048 COMPOSITES FABRICATION PROCESSES
effect and that of V
ƒ
are further illustrated in Fig. 5b, which shows that per-
meability is reduced by a factor of 10 for an increase in V
ƒ
from 0.4 to 0.6.
These two relationships allow some simple generalizations to be stated:
(i) Permeability will be higher for a coarser fiber array.
(ii) Permeability is much higher in the fiber direction than at right angles.
(iii) Permeability decreases sharply as V
ƒ
is increased.
Unfortunately small-diameter fibers at high V
ƒ
tend to give composites with
better mechanical properties, so it is necessary to balance the aims of achieving
high mechanical properties against the requirements of ease of processing. For-
tunately there are a number of ways in which the effective permeability may be
increased without undue performance penalty. These are discussed in a later
section.
Consolidation
This is the process whereby the thickness of the section is reduced to the design
value and porosity and other defects eliminated. When dry or impregnated plies
(or tows) are laid up to form a laminate, the initial lay-up (pack) will be signif-
icantly thicker than ultimately required. This is due to the difficulty in obtaining
perfect contact, to entrapment of air, and sometimes due to an excess of resin
that is often deliberately incorporated. The application of pressure and/or heating
to the pack will cause it to consolidate. Ideally, any entrapped air or other
volatiles will also be eliminated together with any excess resin. Provision for
removal of resin and volatiles may be made by venting the tooling, by the use
of absorbent bleeder fabrics, and/or by the use of vacuum. The consolidation
process requires that resin flows, usually in the thickness direction of the pack.
The process may be partially affected by a preconsolidation operation (debulk-
ing) prior to the main processing operation or as part of the main process.
The principles of consolidation of a simple laminate, with a bleed ply, are
illustrated in Fig 6. Once again the process requires a sufficient time for the flow
processes to occur and this will be affected by the applied pressure and the
temperature. Where preconsolidation is used, care must be taken not to advance
the cure of thermoset systems too far so as to compromise satisfactory final
consolidation and cure.
Effects of Applied Pressure
Examination of the Darcy relationship (Eq. 1) indicates that the rate of infiltra-
tion may be increased by increasing the pressure gradient forcing infiltration.
While this is true, there are some limitations and implications that must be
discussed. First, much composite molding is carried out using simple tooling,
usually also composite, and a vacuum bag. This limits the pressure that may be
applied to less than one atmosphere (
⬇1 bar). Higher pressures may be applied
if an autoclave is employed, typically up to 5 bars. The limitation here is the
pressure capacity of the autoclave vessel, which may be 3 m in diameter. Higher
pressures may be exerted by press, but it should be noted that to apply a pressure
of only 10 bars, which is approximately 1 MPa, a closing force of 100 ton
䡠fis

2 BASIC PRINCIPLES FOR PROCESSING 1049
Absorbent bleeder pack
Resin permeable membrane
Resin flow
Mold surface
Consolidated plies
Excess resin
absorbed into bleeder
Prepreg plies
containing excess resin
Fig. 6 Process of consolidation involves flow in the thickness direction of the laminate.
If a bleeder ply is used, excess resin flows through a resin-permeable release ply and
is absorbed in the porous bleeder.
required for each 1 m
2
projected area of the molding. One implication of using
higher pressures is that the tooling needs to be made more robust. This means
use of steel tools, at a cost of at least five times that of the simple composite
tooling. A further problem is that the application of high compressing pressures
to the reinforcement before it is infiltrated, will tend to consolidate it, increase
the V
ƒ
, and decrease the permeability, so that there is no gain in infiltration rate.
Cure of Thermosetting Resins
Thermosetting resins are cured by a chemical process that is enhanced by raising
the temperature. The detailed chemistry of this process will not be discussed,
but the basic behavior is common to all systems. The resins are usually supplied
in liquid form, typically with the consistency of a light lubricating oil. There
are usually at least two components, which must be mixed in precise proportions
prior to processing. One component is normally the resin and the other may be
designated curing agents, initiators, accelerators, or catalysts. (The term catalyst
is, however, often used loosely in the industry when the ingredients are not
technically catalysts!) Once mixed they are fully formulated and the chemical
process leading to cure is initiated. Resins may be formulated to cure at ambient
temperature (e.g., 15–25
⬚C) or at elevated temperatures (typically 50–250⬚C)
and over time intervals from a few seconds to several hours, even days. Some
systems, notably the unsaturated polyester resins, are more versatile in this con-
text than others.
