Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.

2.5 Ethane (C
2
H
6
), Ethyl Compounds (C
2
H
5
X), and Newman Projections 69
and the dissociation of ethane into two methyl radicals is endothermic by 90 kcal/mol:
ΔH ° 90 kcal/mol
Just as we imagined replacing one of the hydrogens in methane with an X group
to generate the series of methyl compounds, so we can produce ethyl compounds
( ) from ethane. Indeed, we can now generalize: Substituted alkanes
are called alkyl compounds, often abbreviated , where R stands for a
generic alkyl group. Table 2.3 shows some common ethyl compounds and a few of
their physical properties.
R
O
X
CH
3
CH
2
O
X
CH
3
.
+
.
H
3
C
O
CH
3
U
H
3
C
TABLE 2.3 Some Simple Derivatives of Ethane: Ethyl Compounds
Common Name mp (°C) bp (°C) Physical Properties
Ethane 183.3 88.6 Colorless gas
Ethyl alcohol or ethanol 117.3 78.5 Colorless liquid
Ethylamine 81 16.6 Colorless gas/liquid
Ethyl bromide 118.6 38.4 Colorless liquid
Ethyl chloride 136.4 12.3 Colorless gas/liquid
Ethyl cyanide or propionitrile 92.9 97.4 Colorless liquid
Ethyl fluoride 143.2 37.7 Colorless gas
Ethyl iodide 108 72.3 Colorless liquid
Ethanethiol or ethyl mercaptan 144 35 Colorless liquid
CH
2
CH
3
Ethyl cation (reactive intermediate)
CH
2
CH
3
Ethyl anion (reactive intermediate)
CH
2
CH
3
Ethyl radical (reactive intermediate)
.
:
CH
3
CH
2
O
SH
CH
3
CH
2
O
I
CH
3
CH
2
O
F
CH
3
CH
2
O
CN
CH
3
CH
2
O
Cl
CH
3
CH
2
O
Br
CH
3
CH
2
O
NH
2
CH
3
CH
2
O
OH
CH
3
CH
2
O
H
CH
3
CH
2
O
X
(a)
(b)
Replace this H
Replace this H
Replace this H,
or any other H
HH
H
H
C
H
H
C
HH
H
H
C
H
H
C
HH
HX
H
C
H
C
HX
H
H
C
H
H
C
HH
H
HH
H
CC
HX
H
HH
H
CC
Are these two ethyl
compounds the same?
They don't look the sam
e
in this representation,
but they are!
FIGURE 2.24 (a) A two-dimensional
representation of ethane suggests
that there are two different kinds of
ethyl compound, .
(b) We must look at ethane in three
dimensions to see why there is only
one .CH
3
CH
2
O
X
CH
3
CH
2
O
X
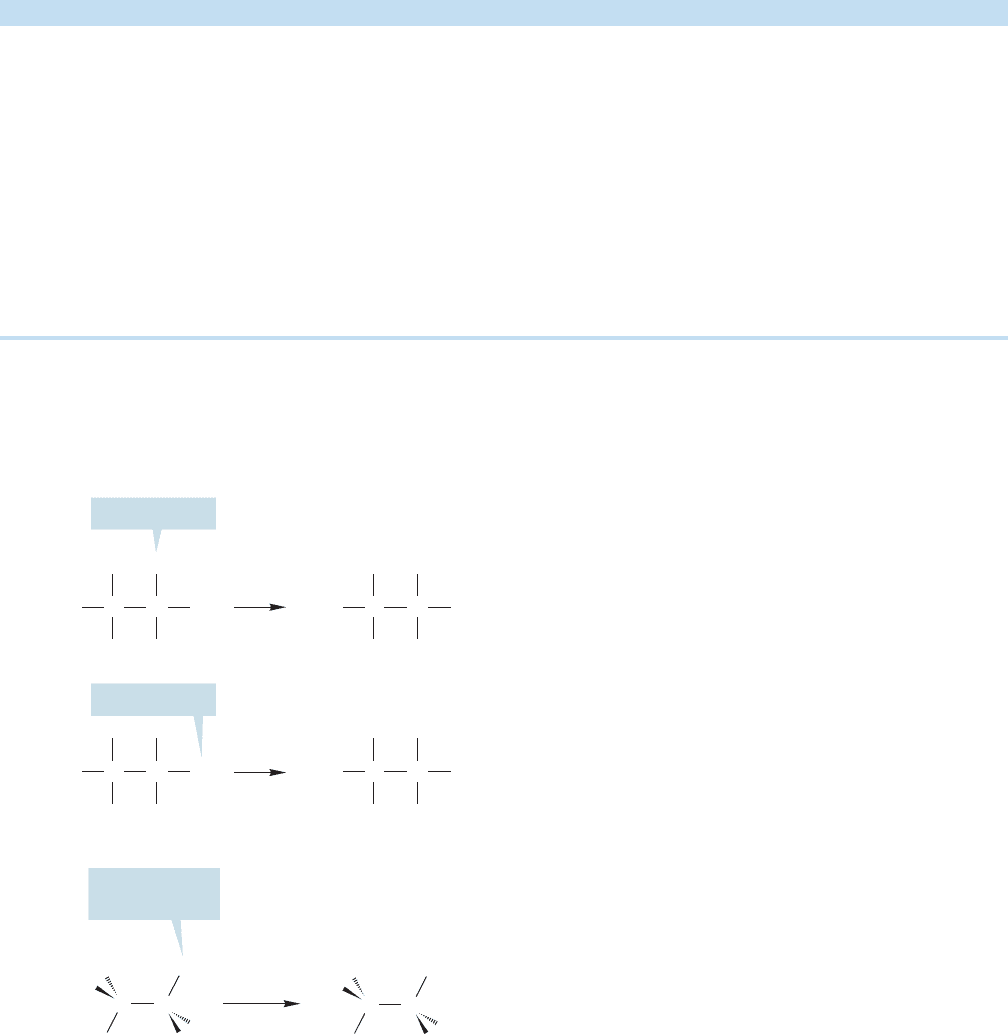
How many different compounds are there? From a two-
dimensional representation of ethane (Fig. 2.24a), there appear to be two ethyl
compounds with the same formula but different structures.Compounds of this type,
which have the same molecular formula but are not the same structure, are called
CH
3
CH
2
O
X
70 CHAPTER 2 Alkanes
isomers (from the Greek words meaning “the same parts”). If the two ethyl com-
pounds of Figure 2.24a existed, they would be isomers of each other. However, the
two different positions shown for X (at the “top” of the molecule and on the “side”)
do not in fact exist. They are artifacts of our attempt to represent a three-dimensional
molecule in two dimensions. This common misconception for beginners demon-
strates how important it is to know the three-dimensional structures of compounds.
