Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.

1.6 Bond Strength 39
energies. These numbers do not have to be known precisely, but it is important to
have a rough idea of the bond strengths of common covalent bonds.
Table 1.9 gives averages over a range of compounds. A few compounds may lie
outside these values. In later chapters more precise specific values will appear.
Let’s consider the simplest molecule, which is H
2
with only one electron and
a positive charge. Even this molecule is bound quite strongly.
10
The small amount
of information already in hand—a molecular orbital picture of H
2
and the bond
strength of the bond (104 kcal/mol)—enables us to
construct a picture of this exotic molecule and to estimate
its bond strength. We can imagine making by allow-
ing a hydrogen atom, to combine with H
, a bare pro-
ton. All the work necessary to create an orbital interaction
diagram for this reaction was done in the construction of
Figure 1.39.We are still looking at the combination of a pair
of hydrogen 1s atomic orbitals, so the building of a diagram
for produces precisely the same orbital diagram,repro-
duced in Figure 1.45. The only difference comes when we
put in the electrons.Instead of having two electrons,as does
H
2
, with one coming from each hydrogen atom, has
only one electron. Naturally, it goes into the lower-energy,
bonding molecular orbital, The electron spin is shown
down, but it could equally well be shown up. There is no
difference in energy between the two spins and we have no
way of knowing which way a single spin is oriented.
What might we guess about the bond energy of this
molecule If two electrons in result in a bond ener-
gy of 104 kcal/mol, it seems reasonable to guess first that
stabilization of a single electron would be worth half the amount, or about
52 kcal/mol.That is, when an atom and an H
ion combine to form the species
we are estimating that 52 kcal/mol of heat is given off. And we are amazing-
ly close to being correct! The molecule is bound by 64 kcal/mol (Fig. 1.45),
which means that the system is 64 kcal/mol more stable than the system of a
separated and H
.H
.
H
2
+
H
2
+
H
2
+
,
H
.
£
B
H
2
+
?
£
B
.
H
2
+
H
2
+
H
.
,
H
2
+
H
O
H
H
2
+
,
10
Here is the answer to Problem 1.22. The simplest molecule is hydrogen (H
2
) minus one electron. Another
electron cannot be removed to give something even simpler because is not a molecule—there are no
electrons to bind the two nuclei.
H
2
2+
TABLE 1.9 Some Average Bond Dissociation Energies
Bond BDE (kcal/mol) Bond BDE (kcal/mol)
96–105 55–57
93–107 175
110–119 143
82–87 173–181
83–90 230
85–96 204
69–75 71
105–115 88
83–85 103
72–74 136H
O
FC
O
Br
H
O
ClC
O
Cl
H
O
BrC
O
F
H
O
IC
O
N
'
C
q
NC
O
O
'
C
q
CC
O
C
C
P
OS
O
H
'
C
P
NO
O
H
'
C
P
CN
O
H
C
O
IC
O
H
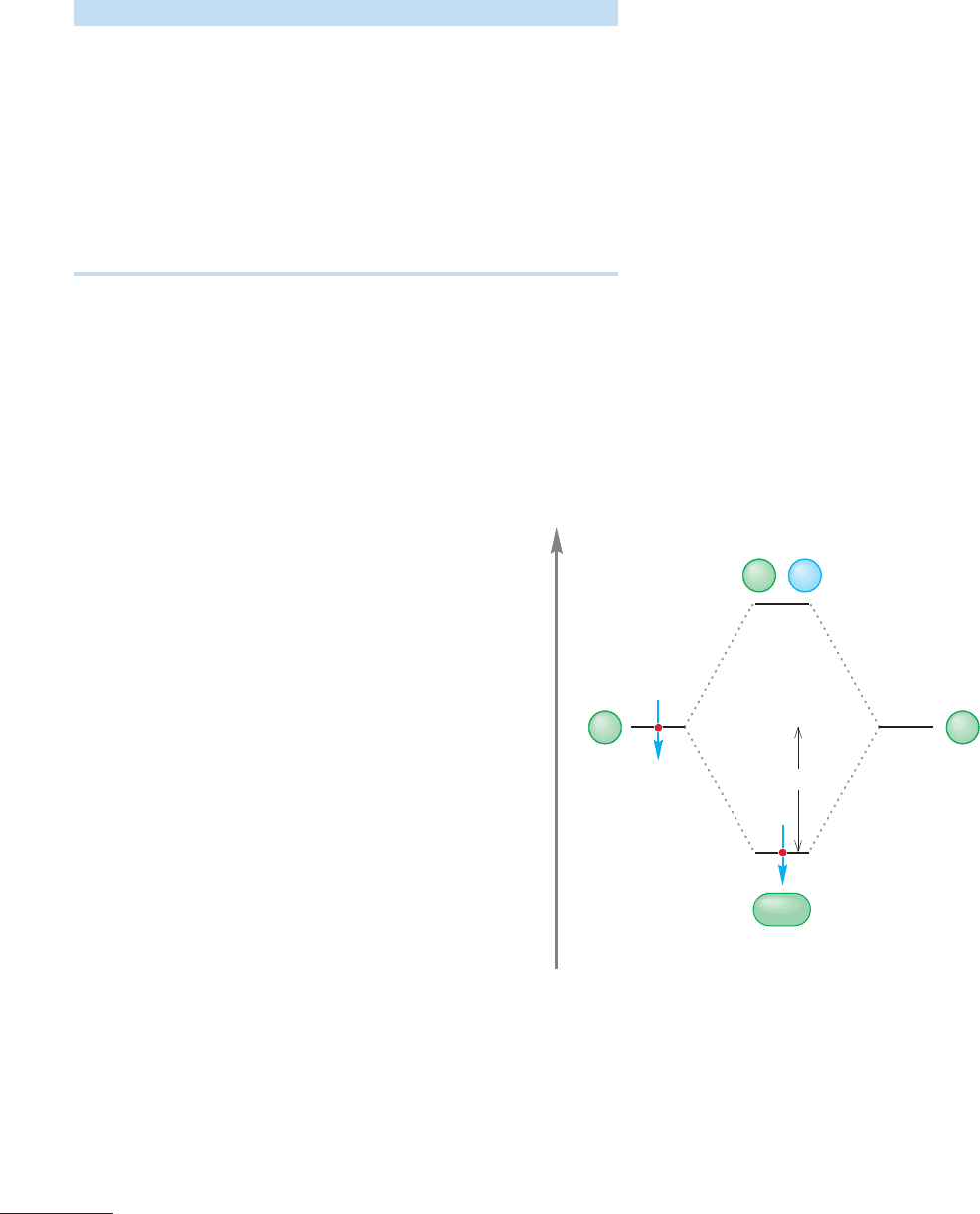
64 kcal/mol
Energy
Φ
A
Φ
B
ψ(H
a
, 1s
1
)
ψ(H
b
, 1s
0
)
+
FIGURE 1.45 An orbital interaction
diagram for .