The curing reaction results in the development of a dense network of chemical
bonds between the original polymer molecules. On the completion of cure the
resin is solid, cannot be melted, and cannot be dissolved in solvents without
degradation. Most of the thermosets used in composites cure to hard rigid solids,
but it is possible to formulate systems to yield softer or even elastomeric prod-
ucts. The cure process occurs in several stages. First there is often an incubation
period when there is little observable change. Then the viscosity is observed to
increase, and heat arising from the reaction(s) will be evolved. This usually

1050 COMPOSITES FABRICATION PROCESSES
results in a rise in temperature, referred to as the cure exotherm. A consequence
of this temperature rise is that the viscosity of the resin will be decreased, which
assists the processes of infiltration and consolidation. The next stage is gelation:
At this point the resin ceases to be liquid and transforms to a solid, initially it
is rubbery but then becomes progressively harder. The evolution of heat is gen-
erally most intense during the period around gelation. As the cure proceeds the
exotherm becomes weaker and the resin becomes fully hardened or vitrified.
Raising the initial cure temperature by heating will accelerate the cure process.
Gelation will occur sooner and the exotherm will be more intense. Care must
be exercised to ensure that the exothermic heat does not lead to the resin/
laminate becoming excessively hot so that degradation occurs. This is often
observed as burnt regions in the thicker sections of a molding where heat transfer
out of the laminate is less favorable. In extreme cases the molding can com-
pletely disintegrate, or even explode, as a result of excess exotherm! It is for-
tunate that the reinforcement, which does not participate in the reactions, acts
to some degree as an internal heat sink.
Three temperature, viscosity and cure profiles for a laminate cured at different
temperatures are shown in Figs. 7a,7b, and 7 c. Note how the time to gelation
is reduced by raising the cure temperature. For reasons of productivity it is
generally desirable to cure as fast as possible, provided excessive exotherm can
be controlled. However, it is also necessary that the resin is at low viscosity for
a sufficient time interval to allow any infiltration or consolidation processes to
be completed. This may be achieved by balancing the cure temperature profile
and the consolidation pressure.
Processing Thermoplastic Matrix Systems
Thermoplastics, with the exception of the monomer polymerization systems, are
not cured but are processed through melt–freeze cycles. Thermoplastics are ei-
ther amorphous or semicrystalline, with degrees of crystallinity of up to about
75%. On heating to above the melting temperature, T
m
, amorphous polymers
soften over a range of temperatures while the semicrystalline materials melt more
sharply and generally to melts of lower viscosity. Overheating leads to degra-
dation, discoloration, and loss of properties. On cooling back through the freez-
ing temperature, the amorphous materials become gradually more rigid, but the
semicrystalline polymers freeze more sharply and recrystallize. The extent of
crystallinity is dependent on the rate of cooling through the critical region. Fast
cooling leads to a fine substructure but lower overall crystallinity, while slow
cooling has the converse effect. In most cases optimum mechanical performance
of the composite requires that the crystallite size and degree of crystallinity be
controlled. It is therefore necessary to exercise control over cooling rates when
processing thermoplastic systems. A further property of amorphous thermoplas-
tics is the glass transition temperature, T
g
. This transition occurs below the
melting point and at T
⬍ T
g
the polymer behaves as a glass. It is hard, often
brittle, and does not creep significantly under load. At T
⬎ T
g
the polymer is
rubbery and much softer, extendible, and creeps under load. The temperature T
g
effectively defines the upper service temperature for amorphous thermoplastics.