As we saw in Figure 2.21a, all six hydrogens in ethane are the same (equivalent) and
therefore there is only one kind of substituted ethyl compound (Fig. 2.24b).Of course
there are different conformations of a substituted ethyl compound. We know about
the staggered and eclipsed conformations and we can call these conformational
isomers, because the two conformations have the same molecular formula but
different structures. However, even though most conformational isomers have
relatively low barriers to rotation, we usually focus on the most stable representa-
tion. In the ethyl series, we will work with the staggered structure.
Summary
Even a molecule as simple as ethane, the two-carbon alkane, has a complex
structure! For example, staggered and eclipsed conformational isomers exist, sep-
arated by a low barrier to rotation (~3 kcal/mol). Because this barrier is so low,
there is rapid rotation around the carbon–carbon bond. All six hydrogens in ethane
are equivalent; there is only one ethane and only one kind of ethyl derivative.You
may need a ball-and-stick model set to convince yourself.
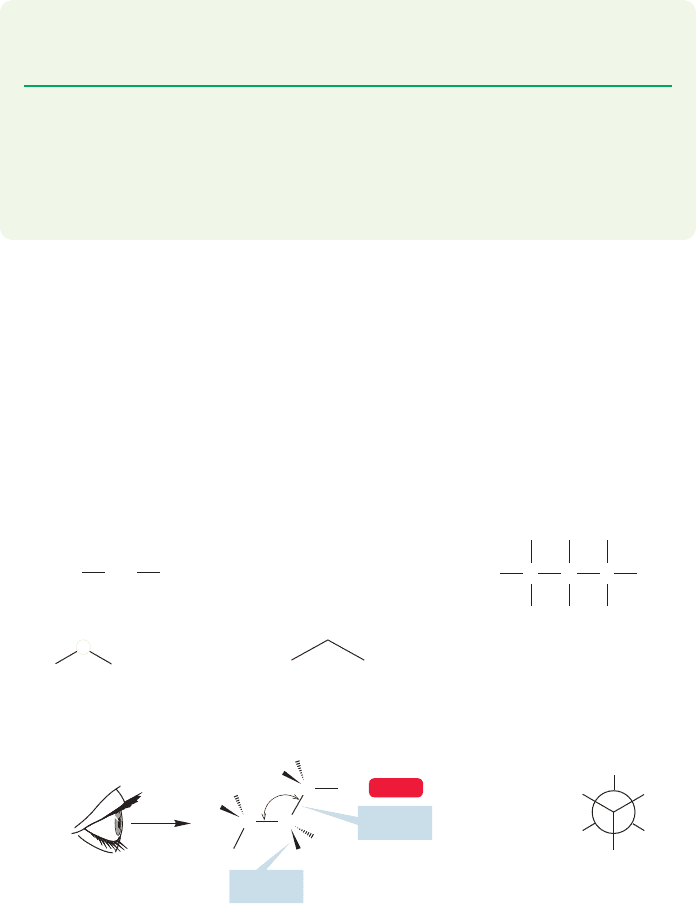
2.6 Structure Drawings
CONVENTION ALERT
In the preceding sections, there are many different representations of the very sim-
ple molecule ethane. Figure 2.25 recapitulates them and adds some new ones.
Methyl groups are sometimes written as Me and ethyl groups as Et, especially in
colloquial use.
C
2
H
6
CH
3
CH
3
H
3
C
(CH
3
)
2
(Me)
2
CH
3
CH
3
Me
Et H
Me
EtH
CH
3
HH
H
H
H
H
H
H
H
H
C
H
H
C
CC
H
H
H
HH
H
FIGURE 2.25 Twelve different
representations of ethane.
The difficulty of representing the real, dynamic, three-dimensional structure of
ethane should be more apparent to you now. In the real world, there is not often the
time to draw out carefully a good representation of even as simple a molecule as
ethane. The solid and dashed wedges of Figure 2.25 are the traditional attempts at
adding a three-dimensional feel to the two-dimensional drawing. More complicat-
ed molecules can raise horrendous problems. Adequate codes are needed, and you
must learn to see past the coded structures to the real molecules both easily and
quickly. It’s worth considering here, at this very early point, some of the pitfalls of
the various schemes.
2.7 Propane (C
3
H
8
) and Propyl Compounds (C
3
H
7
X) 71
First of all, it is not simple to represent even ethane effectively in a linear fash-
ion.Clearly, neither C
2
H
6
nor is as descriptive as ,as those for-
mulas don’t show the connectivity of the atoms in a proper way.The representation
is better, but even this picture lacks three dimensionality. We used this
representation in this chapter, but you will sometimes see the variant .
The two formulations are equivalent, even though the seems to indi-
cate that the bond to the right-hand carbon comes from one of the hydrogens on
the left-hand carbon. Of course, it does not; it is the two carbons that are bonded,
as shows. Yet it is easier to write , and you will see this
form often.
CH
3
O
CH
3
H
3
C
O
CH
3
CH
3
O
CH
3
CH
3
O
CH
3
H
3
C
O
CH
3
H
3
C
O
CH
3
Et
O
H
PROBLEM 2.18 Draw a three-dimensional structure for (Me)
2
CH
2
, (CH
3
)
4
C,
(CH
3
)
3
CH, EtCH
3
, (Et)
2
,CH
3
CH
2
CH
3
, EtMe, MeEt.
PROBLEM 2.19 Start with the two “different”structures in Figure 2.24a and replace
the X group with CH
3
in each. Make three-dimensional drawings of the “two”
molecules you’ve created and convince yourself that both your three-dimensional
drawings represent the same molecule; there is only one CH
3
CH
2
CH
3
. By all
means, use your models.
2.7 Propane (C
3
H
8
) and Propyl
Compounds (C
3
H
7
X)
Propane, the third member of the alkane series, contains three carbon atoms and has
many of the chemical and physical properties of ethane and methane. Figure 2.26
shows several representations for propane, including two new ones. In Figure 2.26d,
only the carbon frame is retained, and all eight hydrogen atoms are implied.