H
2
+
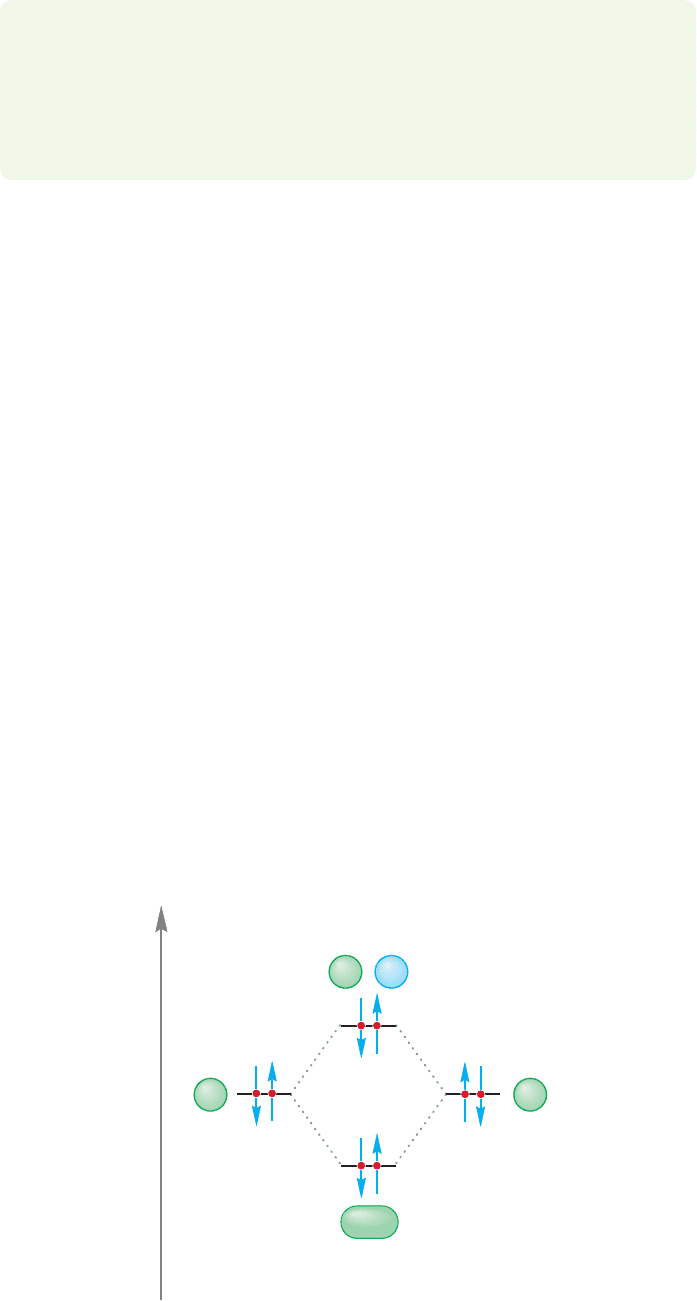
Energy
Φ
A
Φ
B
(He
b
1s
2
)(He
a
1s
2
)
40 CHAPTER 1 Atoms and Molecules; Orbitals and Bonding
PROBLEM 1.27 Although our guess 52 kcal/mol is quite close to the actual value,
it is a bit low. In other words, the molecule is more stable (lower in energy)
than we thought. Why is our estimate of bond strength a little low? To ask the
same question another way, why might the stabilization of two electrons in an
orbital be less than twice the stabilization of one electron in the orbital? Hint:
Consider electron–electron repulsion.
It may seem somewhat counterintuitive that a low-energy (strong) bond is asso-
ciated with a large number for the BDE.The lower the energy of a bond, the high-
er the number representing bond strength! The low-energy, strong bond in H
2
has
a BDE of 104 kcal/mol, for example, whereas the higher-energy, weaker bond in
has a BDE of only 64 kcal/mol. A strong bond means a low-energy, stable
species. The diagram in Figure 1.42 should help keep this point straight. A strong
bond means a more stable bond and it means a lower position on an energy diagram.
Remember the sign convention: The
minus sign tells us that this reaction is exothermic as read from left to right. An
exothermic reaction will have products that are more stable than the starting ma-
terials. The products will be lower than the reactants on the energy diagram.
The antibonding molecular orbital has been empty in all of the examples
we have considered. Why the emphasis on What is an empty orbital, anyway?
The easiest,nonmathematical way to think of in H
2
is as the place the next elec-
tron would go. If the bonding molecular orbital is filled, as it is in H
2
, for exam-
ple (Fig. 1.42), another electron cannot occupy and must go instead into the
antibonding orbital, This would create
Dihelium, He
2
, a strictly hypothetical species, is an example of a molecule in
which electrons must occupy antibonding orbitals. Each He, like each H in H
2
,
brings only a 1s orbital to the mixing. Just as in the molecules H
2
and the
molecular orbitals for He
2
are created by the combination of two 1s atomic orbitals
(Fig. 1.46). Each helium atom brings two electrons to the molecule, though, so the
electronic occupancy of the orbitals will be different from that for H
2
or The
bonding orbital can hold only two electrons (Pauli principle, p. 8) so the next
two must occupy The two electrons in this antibonding orbital are destabiliz-
ing to the molecule, just as the two electrons in are stabilizing. As a result, there
£
B
£
A
.