In the case of the semicrystalline thermoplastics only the amorphous portion is
affected by T
g
, so the effect is less severe. Semicrystalline polymers therefore
have higher useful service temperatures and in the temperature interval between
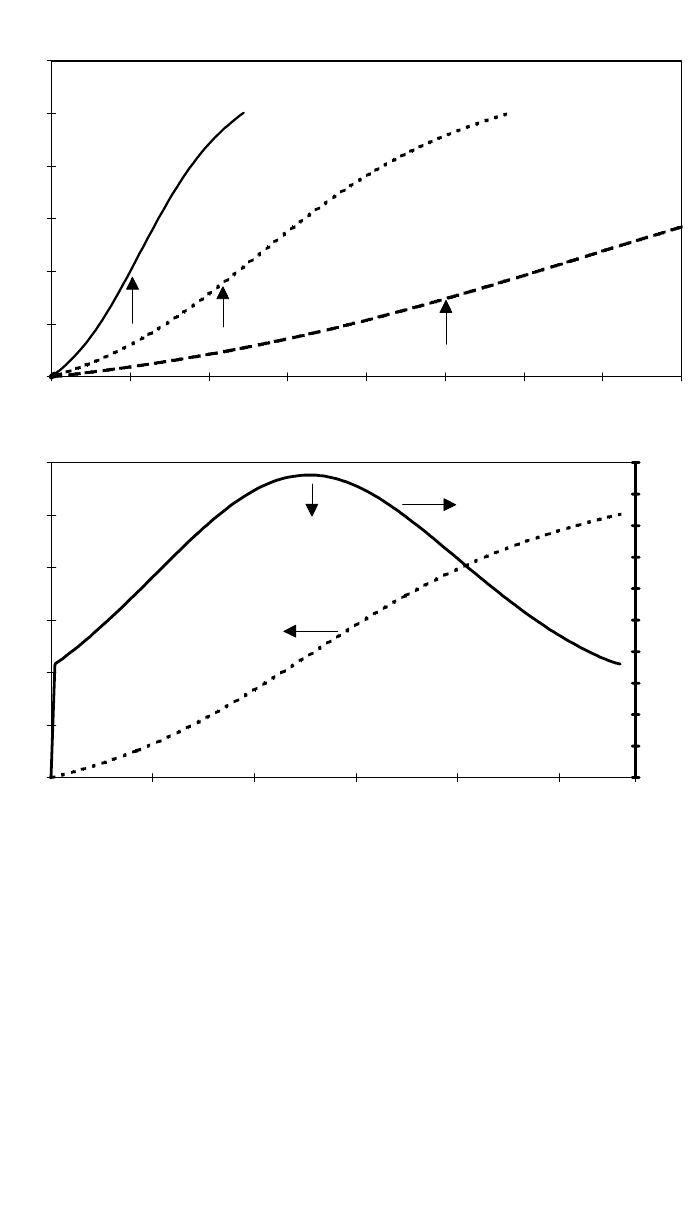
2 BASIC PRINCIPLES FOR PROCESSING 1051
0.0
0.2
0.4
0.6
0.8
1.0
1.2
0 50 100 150 200 250 300 350 400
TIME - min
DEGREE OF CURE
380K = 107°C
400K = 127°C
420K = 147°C
GEL POINT
GEL POINT
GEL POINT
(a)
0.0
0.2
0.4
0.6
0.8
1.0
1.2
0 50 100 150 200 250 300
TIME - min
DEGREE OF CURE
0.000
0.001
0.002
0.003
0.004
0.005
0.006
0.007
0.008
0.009
0.010
RATE OF EXOTHERM
MAX RATE OF HEAT
EVOLUTION
400K = 127°C
ARBITRARY UNITS
(b)
Fig. 7 These figures illustrate the cure characteristics of a typical thermosetting resin, e.g., an
epoxy. (a) Shows the cure profile at three different curing temperatures. The degree of cure is
plotted against time of cure. The cure rate is much faster at the higher temperatures. A temper-
ature increase of only 20⬚C, from 107⬚C to 127⬚C, reduces the gel time from 250 min to just
over 100 min. (b) Cure profile at 127⬚C is shown together with the rate of exotherm. This peaks
soon after gelation. The exotherm rate is higher at higher cure temperatures (not shown). (c)
Resin viscosity profiles for the same resin at the three temperatures is shown. The viscosity in-
creases sharply after the gel point and this defines the workable range, or process window.
T
g
and T
m
are often quite tough. The high melt viscosity of thermoplastics means
that processes dependent on infiltration cannot be used. Most systems are, there-
fore, precompounded or co-mingled.