1.532
(a)
(c)
(b)
(d)
(e)
CH
3
CH
2
CH
3
CH
3
CH
2
CH
3
H
H
H
H
H
H
H
H
C
C
C
112
1.107
=
HH
H
H
C
H
H
C
H
H
C
C
C
C
CH
3
H
HH
H
H
A
A
WEB 3D
FIGURE 2.26 Different
representations of propane.
Not even the carbons are shown in Figure 2.26e, and the reader is left to fill in
mentally all the atoms. This most abstract representation—called a line drawing
of the molecule—is the representation of choice for organic chemists, and we will
72 CHAPTER 2 Alkanes
gradually slip into this way of drawing molecules in subsequent chapters. In a line
drawing of a hydrocarbon,every angle vertex and terminus is a carbon, never a hydro-
gen, and you have to add hydrogens to every carbon so that carbon’s valence of four
bonds is satisfied.
Notice that some, but not all of the schematic representations for propane show
that it is not a strictly linear species. All carbons are hybridized approximately sp
3
,
and the angle is close to 109° (in fact, it is 112°).
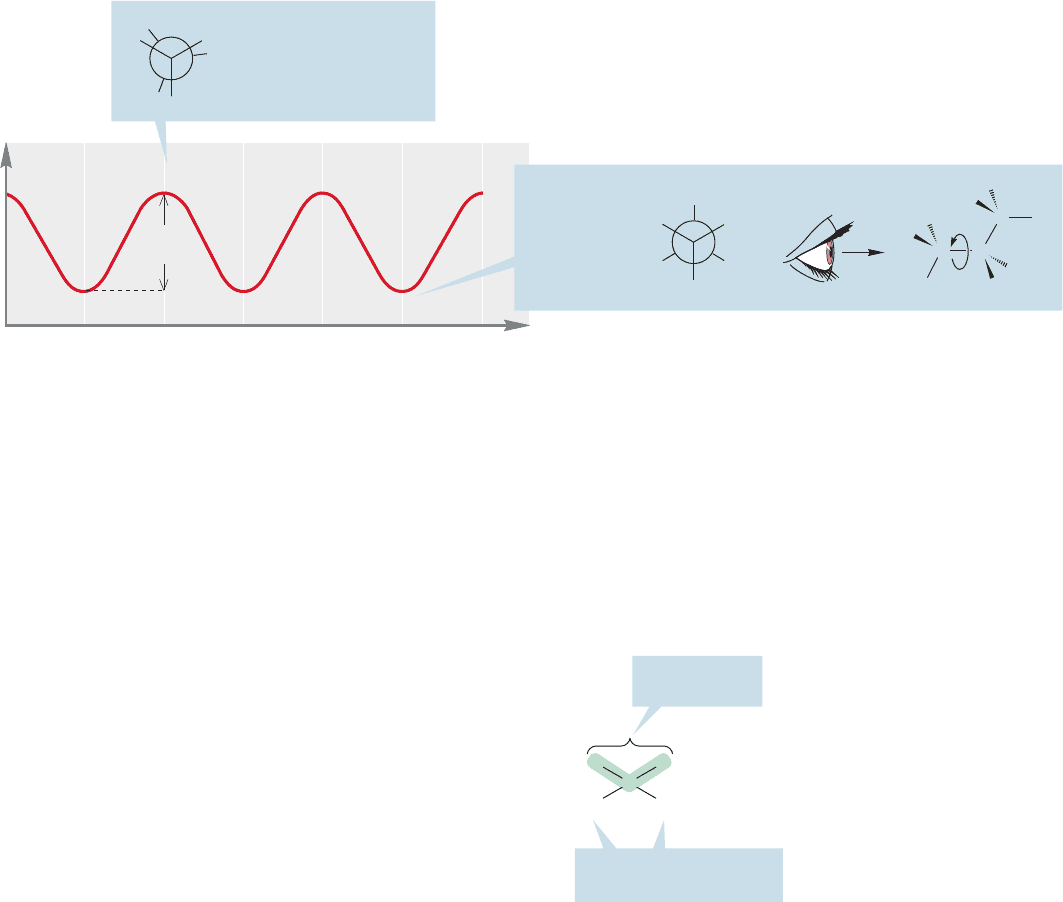
The Newman projection for propane (Figs.2.26 and 2.27), constructed by look-
ing down one of the two equivalent carbon–carbon bonds, shows the structure of
propane particularly well.A plot of energy versus dihedral angle for propane, shown
in Figure 2.27, is similar to that for ethane, except that the rotational barrier of
3.4 kcal/mol is slightly higher. In propane, a large methyl group is eclipsed with a
hydrogen at the top of the barrier (the transition state), and this spatial interaction
accounts for the higher energy.
C
O
C
O
C
Energy
Dihedral angle (θ)
0⬚ 60⬚ 120⬚ 180⬚ 240⬚ 300⬚ 360⬚
TS
SSS
TS TS TS
3.4
kcal/mol
A transition state (TS)
is an energy maximum
not an energy minimum
CH
3
H
H
H
H
H
Staggered
propane (S)
occupies an
energy
minimum
CH
3
H
HH
H
H
H
H
H
H
H
H
H
H
C
C
C
=
FIGURE 2.27 A graph of energy versus dihedral angle (θ)
for propane.
Replacement of a hydrogen in propane with an X group yields propyl deriva-
tives, but the situation is more complicated than in methane or ethane. In both
methane and ethane,all hydrogens are equivalent, and there can be only one methyl
or one ethyl derivative.In propane,there are two different kinds of hydrogen.There
is a central CH
2
group, called a methylene group,and two equivalent methyl groups
at the two ends of the molecule (Fig. 2.28). The carbon and hydrogens in a meth-
ylene group (CH
2
) are not the same as the carbons and hydrogens in methyl
groups (CH
3
).
One methylene
group
Propane
Two equivalent (identical)
methyl groups
C
HH
H
3
CCH
3
FIGURE 2.28 The two types
of hydrogen in propane.
2.8 Butanes (C
4
H
10
), Butyl Compounds (C
4
H
9
X), and Conformational Analysis 73
Isomers
if X is
the same
A series of propyl
compounds
A series of isopropyl
compounds
replace a methylene
hydrogen
replace a methyl
hydrogen
X
C
HH
H
3
CCH
3
C
H
H
3
CCH
3
HH
C
H
H
C
H
3
C
X
WEB 3D
WEB 3D
FIGURE 2.29 Propyl and isopropyl
compounds.
