Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.


2.14 Nuclear Magnetic Resonance Spectroscopy 89
about ethane? There are two carbons here, but they are in identical environments and
thus equivalent: one signal again. How about propane? Three carbons are here, two
methyl groups and a single methylene.Surely,we must observe a different signal for that
CH
2
than for the CH
3
groups. And we do—there are two signals in the
13
C NMR
spectrum for propane,one for the CH
2
and another for the two equivalent CH
3
groups.
Counting the number of signals is trivial if (and only if) we can discern whether
the molecular environments of the carbons are the same or not. Here is a slightly
harder example.The molecule tetrahydrofuran produces two
13
C signals (Fig. 2.56).
It is tempting to say that because there are only methylene (CH
2
) groups in this
molecule,there should only be one signal.But those methylenes are not all the same.
Two of them are adjacent to the oxygen, and two are not. So there are two signals,
one for each set of two different methylenes.
We will pick these points up again as we discuss the various structural types in
the next few chapters, but for the moment, work through the following Problem
Solving box and then try a few problems on your own.
One signal
Another signal
H
2
C
H
2
C
CH
2
O
CH
2
FIGURE 2.56 Tetrahydrofuran has
two different methylene groups.
PROBLEM SOLVING
Nearly everyone has initial problems with seeing differences in the atoms making
up any molecule. Why, for example, are there three different methylene (CH
2
)
groups in heptane? Maybe it is intuitively obvious (maybe!) that the central
methylene group is different from the others, but almost everyone worries about
the other methylene groups.
The best technique to use when you are in doubt is to list all the groups to
which the carbons in question are attached. And be very detailed in making that
list—do not just look at nearest neighbors. Here is an example using heptane:
1234567
CH
3
CH
2
CH
2
CH
2
CH
2
CH
2
CH
3
Carbon 1 methyl: Attached to C CCCCC,same as carbon 7
methyl
Carbon 2 methylene: Attached to C on one side and to C CCCC
on the other, same as carbon 6 methylene
Carbon 3 methylene: Attached to C C and to C C C C, same as
carbon 5 methylene
Carbon 4 methylene: Attached to C C C and to C C C
Carbon 5 methylene: Attached to C C C C and to C C, same as
carbon 3 methylene
Carbon 6 methylene: Attached to C CCCC and to C, same as
carbon 2 methylene
Carbon 7 methyl: Attached to C CCCCC,same as carbon 1
methyl
Therefore, heptane will have four signals in the
13
C NMR. One signal for the
equivalent methyl groups (carbons 1 and 7), one signal for the methylenes of
carbons 2 and 6, one signal for the methylenes of carbons 3 and 5, and one signal
for the carbon 4 methylene.
OOOOO
OOOO
OOOO
OOOO
OOOO
OOOO
OOOOO
PROBLEM 2.34 How many
13
C signals will we see in an NMR spectrum of the
molecules in Figures 2.45–2.47?

90 CHAPTER 2 Alkanes
PROBLEM 2.35 How many
13
C signals will an NMR spectrometer detect in the
molecules of Figure 2.49?
PROBLEM 2.36 How many
13
C signals will an NMR spectrometer detect in the
molecules of Figure 2.52?
Hydrogen NMR (
1
H NMR) spectroscopy is similar to
13
C spectroscopy.The areas
of the signals we observe in
1
H NMR spectroscopy are in the ratio of the numbers of
different hydrogens giving rise to those signals. For example, the signal recorded for
six hydrogens of one kind will give a signal three times as big as a signal for two hydro-
gens and so on.These ratios from the signals are more reliably determined in
1
H NMR
than they are in
13
C NMR. There are other very useful complications introduced by
the abundance of the NMR active isotope (
1
H) in organic molecules,but we can leave
them for Chapter 15. We will use some of the molecules introduced in this chapter
to work through a bit of hydrogen NMR. We’ll find a few more subtleties, but the
overall picture will not be very different from what we have seen for
13
C.