1£
B
2
H
2
+
.
H
2
+
,
H
2
-
1H
2
+ e
-
U
H
2
-
2.£
A
.
£
B
£
B
£
A
£
A
?
1£
A
2
¢H ° =-104 kcal>mol.2 H
.
U
H
O
H,
H
2
+
H
2
+
FIGURE 1.46 An orbital interaction
diagram for He
2
.There is no net
bonding because the stabilization
owing to the pair of electrons in the
bonding molecular orbital is offset by
the destabilization from the two
electrons in the antibonding orbital.
1.7 An Introduction to Reactivity: Acids and Bases 41
is no net bonding in the hypothetical molecule He
2
. The stabilization afforded by
the two electrons in the bonding molecular orbital is cancelled by the destabiliza-
tion resulting from the two electrons in the antibonding molecular orbital. Molecular
helium He
2
is, in fact, unknown.
Summary
The energy of an electron in a bonding molecular orbital generated through
mixing of two orbitals depends primarily on the extent of mixing between the two
orbitals that combine to produce it. Extensive interaction leads to a low-energy
bonding molecular orbital (and a high-energy antibonding molecular orbital).
Thus, one or two electrons in such an orbital will be well stabilized. The bond
joining the two atoms is strong. Orbitals that are orthogonal do not mix.
PROBLEM 1.28 Draw the orbital interaction diagram for
WORKED PROBLEM 1.29 Estimate the bond strength for
Show how you arrived at your estimate.
ANSWER The molecular orbital diagram for this molecule can
be easily derived from Figure 1.46 by removing one electron.
The molecule can be constructed from He and He
.This
molecule will have only three electrons. Assuming that an elec-
tron in the antibonding orbital destabilizes the molecule
about as much as one in the bonding orbital stabilizes, there
is one net bonding electron in this molecule (2 bonding elec-
trons – 1 antibonding electron 1 net bonding electron). The
should have a bond strength about the same amount as
another molecule with a single electron in the bonding
molecular orbital. This estimate turns out to be correct. The
bond energy for has been experimentally measured to be
about 60 kcal/mol.
PROBLEM 1.30 Use the answer to Problem 1.27 to work out the answer to a more
subtle question. In He
2
, both the bonding and the antibonding molecular orbitals
are filled with two electrons. Consider electron–electron repulsion to explain why