PROBLEM 2.20 Make a three-dimensional drawing of propyl alcohol
( ) and one of the related isopropyl alcohol
().CH
3
O
CHOH
O
CH
3
CH
3
O
CH
2
O
CH
2
O
OH
Butane
Isobutane
CH
3
H
3
C
CH
CH
3
H
3
C
CH
CH
2
H
3
C
CH
2
CH
3
CH
2
H
3
C
CH
2
X = CH
3
X = CH
3
X CH
3
X
WEB 3D
WEB 3D
FIGURE 2.30 If X CH
3
,we
produce the isomers butane and
isobutane.
HH
H
H
C
H
H
C
H
H
C
H
H
C
CH
3
CH
2
CH
2
CH
3
CH
3
CH
2
CH
2
CH
3
C
H
H H
H
C
CH
3
H
3
C
FIGURE 2.31 Different representations of butane.
(Fig. 2.31) provides a nice example of conformational analysis, the study of
the relative energies of conformational isomers. Let’s begin this analysis by con-
structing a Newman projection by looking down the bond of butaneC(2)
O
C(3)
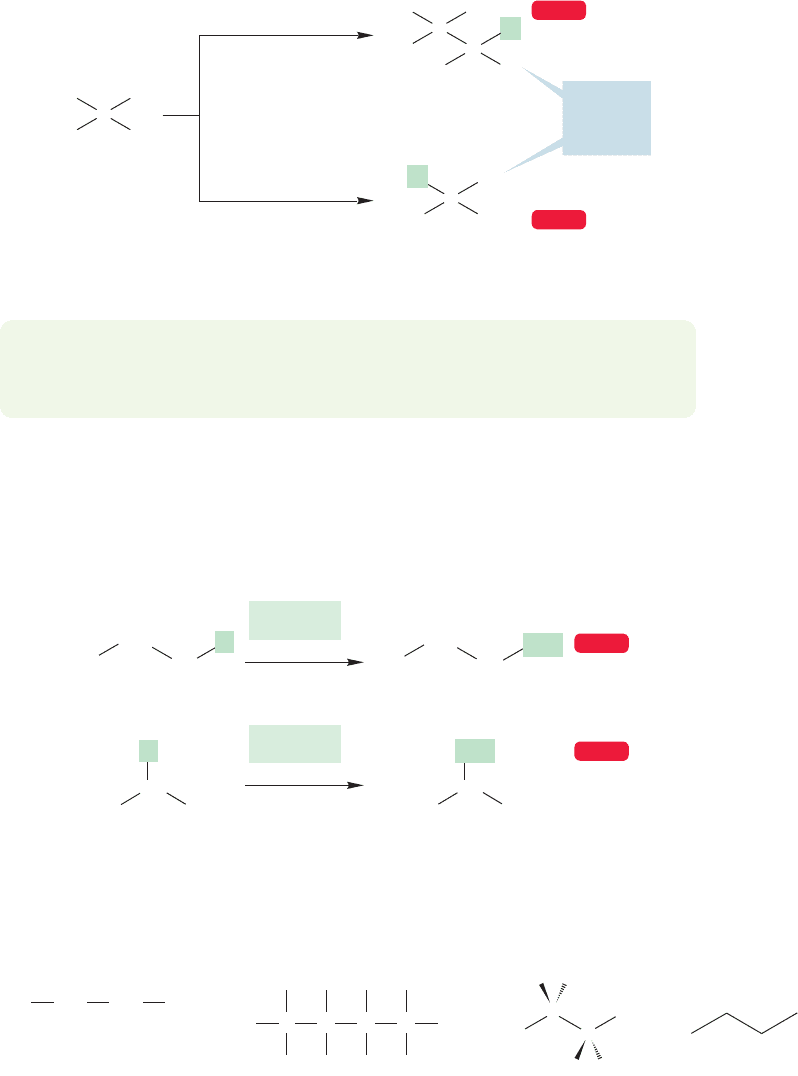
Either of these two kinds of hydrogen can be replaced by an X to give a propyl
compound (Fig. 2.29). The linear compound , in which
X replaces a methyl hydrogen, is called propyl X (in the old days, n-propyl X). The
branched compound , in which X replaces a hydrogen on the
methylene carbon, is called isopropyl X (and sometimes i-propyl X). Propyl com-
pounds and isopropyl compounds are structural isomers of each other; they are
compounds with the same formula but different structures.
CH
3
O
CHX
O
CH
3
CH
3
O
CH
2
O
CH
2
O
X
2.8 Butanes (C
4
H
10
), Butyl Compounds (C
4
H
9
X),
and Conformational Analysis
If, in the two propyl isomers on the right in Figure 2.29, we let X be methyl, we
generate the isomers butane and isobutane (Fig. 2.30). The structure of butane
74 CHAPTER 2 Alkanes
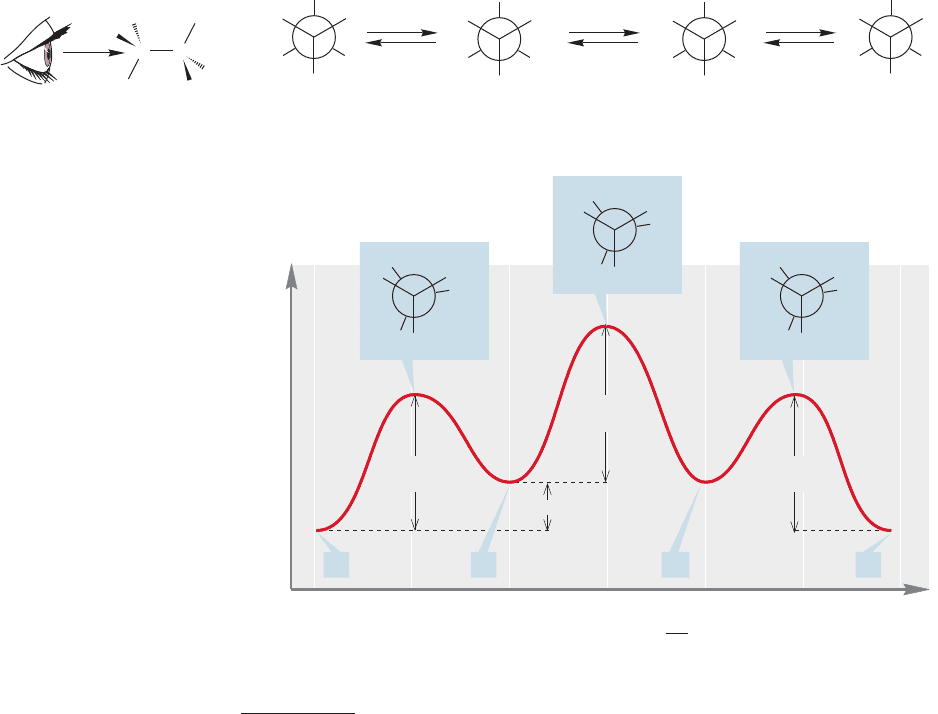
(Fig. 2.32a). The notation C(2), C(3), and so on will be used to refer to the carbon
atom number. If we make the reasonable assumption that the relatively large methyl
groups attached to these two carbons are best kept as far from each other as possi-
ble, we have the most stable staggered conformation A in Figure 2.32a, which is
called the anti form. As in ethane (see Fig. 2.22), a 120° clockwise rotation of the
bond keeping C(2) fixed,passes over a transition state (an energy max-
imum); this is the first transition state in Figure 2.32b. As the rotation continues,
the molecule acquires a new staggered conformation, B. Conformation B is less sta-
ble than A because in B the two methyl groups are closer to each other than they
are in A. Conformation B is called gauche-butane, and this kind of methyl–methyl
interaction is a gauche interaction. The gauche form of butane (B) is about 0.6
kcal/mol higher in energy than the anti form (A).