Heat Transfer
It will have become apparent from the preceding discussion that most processing
operations involve heat flow, to manage heating, cooling, or to control exotherm
or crystallinity. Polymer resins are generally poor thermal conductors and are
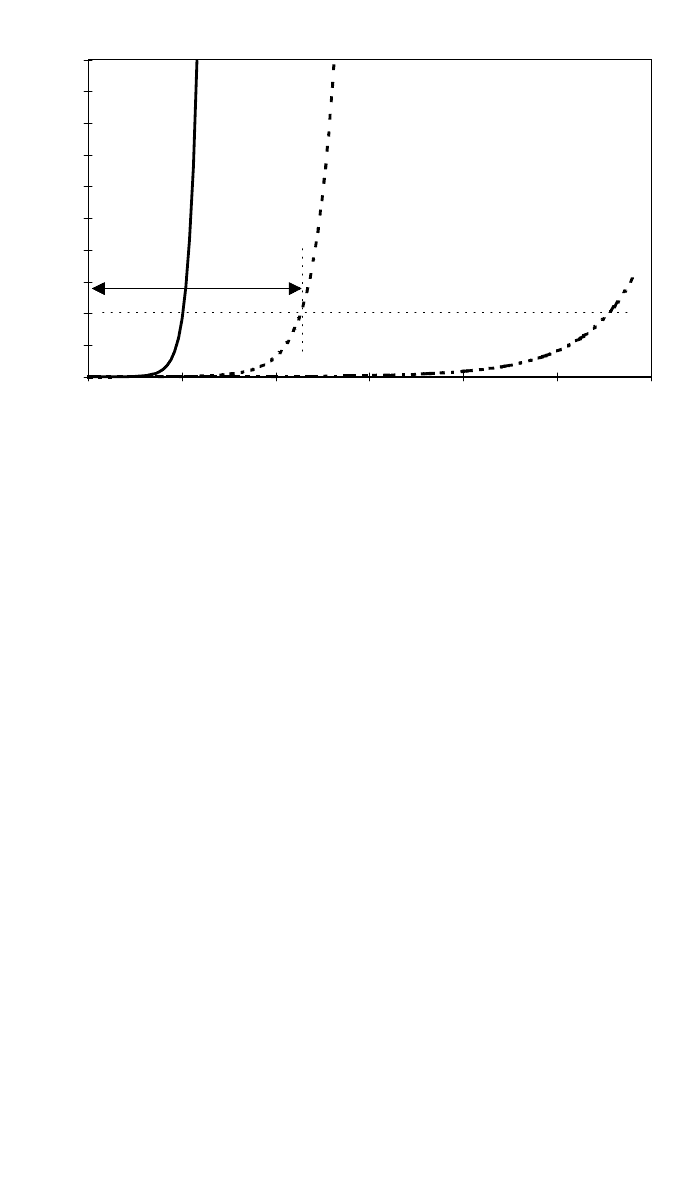
1052 COMPOSITES FABRICATION PROCESSES
0
50
100
150
200
250
300
350
400
450
500
0 50 100 150 200 250 300
TIME - min
VISCOSITY - Pa s
420K = 147°C
400K = 127°C
380K = 107
o
C
RESIN WORKABLE UP TO THIS
VISCOSITY
PROCESS
WINDOW @ 400K
(c)
Fig. 7 (Continued )
sensitive to overheating. Unimpregnated fiber arrays are also very poor conduc-
tors, although some fibers themselves are quite good. The materials used for
tooling may also be poor conductors. This applies particularly to composite
tooling, which is very widely used, also to plaster and concrete. The problem is
often increased by the use of vacuum bags, porous breathers, and bleed plies
over the laminate on the tool. Metal tooling is the exception, but this is only
generally used for the higher production rate processes. It follows that consid-
erable care is needed to manage the heat input and output during processing and
that the time required for heat transfer is a significant and sometimes critical
part of most processing operations. Problems may be minimized by avoidance
of thick sections and by the incorporation of internal heating or cooling into
mold tools.
Flow Processes during Molding
In all processes some flow of resin will occur, but the flow or movement of the
reinforcement warrants further consideration. At one extreme there are processes
that involve vertical consolidation only with no relative movement of the rein-
forcement in other directions. The other extreme is when molding with a com-
pound, such as BMC, where the whole charge, resin plus fiber, flows in all
directions to fill the mold. In reinforcements incorporating straight continuous
fibers, no movement can occur in the fiber direction. Any significant movement
normal to the fibers will result in splitting if there is extension or wrinkling if
there is compression. On the other hand limited shear deformation is possible.
If there is biaxial continuous reinforcement, as in a woven cloth, no stretch can
be accommodated in either direction, compression will result in wrinkling but
again some shear movement is possible.