PROBLEM 2.37 What will be the ratios of the signals in the
1
H NMR spectra for
propane (Fig. 2.55), and THF (Fig. 2.56)?
PROBLEM 2.38 How many signals would be seen in the
1
H NMR spectrum for
heptane (Fig. 2.44)? What will the relative sizes of these signals be?
PROBLEM 2.39 How many signals would be seen in the
1
H NMR spectrum for
cis-1,2-dimethylcyclopropane (Fig. 2.51)? Caution: This question is a bit tricky—
look carefully at a model, or use the website to see cis-1,2-dimethylcyclopropane
in three dimensions.
2.15 Acids and Bases Revisited: More Chemical
Reactions
In Section 1.7 (p.41), we introduced acids and bases. Now we know quite a bit more
about structure and can return to the important subject of acids and bases in greater
depth. In particular, we know about carbocations and carbanions, which play an
important role in acid–base chemistry in organic chemistry. The Lewis definition
of acids and bases is far more inclusive than the Brønsted
7
definition, which focus-
es solely on proton donation (Brønsted acid) and acceptance (Brønsted base).The
archetypal Brønsted acid–base reaction is the reaction between KOH and HCl to
transfer a proton from HCl to HO
. This reaction is a competition between the
hydroxide and the chloride for a proton. In this case, the stronger base hydroxide
wins easily (Fig. 2.57).
7
These acids and bases were named after Johannes Nicolaus Brønsted (1879–1947).
K
+
K
+
OH
..
..
..
..
HCl
HOH
..
..
..
..
..
Cl
..
..
..
++
–
–
FIGURE 2.57 The two Brønsted
bases, HO
and Cl
, compete for the
proton, H
.
WEB 3D
2.15 Acids and Bases Revisited: More Chemical Reactions 91
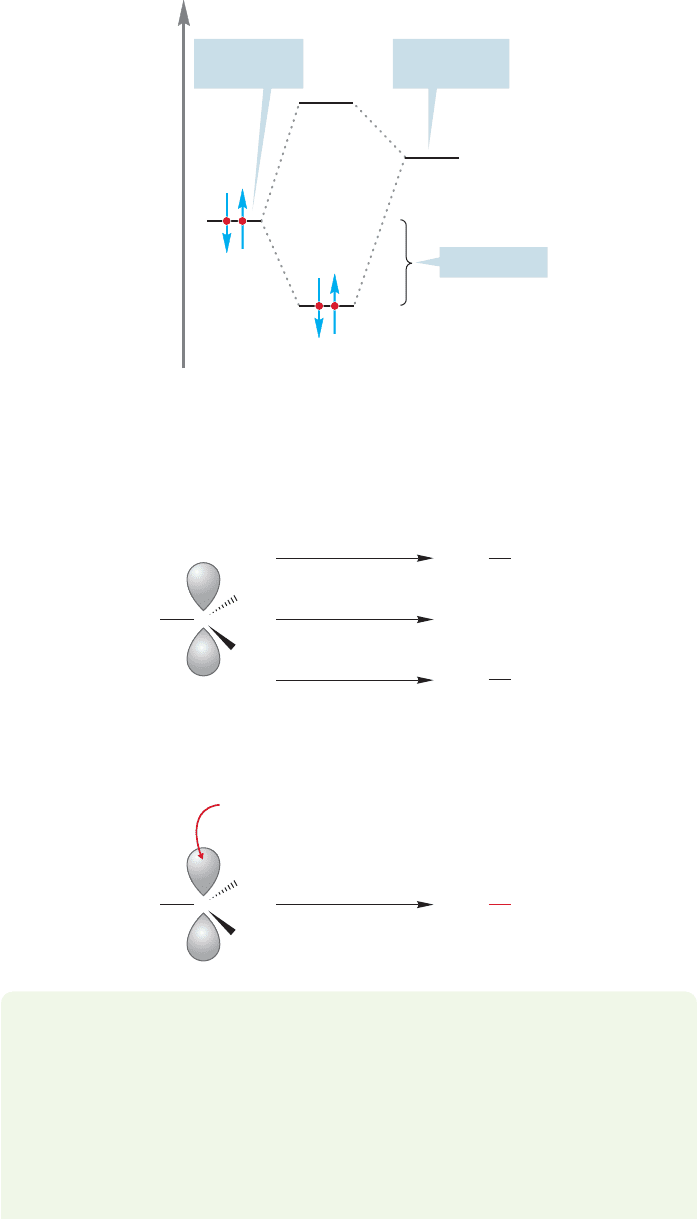
In the Lewis description of acids and bases, any reaction between an empty
orbital and a reactive pair of electrons in a filled orbital is an acid–base reaction.