6
The eclipsed transition state for
the interconversion of staggered conformation A and staggered conformation B lies
about 3.4 kcal/mol higher than A.
C(2)
O
C(3)
(a)
Rotation about the central C
C bond
Energy
–180 +180–120 –60 0 +60 +120
3.8
kcal/mol
rotate
120
rotate
120
rotate
120
A
A
B'
0.6 kcal/mol
3.4
kcal/mol
A
anti Form
CH
3
H
3
C
H
H
H
H
CC
CH
3
H
HH
H
CH
3
A
anti Form
CH
3
H
HH
H
CH
3
B
gauche Form
H
CH
3
HH
H
CH
3
B'
gauche Form
H
H
HH
H
3
C
CH
3
B
3.4
kcal/mol
H
CH
3
H
CH
3
H
H
H
3
C
CH
3
H
H
H
H
H
CH
3
H
H
H
3
C
H
(b)
FIGURE 2.32 (a) Newman projections
for butane.The staggered
conformations are shown. (b) A
graph of dihedral angle (θ) versus
energy for butane as the molecule
changes through rotation about the
bond. All three eclipsed
conformations are shown.
C(2)
O
C(3)
6
This number, 0.6 kcal/mol, is somewhat controversial. Most books give this value as 0.9 kcal/mol. That
0.9 figure is correct for the gas phase, but is not correct in solution. Indeed, the value is different in differ-
ent solvents. It seemed best to give a number for solution, in which most chemistry takes place. You will
find slight differences in almost every version of the curve in Figure 2.32, but the big picture—the gener-
al shape of the curve—will not vary.The reasons for the difference between the solution and the vapor phase
are complex, but if you would like to read more, see E. E. Eliel and S. H. Wilen, Stereochemistry, Wiley,
1994, pp. 600 ff.
2.8 Butanes (C
4
H
10
), Butyl Compounds (C
4
H
9
X), and Conformational Analysis 75
PROBLEM 2.21 Recall from Figure 2.22 that the staggered ethane conformation is
2.9 kcal/mol more stable than its eclipsed conformation. Given that value and the
information that the transition state for the interconversion of staggered confor-
mations A and B in butane is 3.4 kcal/mol higher in energy than A (Fig. 2.32b),
calculate how much energy each eclipsed interaction in the
transition state for the interconversion of A and B is worth. How much energy is
the eclipsed interaction in the transition state for the con-
version of B to B′ worth?
PROBLEM 2.22 Draw Newman projections constructed by looking down the
indicated carbon–carbon bond in the following molecules. For the molecule on
the right, make a graph of energy versus dihedral angle.
C
O
CH
3
/C
O
CH
3
C
O
CH
3
/C
O
H
Another 120° clockwise rotation brings us to a second gauche-butane, B′.
The transition state for the conversion of B to B′, the second transition state in
Figure 2.32b, involves an eclipsing of two bonds and lies 3.8 kcal/mol
above B and B′, a total of about 4.4 kcal/mol above A. A final 120° clockwise rota-
tion returns us to A.