Reinforcements containing discontinuous fibers, however, are free to flow to
a much greater extent. Sheet molding compound, with chopped roving reinforce-
ment, will flow in three dimensions and allow features such as ribs and bosses

2 BASIC PRINCIPLES FOR PROCESSING 1053
to be molded without deliberate placement of reinforcement at these features.
Continuous random mat will also flow but to a lesser degree. Clearly it is im-
portant to select reinforcement appropriate to the degree of flow necessary to
form the part. See the comment in the following section concerning thermo-
plastic matrix systems.
Drape
This is related to flow but is concerned with the extent to which a reinforcement
will comply to a complex molding surface. A uniaxial prepreg can only be
successfully draped on single curvature surfaces. Double curvature requires ex-
tension or contraction in the lateral direction leading to splits or wrinkles. Double
curvature panels can, however, be formed by use of narrow tapes with small
gaps or overlaps accommodating the misfit. Biaxial continuous fiber reinforce-
ment, in principle, suffers from a similar limitation but can, in fact, accommodate
some draping by tow slippage and shear. Satin and twill weaves have signifi-
cantly better drape characteristics than plain weaves and lighter fabrics drape
more efficiently than heavy. Random mats drape very well, but this is affected
by the type of binder used. A strong binder will prevent effective drape. If the
binder is resin soluble, draping can be effected after the resin has been applied.
With CSM there is a possibility of tearing if the binder is too weak. This leads
to depletion of reinforcement in the torn areas. Continuous strand mat is resistant
to tearing, it drapes well but cannot flow as much as CSM. Both CSM and CRM
are used in precompounded thermoplastic sheet systems. They cannot flow in
any way until the matrix has been melted, but then behavior is similar to that
in thermosets.
2.3 Design of Reinforcements to Enhance Permeability
Following from the discussion of infiltration in Section 2.2, it would appear that
the objectives of obtaining high mechanical properties by maximizing V
ƒ
and
benefiting from high permeability are mutually exclusive. Tightly packed arrays
of small-diameter fibers are highly impermeable, especially in the directions
normal to the fiber axes. The effect of V
ƒ
on permeability is shown in Fig. 5b
for axial and transverse infiltration. The time to infiltrate a 250-mm-long uniform
array of 10-
m-diameter fibers, at a V
ƒ
of 0.5, under a pressure of 2 bars with
a resin of 0.1 Pa/s is calculated to be in excess of 200 h, i.e., practically im-
possible! However if the fibers are arranged as tightly packed tows, of 1 mm
diameter and packed with a V
ƒ
of 0.7 within the tows, and the tows then packed
with a packing factor of 0.7, to give an overall V
ƒ
of 0.49 (⬇0.5), as shown in
Fig. 8, under similar conditions the infiltration time of the array is about 5 s.
Furthermore the transverse infiltration of the tows takes only a further 15 s
because the distance to infiltrate is only about 0.5 mm (half the tow diameter),
so that complete infiltration takes about 20 s. This is a much more realistic
prospect. The key to high permeability with high volume fraction is to provide
a network of relatively large passages through the reinforcement, between tows,
or simply by leaving some gaps between tows. The resin can then quickly in-
filtrate through these large passages by axial macroinfiltration, and then infiltrate
the tows by transverse microinfiltration. These infiltration passages may be in-
corporated into the reinforcement during the weaving operation.