the stabilization of the two electrons in is less than the destabilization of the
electrons in
1.7 An Introduction to Reactivity: Acids and Bases
Even at this early point we can begin to consider what many see as the real business of
chemistry—reactivity. The orbital interaction diagrams we have just learned to draw
lead us to powerful unifying generalizations. Remember that we can fit no more than
two electrons into any atomic or molecular orbital (p. 8). There are two ways to pro-
vide the electrons that fill the bonding molecular orbital so as to stabilize two electrons.
£
A
.
£
B
He
2
+
H
2
+
,
He
2
+
£
B
£
A
He
2
+
He
2
+
.
He
2
+
.
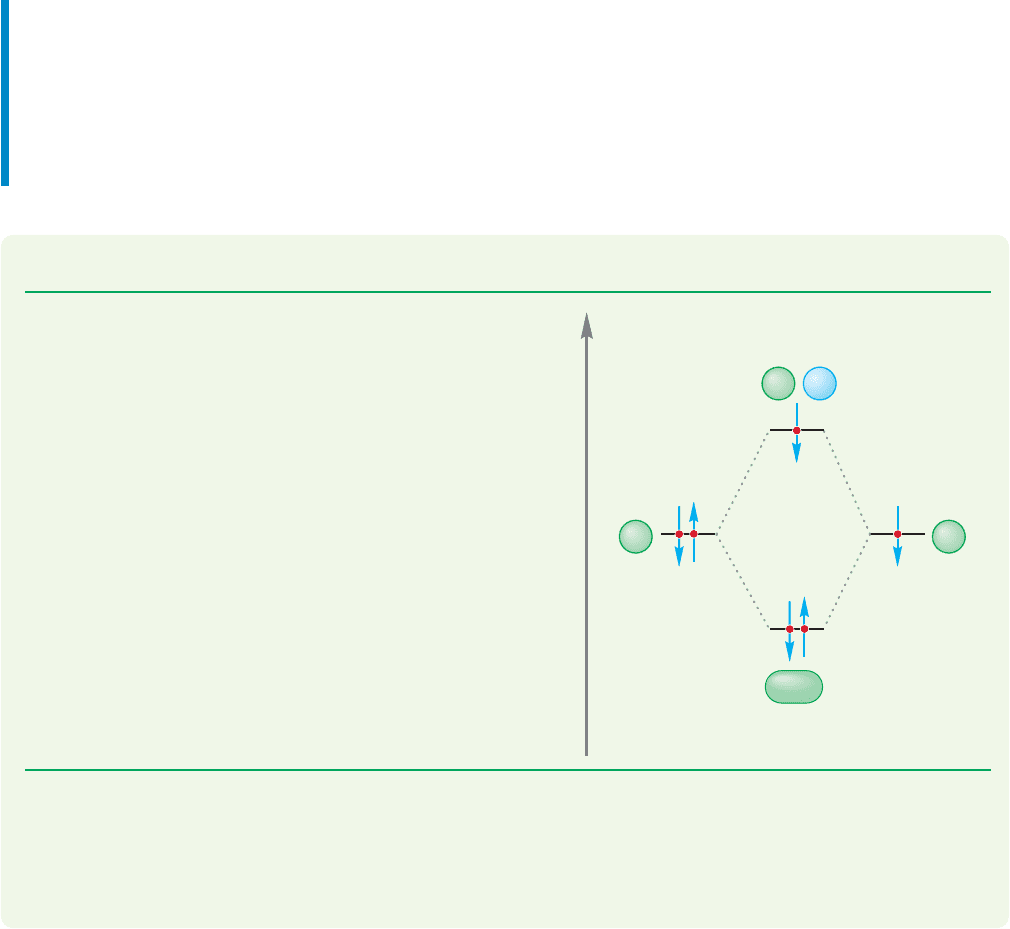
Energy
ψ(He
a
, 1s
2
)
Φ
B
Φ
A
ψ(He
b
, 1s)
+
42 CHAPTER 1 Atoms and Molecules; Orbitals and Bonding
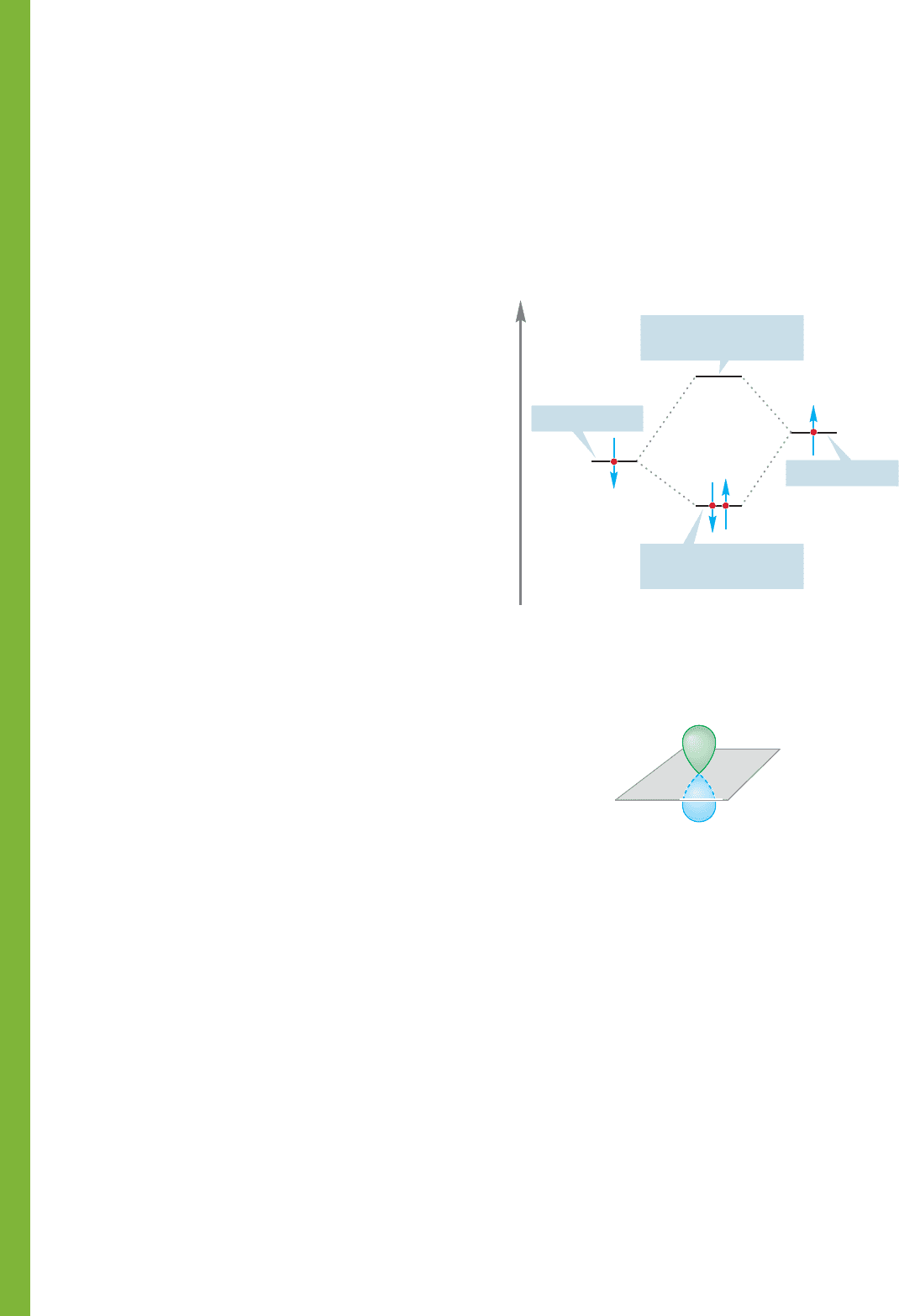
Figure 1.47a shows a pair of
electrons, each in a singly
occupied orbital, combining
to produce a bond in which
the two electrons are stabi-
lized (both electrons move
down to a lower energy
value). Alternatively, we
could imagine the situation
outlined in Figure 1.47b, in
which a filled orbital mixes
with an empty orbital to
produce a similar stabiliza-
tion. An atom or molecule that is a two-electron donor is called a Lewis base.An
atom or molecule that is a two-electron acceptor is called a Lewis acid.These labels
were introduced by G. N. Lewis (p. 14).
Both these bond-forming scenarios are common,but the scenario in Figure 1.47b
is especially important. We have already seen an example of the process shown in
Figure 1.47a, when we considered a pair of hydrogen atoms combining to form H
2
(Fig. 1.42). We have not yet seen any real examples of the Lewis acid–Lewis base
situation, but we will encounter hundreds as we work through this book.
You are probably familiar with Brønsted acids and bases (proton donors and
acceptors, respectively), but the situation described by Figure 1.47b is much more
general. It would seem that a stabilizing (energy-lowering) bond-forming interac-
tion can take place whenever a filled orbital overlaps with an empty orbital. We will
give this important idea the careful treatment it deserves in Chapters 3 and 7, but
it is worth thinking about it a bit right now.