C
O
CH
3
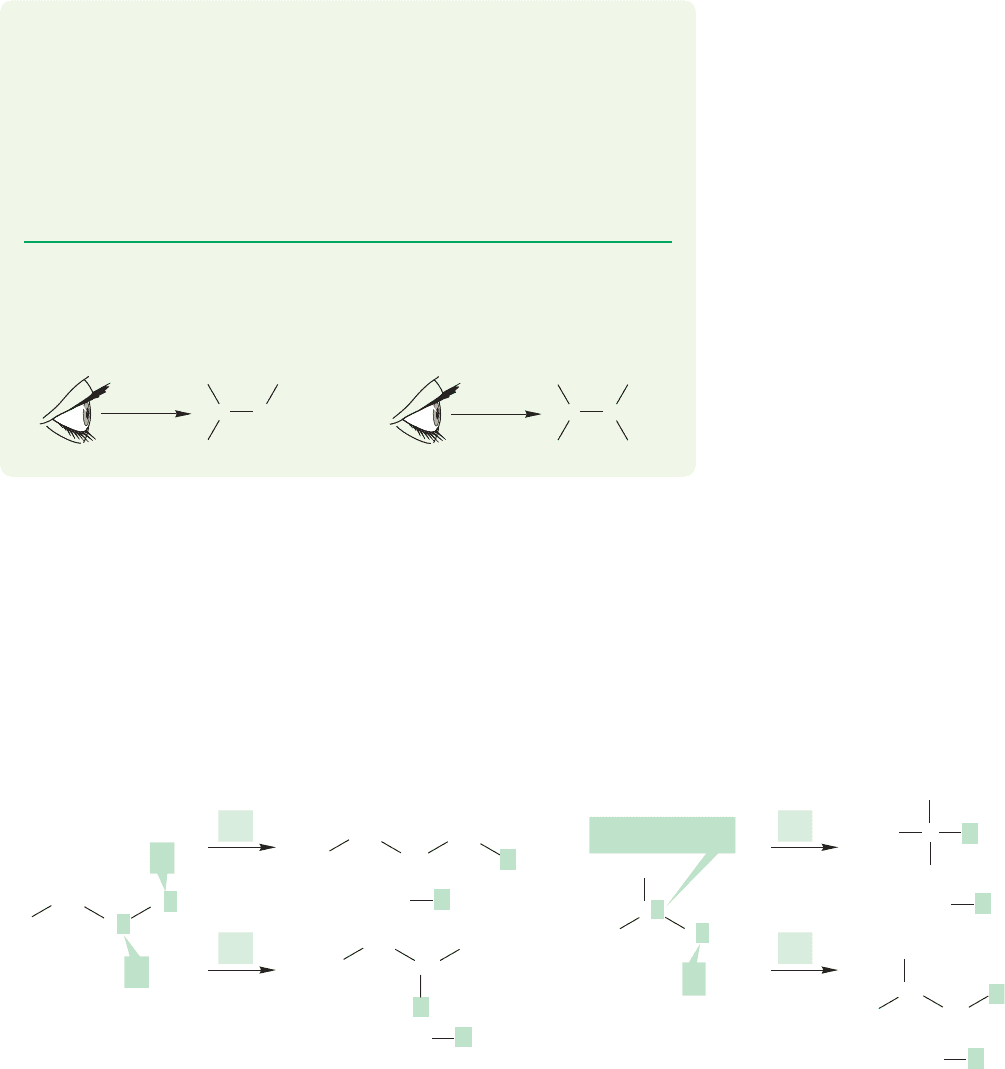
We start our analysis of branched compounds with the butyl compounds,
. Butane contains two kinds of hydrogen atoms in its pairs of equivalent
methylene (CH
2
) groups and methyl (CH
3
) groups. Replacement of hydrogen a or
b produces two types of butyl compounds, shown in Figure 2.33a and b. Similarly,
isobutane contains a single methine group (CH) and three equivalent methyl groups
and also yields two types of butyl compounds (Fig. 2.33c and d).
The four different types of butyl compounds are called butyl, isobutyl, sec-butyl
(short for secondary-butyl),and tert-butyl (or sometimes t-butyl,short for tertiary-butyl).
C
4
H
9
O
X
CH
H
3
C
H
3
C
CH
3
CH
3
HC
HC
CH
2
H
3
C
H
3
C
CH
3
Butyl
Butane
Isobutane
H
3
C
CH
2
CH
2
CH
3
H
3
C
CH
3
CH
3
CH
H
3
C
CH
2
CH
2
CH
2
X
sec-Butyl
H
3
C
CH
2
CH
CH
3
X
X
tert-Butyl X
X
a
b
d
c
a
b
c (methine group)
d
C
CH
3
CH
3
H
3
C
X
Isobutyl
X
X
H
3
C
CH
2
CH
3
CH
FIGURE 2.33 (a) and (b) Replacement of one of the hydrogens of butane with X yields two kinds of butyl derivatives,
and . (c) and (d) Replacement of hydrogen with X in isobutane yields two more butyl
compounds, tert- and .isobutyl
O
Xbutyl
O
X
sec-butyl
O
Xbutyl
O
X
76 CHAPTER 2 Alkanes
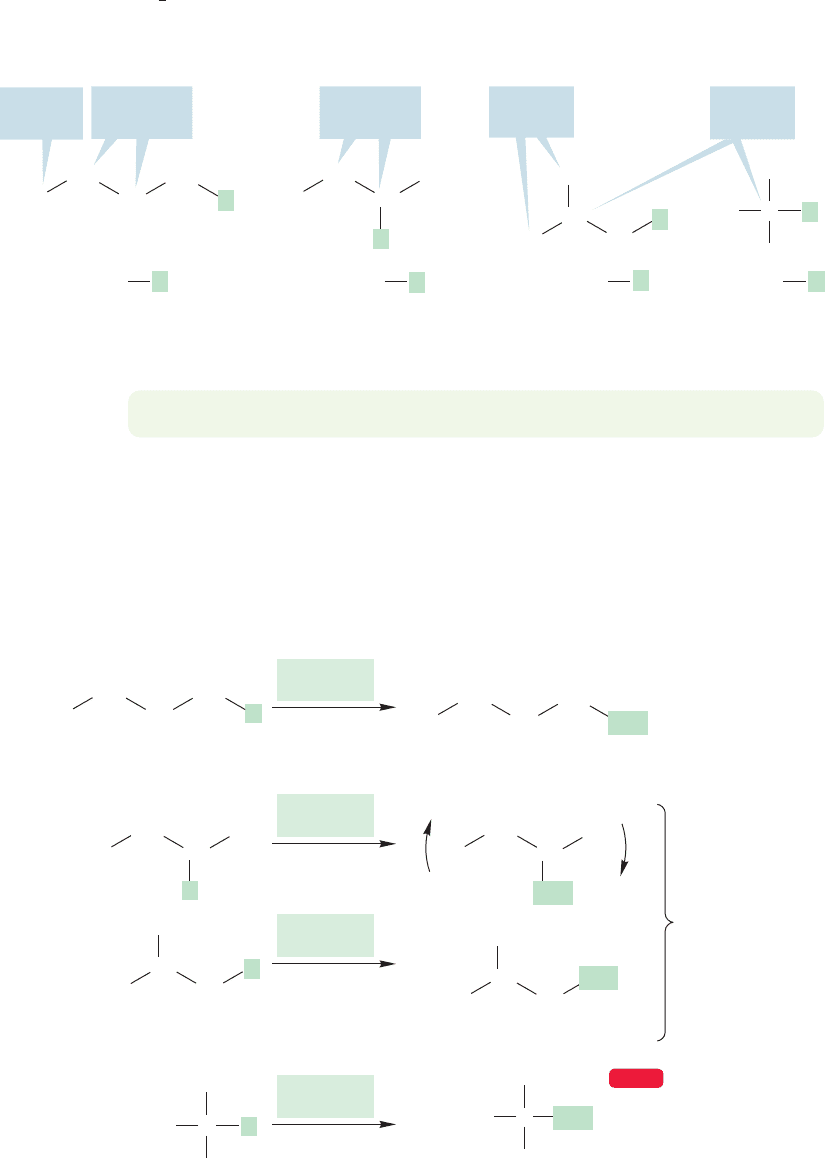
To see what the prefixes sec- and tert- mean, we must introduce new and important
terminology (Fig. 2.34). A primary carbon is a carbon that is attached to only one
other carbon atom. A secondary carbon is a carbon attached to two other carbons,
and a tertiary carbon is a carbon attached to three other carbons.A quaternary (not
quar
ternary) carbon (not shown in Figure 2.34, but we’ll see one in a moment) is
a carbon that is attached to four other carbons.Thus, the names sec-butyl and tert-
butyl tell you something about the structure.