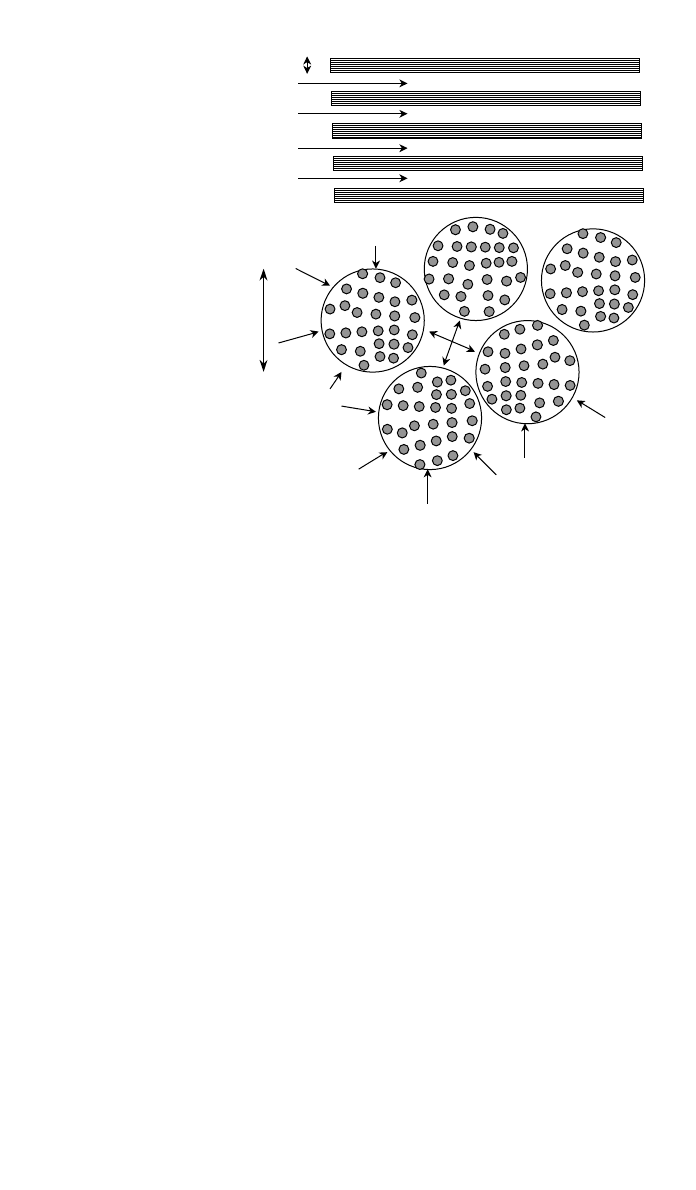
1054 COMPOSITES FABRICATION PROCESSES
d
d
MACRO FLOW:
Longitudinal flow between
fibre bundles.
MICRO FLOW:
Transverse flow into
fibre bundles.
Much slower but distance
only bundle diameter.
Fig. 8 Illustrates the principles of macro- and microflow in an array of fiber bundles. This is the
normal structure of fiber composites. Flow is fast through the relatively large channels between
the bundles. Final infiltration is accomplished by microflow in the transverse direction into the
tightly packed fiber bundle. This is much slower but the infiltration distance is very short.
Another method of speeding up infiltration is to incorporate layers of open
reinforcement, such as CSM, between layers of heavier material such as woven
roving or noncrimp fabric. The distributor ply allows the whole area of the
molding to be infiltrated so that the heavy reinforcement only has to be infiltrated
through its thickness direction. Again the key is to minimize the distance through
which tightly packed fibers need to be infiltrated. This principle is further ex-
ploited in the SCRIMP and RFI processes discussed in later sections.
2.4 Tooling
Most composite laminates are of shell form, often with additional detail such as
stiffening ribs, bosses, and cutouts. These forms may be molded using single-
sided tools of either male or female form. The choice is usually governed by
the geometry of the piece, the logistics of the laminating process, and which
side constitutes the prime surface where dimensions and surface finish need to
be controlled. Ribs or bosses may be incorporated either by cutting these features
into the tool face or, more usually, by building up on the back surface using
additional tool pieces where necessary. Simple one-sided tools may be fabricated
using composites by taking an impression off a master form, usually termed the
plug. The basic impression is a thin shell that is then backed up with stiffeners
and supporting structure to render it sufficiently robust to withstand the chosen
molding process. The mold materials may be E-glass with unsaturated polyester,
vinyl ester or epoxy resin matrices, or carbon fiber with epoxy backed up with
timber or metallic supporting structures. The advantage of carbon fiber is its
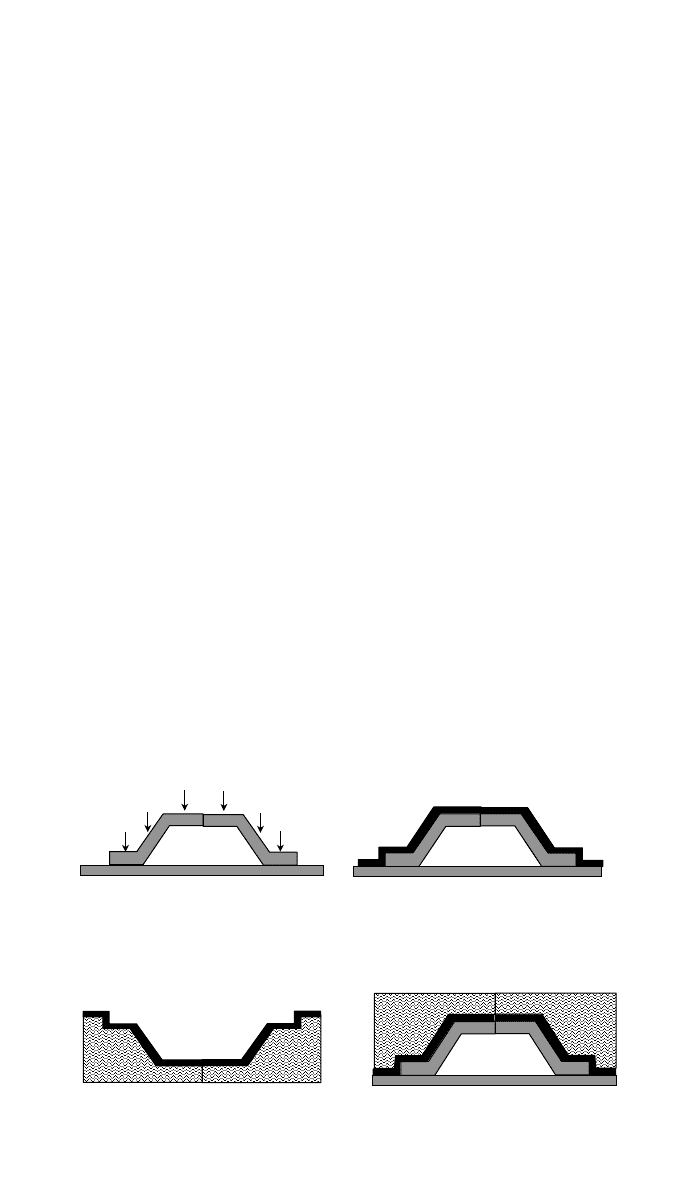
2 BASIC PRINCIPLES FOR PROCESSING 1055
1. Master form - “Plug”
2. CFRP shell laminated onto plug