Organic chemists use the terms electrophile for Lewis acid and nucleophile for
Lewis base. The combination of a Lewis base and a Lewis acid (or nucleophile
electrophile), conceptualized in the orbital interaction diagram of Figure 1.47b, leads
to formation of a covalent bond.The concepts that “Lewis bases react with Lewis acids”
and “the interaction of a filled orbital with an empty orbital leads to bond formation
and stabilization of two electrons”run throughout organic (and other) chemistry.These
two concepts allow us to generalize and not to be swamped by all the prolific detail
soon to come. Nucleophile electrophile is the unifying theme of organic chemistry.
FIGURE 1.47 Two interactions that
lead to stabilization of a pair of
electrons.
11
By Walter Kauzmann (1916–2009) in an early book,Quantum Chemistry, Academic Press, New York, 1957.
Energy
Stabilization
(a) (b)
PROBLEM 1.31 Identify the Lewis base (nucleophile) and Lewis acid (electrophile)
in each of these reactions. Write the molecule formed in each case.
(a) (b)
(c) (d)
H
3
C
+
:
NH
3
U
?H
3
C
+
-
:
CH
3
U
?
H
+
-
:
O
.
.
.
.
H
U
?H
+
H
:
-
U
?
1.8 Special Topic: Quantum Mechanics and Babies
We might imagine that molecular orbitals and quantum mechanics,so critical in the
microscopic world, would have little real influence on our day-to-day lives because
we really do live in a world of baseballs, not electrons. Or so it would seem. Yet it
has been argued
11
that “a new-born baby becomes conscious of . . . the consequences

1.9 Summary 43
[of the Pauli principle] long before he finds it necessary to take account of the con-
sequences of Newton’s laws of motion.” How so? Ernest Rutherford (p. 2) told us
long ago that an atom is essentially empty space because the nucleus is very small
compared to the size of a typical atom, and electrons are even tinier than the nucle-
us. Why is it then, that when “solid” objects come together they do not smoothly
pass through one another, as they sometimes do in 3
A.M. science fiction movies?
The answer is that the apparent solidity of matter is the result of Pauli forces.
When your hand encounters the table top, the electrons in the atoms of your hand
and the electrons in the atoms of the table top have the same spins and nearly the
same energies. The Pauli principle ensures that these electrons cannot occupy the
same regions of space, that the table top feels solid to your touch, even though
Rutherford showed us that it is not. Were it not for the Pauli principle our appre-
hension of the world would be vastly different.
Atomic orbitals (mathematically described by wave functions)
are defined by four quantum numbers, n, l, m
l
, and s. Electrons
may have only certain energies determined by these quantum
numbers. Orbitals have different, well-defined shapes: s orbitals
are spherically symmetric, p orbitals are roughly dumbbell
shaped, d and f orbitals are more complicated. An orbital may
contain a maximum of two electrons.
Some molecules cannot be well described by a single Lewis
structure but are better represented by two or more different
electronic descriptions. This phenomenon is called resonance.Be
sure you are clear on the difference between an equilibrium
between two different molecules and the description of a single
molecule using a number of different resonance forms.
When two orbitals overlap, two new orbitals are formed:
one lower in energy than the starting orbitals, the other higher
in energy than the starting orbitals. Electrons placed in the new,
lower-energy orbital are stabilized because their energy is low-
ered. The overlap of atomic orbitals to form molecular orbitals
and the attendant stabilization of electrons as they move from
the atomic orbitals to the lower-energy molecular orbitals are
the basis of covalent bonding.
This orbital-forming process can be generalized: when you
mix n orbitals into the calculation, the mathematical solution
will produce n new orbitals.
anion (p. 4)
antibonding molecular orbital (p. 32)
arrow formalism, curved arrow formalism,
or electron pushing (p. 23)
atom (p. 2)
atomic orbital (p. 3)
aufbau principle (p. 8)
bond dissociation energy (BDE) (p. 37)
bonding molecular orbital (p. 32)
cation (p. 4)
covalent bond (p. 6)
delocalization (p. 27)
dipole moment (p. 14)
electron (p. 2)
electron affinity (p. 4)
electronegativity (p. 15)
electrophile (p. 42)
endothermic reaction (p. 36)
enthalpy change (¢H°) (p. 36)
exothermic reaction (p. 36)
formal charge (p. 20)
functional group (p. 22)
Heisenberg uncertainty principle (p. 2)
heterolytic bond cleavage (p. 37)
homolytic bond cleavage (p. 37)
Hund’s rule (p. 9)
ion (p. 4)
ionic bond (p. 5)
ionization potential (p. 4)
Lewis acid (p. 42)
Lewis base (p. 42)
Lewis structure (p. 14)
lone-pair electrons (p. 14)
molecular orbital (p. 3)
node (p. 6)
nonbonding electrons (p. 14)
nonbonding orbital (p. 32)
nucleophile (p. 42)
nucleus (p. 2)
octet rule (p. 4)
orbital (p. 3)
orbital interaction diagram (p. 33)
orthogonal orbitals (p. 34)
paired spin (p. 8)
parallel spin (p. 10)
Pauli principle (p. 8)
polar covalent bond (p. 14)
quantum numbers (p. 6)
resonance arrow (p. 23)
resonance forms (p. 22)
tautomers (p. 29)
unpaired spin (p. 8)
valence electrons (p. 16)
wave function (ψ) (p. 6)
weighting factor (p. 26)
Key Terms
1.9 Summary
New Concepts
44 CHAPTER 1 Atoms and Molecules; Orbitals and Bonding
There are no reactions to speak of yet, but we have developed a
number of tools in this first chapter.
Lewis structures are drawn by representing each valence
electron by a dot. These dots are transformed into vertical arrows
if it is necessary to show electron spin. Exceptions are the ls
electrons, which are held too tightly to be importantly involved
in bonding except in hydrogen. These electrons are not shown in
Lewis structures except in H and He. Somewhat more abstract
representations of molecules are made by showing electron pairs
in bonding orbitals as lines—the familiar bonds between atoms.