PROBLEM 2.23 Draw two compounds containing quaternary carbons.
2.9 Pentanes (C
5
H
12
) and Pentyl Compounds
(C
5
H
11
X)
We might now anticipate that four pentane compounds would emerge when we
transform our four butyl X compounds into five-carbon compounds by letting
X CH
3
(Fig. 2.35). Yet only three different pentanes exist! Somehow the
O
Primary
carbons
Secondary
carbons
Secondary
carbons
Tertiary
carbons
Primary
carbon
Butyl
H
3
C
CH
2
CH
2
CH
2
X
sec-Butyl
H
3
C
CH
2
CH
CH
3
X
X tert-Butyl XX
C
CH
3
CH
3
H
3
C
X
Isobutyl X
X
H
3
C
CH
2
CH
3
CH
FIGURE 2.34 The four kinds of butyl compounds used to illustrate the bonding in primary, secondary,
and tertiary carbons (X is not carbon or hydrogen).
H
3
C
H
3
C
CH
2
X
X
CH
2
CH
2
C
H
3
C
CH
2
CH
3
CH
H
3
C
CH
2
CH
2
CH
2
H
3
C
CH
CH
2
CH
3
H
3
C
CH
CH
2
CH
3
H
3
C
CH
2
CH
3
CH
CH
3
CH
3
C
CH
3
CH
3
H
3
CX
Pentane
Neopentane
Isopentane
X = CH
3
X = CH
3
X = CH
3
X = CH
3
These are the
same compound,
isopentane; rotate
the top figure 180⬚
as shown by the
arrows
X
CH
3
CH
3
CH
3
CH
3
WEB 3D
FIGURE 2.35 If we let X CH
3
,it
appears as though four C
5
H
12
compounds should exist. Yet there are
only three C
5
H
12
compounds.
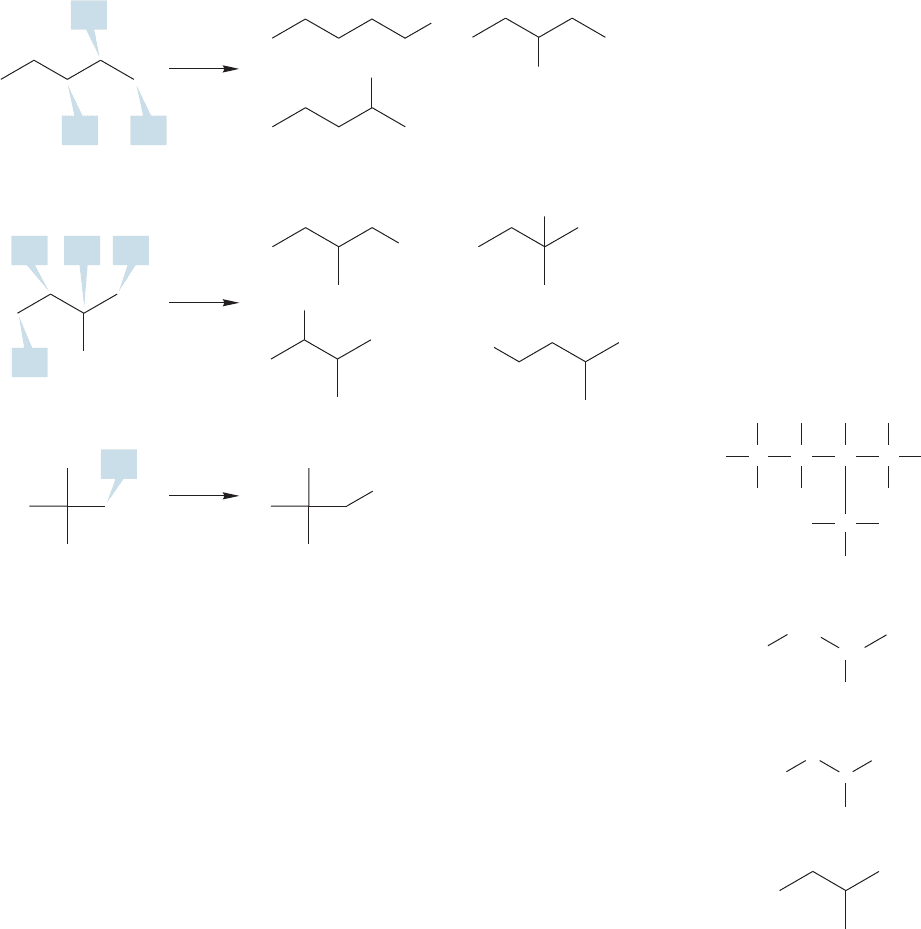
2.9 Pentanes (C
5
H
12
) and Pentyl Compounds (C
5
H
11
X) 77
technique of generating larger alkanes by letting a general X become a methyl group
has failed us.The problem lies again in the difficulty of representing three-dimensional
structures on a two-dimensional surface. Two of the four structures in Figure 2.35
represent the same compound, even though in the two dimensional representation
they look different. The common names for the branched pentanes are isopentane
and neopentane. We will not usually use these common names. Section 2.10 will
describe a more systematic procedure for naming alkanes, including these two.
Even though there are only three pentane compounds—pentane, isopentane, and
neopentane—Figure 2.36 shows that replacing one H in a pentane leads to no fewer
than eight pentyl derivatives ( )! In the next section,we’ll see how to name
these derivatives.
C
5
H
11
O
X
(1)
(3)
Pentane
X
(2)
X
X
(4)
(5)
Isopentane
(7)
X
(6)
X
X
Neopentane
X
(8)
Eight pentyl
derivatives
(5) (4)
(8)
(2)
(6)
(7)
(3) (1)
X
FIGURE 2.36 The eight pentyl
derivatives formed by replacing
hydrogens at the three different
positions of pentane, the four
different positions in isopentane, and
the lone position of neopentane.