3. Back-up structure added4. Completed female half-tool
Prime surface
Fig. 9 Composite tool may be manufactured by laminating a CFRP shell onto a master form
or plug. This is then backed-up by a suitable structure to provide the necessary robustness for
subsequent molding operations.
greater stiffness, better thermal conductivity, and, most significant, much lower
coefficient of thermal expansion. This facilitates molding to closely specified
dimensions and is particularly relevant when the molding is also carbon fiber
reinforced. In contact molding, laminates are built up on the mold surface and
simply hand consolidated without application of additional pressure. Alterna-
tively the mold may be vacuum bagged so that one atmosphere of consolidating
pressure may be used. In some instances single-sided tooling may be press
consolidated using an elastomeric counter tool, but note that a more robust sup-
porting structure may be necessary. Single-sided tooling is widely used, with a
vacuum bag for autoclave cure. Some of these options are illustrated in Figs. 9–
11. Double-sided or matched tooling may also be fabricated using composites.
This is again usually used in a press, and therefore size and consolidating pres-
sure will be determined by the equipment available.
Composite tooling is suitable for short to medium production runs. Typically
a tool may be used to mold up to 100 parts before needing extensive reworking.
This figure can, however, vary very widely depending on the complexity of the
molding and the consolidating and cure conditions. Where long runs are re-
quired, the options are multiple tools or metallic tooling. Metallic tools are
inevitably much more costly than composite, but they are more robust and more
durable. A serious problem is the mismatch in thermal expansion between the
composite part and the tool, which causes difficulty in establishing dimensional
control. The options for metal tooling are cast-zinc alloy, cast or fabricated
aluminum alloy, cast iron, fabricated steel, and Invar. The choice depends on the
part size and complexity, surface finish requirements, and thermal expansion
considerations. Invar has the great advantage of virtually zero thermal expansion
over typical molding temperature ranges (20–150
⬚C). It is an alloy of nickel and
iron and may be fabricated using standard steel practices, the cost is much higher
than that of steel. Metal tooling is generally necessary for all high-pressure