The double-headed resonance arrow is introduced to cope
with those molecules that cannot be adequately represented by a
single Lewis structure. Different electronic representations, called
resonance forms, are written for the molecule. The molecule is
better represented by a combination of all the resonance forms.
Be very careful to distinguish the resonance phenomenon from
chemical equilibrium. Resonance forms give multiple descrip-
tions of a single species. Equilibrium describes two (or more)
different molecules.
The curved arrow formalism is introduced to show the flow
of electrons. This critically important device is used both in
drawing resonance forms and in sketching electron flow in reac-
tions throughout this book.
The formation of a bonding molecular orbital (lower in
energy) and an antibonding molecular orbital (higher in energy)
from the overlap of two atomic orbitals can be shown in an
orbital interaction diagram (Fig. 1.48). Electrons are represented
by vertical arrows which also show electron spin ( or ).
Remember that only two electrons can be stabilized in the
bonding orbital and that two electrons in the same orbital must
have opposite spins.
[\
Reactions, Mechanisms, and Tools
New molecular orbital 2
antibonding
New molecular orbital 1
bonding
Atomic orbital 1
Atomic orbital 2
Energy
FIGURE 1.48 The overlap of two atomic orbitals produces two
new molecular orbitals.
Here, and in similar sections throughout the book, we will take
stock of some typical errors made by those who attempt to
come to grips with organic chemistry.
Electrons are not baseballs. Nothing is harder for most stu-
dents to grasp than the consequences of this observation.
Electrons behave in ways that the moving objects in our ordi-
nary lives do not. No one who has ever kicked a soccer ball or
caught a fly ball can doubt that on a practical level it is possible
to determine both the position and speed of such an object at
the same time. However, Heisenberg demonstrated that this is
not true for an electron. We cannot know both the position and
speed of an electron at the same time.
Baseballs move at a variety of speeds (energies), and a base-
ball’s energy depends only on how hard we throw it. Electrons
are restricted to certain energies (orbitals) determined by the val-
ues of quantum numbers. Electrons behave in other strange and
counterintuitive ways. For example, we have seen that a node is a
region of space of zero electron density. Yet, an electron occupies
an entire 2p orbital in which the two halves are separated by a
nodal plane. A favorite question, How does the electron move
from one lobe of the orbital to the other? simply has no mean-
ing. The electron is not restricted to one lobe or the other but
occupies the whole orbital (Fig. 1.49). Mathematics makes these
properties seem inevitable; intuition, derived from our experi-
ence in the macroscopic world, makes them very strange.
It is easy to confuse resonance with equilibrium. On a mun-
dane, but nonetheless important level, this confusion appears as a
misuse of the arrow convention. Two arrows separate two entirely
different molecules (A and B), each of which might be described
by several resonance forms.The amount of A and B present at
equilibrium depends on the equilibrium constant. The double-
headed resonance arrow separates two different electronic
descriptions (C and D) of the same species, E (Fig. 1.29).
Constructing molecular orbitals through combinations of
atomic orbitals (or other molecular orbitals) can be daunting, at
least in the beginning. Remember these hints:
1. The number of orbitals produced at the end must equal the
number at the beginning. If you start with n orbitals, you
must produce n new orbitals. Here is a way to check your
work as it proceeds.
Common Errors
.
FIGURE 1.49 An electron occupies an entire 2p (or other)
orbital—it is not restricted to only one lobe.

1.10 Additional Problems 45
2. Keep the process as simple as you can. Use what you know
already, and combine orbitals in as symmetrical a fashion as
you can.
3. The closer in energy two orbitals are, the more strongly
they interact. At this level of theory, you need only interact
the pairs of orbitals closest in energy to each other.
4. When orbitals interact in a bonding way (same sign of the
wave function), the energy of the resulting orbital is lowered;
when they interact in an antibonding way (different signs of
the wave function), the energy of the resulting orbital is raised.
5. To order the product orbitals in energy, count the nodes. For
a given molecule, the more nodes in an orbital, the higher it
is in energy.
These methods will become much easier than they seem at
first, and they will provide a remarkable number of insights into
structure and reactivity not easily available in other ways.
The sign convention for exothermic and endothermic
reactions seems to be a perennial problem. In an exothermic
reaction, the products are more stable than the starting materials.
Heat is given off in the reaction and is given a minus sign.
Thus, for the reaction An
endothermic reaction is just the opposite. Energy must be
applied to form the less stable products from the more stable
starting material. The sign convention gives a plus sign.
Thus, ¢H ° =+x kcal>mol.A + B
U
C,
¢H °
¢H ° =-x kcal>mol.A + B
U
C,
¢H °
PROBLEM 1.32 Draw Lewis dot structures for the following
compounds:
(a) CH
3
NO
2
(nitromethane, used for fuel in stock car racing)
(b) (vinyl chloride used to make polyvinyl
chloride)
(c) CH
3
CO
2
H (acetic acid, the acid in vinegar)
(d) HOSO
2
OH (sulfuric acid, H
2
SO
4
, the world’s most widely
used industrial chemical)
See the inside front cover for structures, if you don’t know them.
PROBLEM 1.33 Draw two resonance structures for each of the
compounds in the previous problem. Show the arrow pushing
for interconverting the resonance forms for each compound.
PROBLEM 1.34 Use the arrow formalism to write structures
for the resonance forms contributing to the structures of the
following ions:
..
..
Carbonate ion
C
OO
O
(a)
..
..
..
..
..
..
–
–
Sulfate ion
(b)
O
..
..
..
O
..
..
..
..
..
..
..
S
O
O
––
Nitrate ion
(c)
+
..
..
N
OO
O
..
..
..
..
..
..
––
Guanidinium ion
(d)
....
+
C
NH
2
H
2
N
NH
2
A vinyl ammonium ion
(e)
+
C
N(CH
3
)
3
H
2
C
H
CH
2
P
CHCl
PROBLEM 1.35 Use the arrow formalism to write resonance
forms contributing to the structures for the following molecules:
1.10 Additional Problems
..
..
..
(e)
(f)
..
–
..
..
..