One important lesson to be learned from Figure 2.35 is that, if you let it, the two-
dimensional page will lie to you more often than not. As we have already seen, there
is a tension between our need to talk quickly and efficiently to one another and the
accurate representation of these complicated organic structures.This tension is at least
partly resolved by using codes, but the price of this use is that you must always be able
to see past the code to the real, three-dimensional structure if you expect to be able to
think effectively about organic chemistry. Because these codes become quite abstract,
it is worth going through the process of developing the abstraction one more time.
Consider the representations for isopentane in Figure 2.37. The first drawing
(Fig. 2.37a) only vaguely represents the real molecule. One then goes to the more
schematic structure of Figure 2.37b, and then to a structure in which the hydro-
gens are left out (Fig. 2.37c). Finally, one removes even the carbon labels, as in
Figure 2.37d, to give the ultimate abbreviation for the structure of isopentane. Here
nothing survives from the original picture except a representation of the backbone.
You must be able to translate these highly schematic structures into three dimen-
sions and to see the way these skeletal pictures transform into molecules.
(d)
(a)
H
H
H
H
C
H
H
C
H
H
H
H
H
H
C
C
C
(c)
C
C
C
C
C
(b)
CH
CH
3
CH
2
H
3
C
CH
3
FIGURE 2.37 Four increasingly
abstract representations of
isopentane.
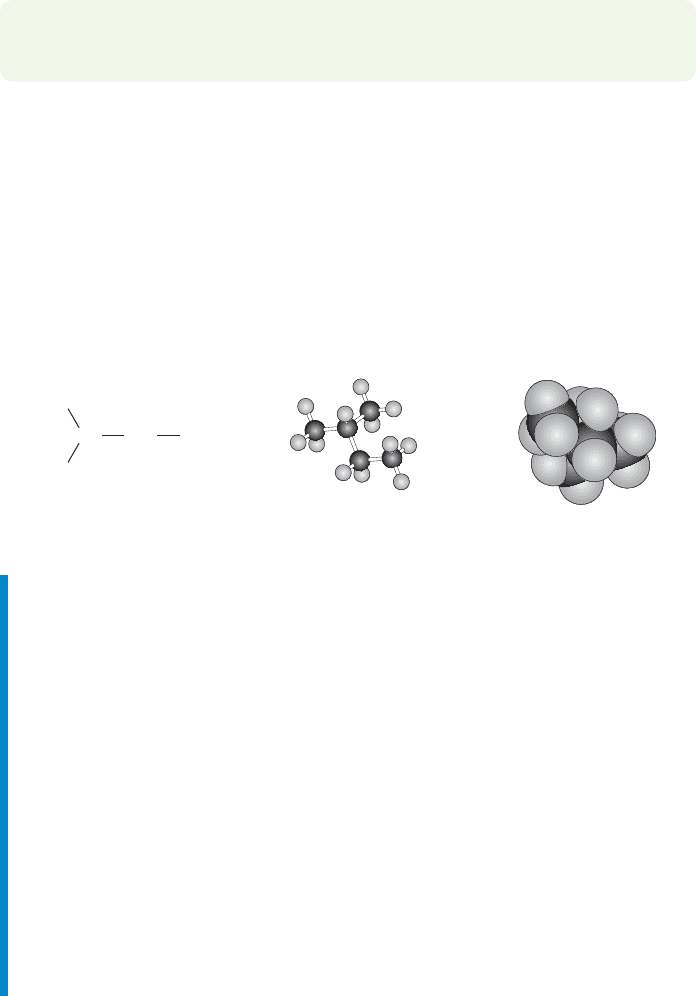
78 CHAPTER 2 Alkanes
H
3
C
H
3
C
CH
2
CH
3
Ball-and-stick
model
Space-filling
model
CH
FIGURE 2.38 Views of isopentane.
PROBLEM 2.24 Draw all the isomers of pentane, first in the format used in
Figure 2.37b and then in the format used in Figure 2.37d.
Actually, there is even more to be done in our escape from the two dimensions
of a book page. Line drawings can be approximated with models in which balls and
sticks are used to represent atoms and the bonds between atoms. Other models con-
centrate on the bonds and leave it to you to imagine the atoms. Regardless of what
kind of models your class uses, they probably do a relatively poor job of showing the
steric requirements of the real molecules.When we refer to steric requirements, we
mean the volumes of space that atoms occupy. Chemists have developed space-filling
models, which attempt to show the volumes of space carved out by the atoms.
Figure 2.38 shows some representations of isopentane.
Summary
There are several ways to represent molecules as summarized in Figures 2.37
and 2.38.The line drawings are the preferred choice for most cases.The conven-
tion of dashes and wedges gives more detail about the three-dimensional struc-
tures. Propane (C
3
H
8
) has staggered and eclipsed conformations and the barrier
between them is relatively low (3.4 kcal/mol). Two isomers are possible for sub-
stituted propane ( ), with the common names propyl and isopropyl.
Conformational analysis of butane (C
4
H
10
) introduces several new concepts.
Butane has anti and gauche forms. The energy diagram for rotation around the
central bond of butane (Fig. 2.32b) shows the effects of the steric interactions of
eclipsing methyl groups. There are four isomers for the substituted butanes
( ), with the common names of butyl , sec-butyl , tert-butyl ,
and isobutyl . Pentane has two structural isomers isopentane and neopentane.
There are eight isomers of the substituted pentanes ( ). A helpful tool
for distinguishing between the different substituted compounds is the very impor-
tant system of classifying carbons as primary, secondary, and tertiary.
C
5
H
11
O
X
O
X
O
X
O
X
O
XC
4
H
9
O
X
C
3
H
7
O
X
2.10 The Naming Conventions for Alkanes
Clearly, things are rapidly getting out of hand. Although it is not too difficult to
remember the four types of butyl compounds (Fig.2.34),remembering the eight types
of pentyl compounds (Fig. 2.36) constitutes a tougher task—and the problem rap-
idly gets worse as the number of carbons increases. A system is absolutely necessary.
In practice, the old common, nonsystematic names are retained through the butanes,
and for a few other old favorite molecules. Once we reach five carbons, a systematic
naming protocol developed by the International Union of Pure and Applied
Chemistry (IUPAC) takes over.The IUPAC system is designed to handle any organ-
ic structure. There are IUPAC names for all the common names we have learned.