NO
+
–
CCH
3
CH
3
+
NC
CH
3
NH
3
C
H
3
C
(d)
(c)
–
.. ..
..
CH
3
C
+
NNCH
3
+
–
NCH
3
C
H
3
C
H
3
C
C
..
..
+
–
NH
2
C
(a) (b)
N
..
..
..
..
NH
3
C
N
+
–
N
..
..
..
PROBLEM 1.36 Draw three resonance structures for each of
the following:
(a)
CH
2
NO
2
(b) CH
3
CO
2
CH
3
(c) (d) HOSO
2
O
PROBLEM 1.37 Draw resonance forms for the following cyclic
molecules:
H
H
H
H
H
H
HH
H
H
H
H
(a) (b)
(c)
..
CC
C
H
HH
–
H
H
C
C
CC
C
C
+
C
C
CC
C
C
C
+
-
CH
2
CO
2
-

46 CHAPTER 1 Atoms and Molecules; Orbitals and Bonding
PROBLEM 1.38 Draw resonance forms for the following
acyclic molecules:
PROBLEM 1.39 Ozone (O
3
) resembles the molecules in
Problem 1.35. These days it has a rather bad press, as it is
present in too small an amount in the stratosphere and too
great an amount in cities. Write a Lewis “dot” structure for
ozone and sketch out contributing resonance forms. Write one
neutral resonance form. Be careful with this last part; the
answer is tricky.
PROBLEM 1.40 Draw two resonance structures for each of the
compounds shown below.
PROBLEM 1.41 Which “resonance structure” does not contribute
to the molecule CH
3
NOCH
2
. Why doesn’t it contribute?
PROBLEM 1.42 Add charges to the following molecules where
necessary:
PROBLEM 1.43 Add charges to the following molecules where
necessary:
(a) (b) (c)
(d) (e) (f)
H
3
CH
O
H
..
..
H
3
C
O
H
3
C
H
O
..
..
..
..
H
3
CH
S
H
..
..
H
3
C H
3
C
H
S
..
..
..
..
S
(a) (b) (c) (d)
H
2
CCH
2
O
..
..
..
..
..
..
..
..
H
2
CCH
2
N
H
2
CCH
2
Br
H
2
CCH
2
S
(a)
H
3
CH
3
CH
3
CCH
2
N
O
–
–
++
(b)
CH
2
N
..
..
..
O
..
..
(c)
CH
2
N
O
..
..
..
(a)
H
3
CC
C
HO
C
H
CH
3
(b)
H
3
C
O
N
C
O
O
O
(a)
H
2
CC
C
HH
C
H
–
CH
2
(b)
H
2
C
C
C
HH
C
H
CH
2
..
–
..
PROBLEM SOLVING
What to do with a “strange”atom like S or P in Problem
1.43? Organic chemistry is largely, but not entirely,
confined to the second row of the periodic table, so what
do you do when you encounter some atom that is not in
the second row? First, don’t panic—if you know the
second row of the periodic table, you can deal with most
of the “strange” atoms you will see. But you do have to
know a few more things. First, P, Si, and S sit right below
N, C, and O. Second, the halogens form a series: F, Cl,
Br, I. If you know the electron counts for the second-row
elements, you don’t have to go through the more
elaborate counting necessary to deal with elements in
subsequent rows. If neutral trivalent N has a pair of
nonbonding electrons, so does P. If neutral divalent O
has two pairs of electrons, so does S. Therefore, HOH
and HSH are related. If tetravalent N is positively
charged, as in H
4
N
, so is tetravalent P as in H
4
P
.
We’ll deal later with atoms further down in the
periodic table.
(g) (h) (i)
(j) (k) (l)
H
3
CH
H
..
..
H
3
C
H
N
H
3
C
H
H
..
..
H
3
CH
P
N
..
P
..
H
3
C
H
H
NH
H
3
C
H
H
PH
PROBLEM 1.44 Determine the formal charge, if there is one,
for each of the nitrogens in the following molecules:
H
H
H
HH
(a)
N
C
CC
C
N
..
H
H
H
(b)
H
H
H
H
H
H
N
C
CC
C
N
..
..
..
(d)
H
H
H
(c)
N
..
H
N
H
H
N
H
N
..
..
..
..
H
N
1.10 Additional Problems 47
PROBLEM 1.53 Would you expect formaldehyde, shown below,
to have a greater dipole moment than carbon monoxide (see
Problem 1.52)? Why or why not?
PROBLEM 1.54 Consider three possible structures for methyl-
ene fluoride (CH
2
F
2
), one tetrahedral (structure A), the others
flat (structures B and C). Does the observation of a dipole
moment in CH
2
F
2
allow you to decide between structures A
and B? What about structures A and C?
PROBLEM 1.55 Draw the electron pushing for the homolytic
cleavage of Br
2
. Draw the electron pushing for heterolytic cleav-
age of Br
2
.
PROBLEM 1.56 While wandering in an alternative universe
you find yourself in a chemistry class and, quite naturally, you
glance at the periodic table on the wall. It looks (in part) like
this:
Deduce the allowed values for the four quantum numbers in
the alternative universe.
PROBLEM 1.57 In a different alternative universe, the follow-
ing restrictions on quantum numbers apply:
n 1, 2, 3, . . .
l n 1, n 2, n 3,...,0
m
l
l 1, l,...,0,...,l 1
Call the elements in this universe 1, 2, 3, . . . and assume that
Hund’s rule and the Pauli principle still apply.
(a) For n 1, 2, and 3 show what orbital subshells are available
and label them as s, p, d, and so on.
(b) How many electrons can be accommodated in the first three
shells (n 1, 2, and 3) in this universe?
(c) Provide electronic descriptions for elements 1 through 14 in
this universe. (In our universe Li would be written 1s
2
2s.)
(d) Construct a periodic table for elements 1 through 23 in this
universe.
s = 앐
1
2
1
H
2
He
3
Li
4
Be
5
B
6
C
13
Al
14
Si
7
N
8
O
15
P
16
S
9
F
10
Ne
17
Cl
18
Ar
11
Na
12
Mg
19
K
20
Ca
C
A
F
F
H
H
B
C
H
F
F
H
C
C
H
H
F
F
C
H
H
..
..
O
PROBLEM 1.45 Determine the formal charges, if any, for the
molecules shown below.
PROBLEM 1.46 Write Lewis dot structures for the neutral
diatomic molecules F
2
and N
2
. In F
2
, there is a single bond
between the two atoms, but in N
2
there is a triple bond between
the two atoms.
PROBLEM 1.47 Atomic carbon can exist in several electronic
states, one of which is (of course) lowest in energy and is called
the “ground state.” Write the electronic description for the
ground state and at least two higher energy, “excited states.”
PROBLEM 1.48 Write the electronic configurations for the fol-
lowing ions:
(a) Na
(b) F
(c) Ca
2
PROBLEM 1.49 Write the electronic configurations for the atoms
in the fourth row of the periodic table,
19
K through
36
Kr. Hint:
The energy of the 3d orbitals falls between that of the 4s and 4p
orbitals. Don’t worry about the m
l
designations of the 3d orbitals.
PROBLEM 1.50 Write electronic configurations for
14
Si,
15
P,
and
16
S. Indicate the spins of the electrons in the 3p orbitals
with a small up or down arrow.
PROBLEM 1.51 There is an instrument, called an electron spin
resonance (ESR) spectrometer, that can detect “unpaired spin.”
In which of the following species would the ESR machine find
unpaired spin? Explain.
(a) O (b) O
(c) O
2
(d) Ne
(e) F
PROBLEM 1.52 For the Lewis structure of carbon monoxide
shown below, first verify that both the carbon and the oxygen
atoms are neutral.
Second, indicate the direction of the dipole moment in this
Lewis structure:
As you have just shown, based on this Lewis structure, carbon
monoxide should have a substantial dipole moment. In fact, the
experimentally determined dipole moment is very small, 0.11 D.
Draw a second resonance structure for carbon monoxide, verify the
presence of any charges, and indicate the direction of any dipole in
this second resonance form. Finally, rationalize the observation of
only a very small dipole moment in carbon monoxide.
:
C
P
O
.
.
:
(a)
C
H
H
H
Al
H
H
(b)
C
H
H
H
Al
HH
H
(c)
H
H
Al
H
(d)
H
H
Al
HH
48 CHAPTER 1 Atoms and Molecules; Orbitals and Bonding
PROBLEM 1.58 In Problem 1.25, you looked at two possible
interactions of a pair of 2p orbitals: side-by-side and
perpendicular, or end-on. Now let a pair of 2p orbitals
interact end to end, and draw the two new molecular orbitals
produced.
PROBLEM 1.59 Indicate whether the following reactions are
exothermic or endothermic. Estimate by how much. Use
Table 1.9 (p. 39) and 66 kcal/mol for the “double” part of
.
PROBLEM 1.60 Let’s extend our discussion of a
little bit to make the orbitals for the molecule linear
HHH. Use the molecular orbitals for H
2
and the 1s atomic
orbital of H. Place the new H in between the two hydrogen
atoms of . Watch out for net-zero (orthogonal)
interactions!
(a) How many orbitals will there be in linear HHH?
(b) Use the molecular orbitals of H
2
and the 1s orbital of
hydrogen to produce the molecular orbitals of linear HHH.
Sketch the new orbitals.
(c) Order the new orbitals in energy (count the nodes).
HH
H
2
, ⌽
B
, ⌽
A
HHH
H, 1s
H
H Atomic
orbital
+
–
Molecular
orbitals
Molecular orbitals
for HHH
H
2
+
–
H
O
H
H
O
H
HH
(a)
(b)
(c)
(d)
H
3
CH
3
C
H
3
C
H
H
2
C
H
2
C
CH
2
.
H
3
C
.
H
2
CCH
2
CH
2
H H
H
2
C
CH
2
H
Br
+
+
+
..
.
..
..
Cl
..
.
..
..
Br
..
..
..
Cl
..
..
..
Cl
..
..
..
Cl
..
..
..
+
C
P
C
+
–
The following molecular orbital problems are more challeng-
ing than the earlier ones, and may be fairly regarded as
“special topics.” Nonetheless, they do provide some remark-
able insights, and for those who like orbital manipulation,
they will actually be fun. Just remember the “rules” outlined
on p. 34.
PROBLEM 1.61 Make a set of molecular orbitals very much like
the ones you made for linear HHH in Problem 1.60, but this
time use 2p orbitals, not 1s orbitals. Place one 2p orbital
between the other two. Use the molecular orbitals 2p 2p and
2p 2p that you constructed in Problem 1.25. Order the new
orbitals in energy.
PROBLEM 1.62 One can generate the molecular orbitals for
triangular H
3
simply by bending the orbitals generated in
Problem 1.60 to transform the linear molecule into the
bent one.
(a) Bend the three molecular orbitals for HHH to make the
orbitals for the triangle. Make careful drawings. Note that
as the old H(1) and H(3) come closer together in the
triangle they will create a new bonding or antibonding
interaction.
(b) Order the new molecular orbitals in energy by counting the
nodes.
PROBLEM 1.63 Which will be lower in energy, linear or trian-
gular What a sophisticated question! Yet, given the
answer to Problems 1.60 and 1.62, it is easy.
PROBLEM 1.64 In the chapter, we made the two molecular
orbitals for H
2
(p. 34), and in Problem 1.60 we used the two
molecular orbitals of H
2
and a 1s orbital to make HHH.This
time, generate the molecular orbitals for HHHH, linear H
4
,
from the molecular orbitals of two H
2
molecules placed end to
end. Remember: At this level of “theory,” you need only interact
orbitals closest in energy.
Order the new molecular orbitals in energy by counting nodes,
and place the proper number of electrons in the orbitals.
HHHH
+
–
HHHH
H
3
+
?
+
–