Another way of putting this is to point out that the interaction between an empty
orbital (Lewis acid) and a filled orbital (Lewis base) is stabilizing (Fig. 2.58).
Consider the methyl cation (Fig. 2.59). That empty 2p orbital makes
CH
3
a
powerful Lewis acid,very reactive toward all manner of Lewis bases with their elec-
tron pairs ready to react. Figure 2.59 gives three examples of such Lewis acid–Lewis
base reactions, each of which fits the orbital interaction diagram of Figure 2.58.
In Figure 2.60,the curved arrow formalism shows the electron flow as the new bond
is formed in this illustrated nucleophile–electrophile (Lewis base–Lewis acid) reaction.
WORKED PROBLEM 2.40 Write arrow formalisms and products for the following
reactions. Identify the participants as acids and bases. Sketch in the empty orbital
for each Lewis acid, and fill in the complete Lewis dot structures for the Lewis
bases. Remember that charge must be conserved in each of these reactions.
Energy
Empty orbital
(Lewis acid)
Filled orbital
(Lewis base)
Stabilization
FIGURE 2.58 The interaction of a
filled orbital (Lewis base) with an
empty orbital (Lewis acid) is
stabilizing.
..
H
–
..
..
..
HO
–
..
..
..
..
Cl
–
CH
4
..
..
..
ClH
3
C
..
..
OHH
3
C
H
+
C
H
H
FIGURE 2.59 Three Lewis bases
reacting with the methyl cation, a
Lewis acid.
..
..
..
ClH
3
C
H
H
..
..
..
..
Cl
–
H
+
C
FIGURE 2.60 The curved arrow
formalism for the formation of
methyl chloride from the methyl
cation (Lewis acid) and a chloride
ion (Lewis base).
(a) (CH
3
)
3
C
Br
(b) (CH
3
)
3
C
NH
3
(c) H
NH
2
*(d) H
3
BHO
U
U
U
U
(continued)
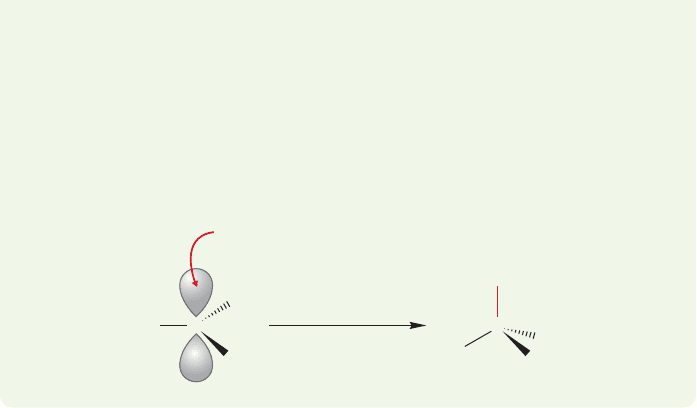
ANSWER (d) Hydroxide must be the Lewis base, but where is the empty orbital?
Where is the Lewis acid? Recall the structure of the sp
2
hybridized borane
(p. 56). Boron is neutral, but there is an empty 2p orbital on boron, and borane is
very definitely a Lewis acid. Reaction with the Lewis base hydroxide looks just
like the reaction of the methyl cation with hydroxide (Fig. 2.59) except that the
product is negatively charged.
92 CHAPTER 2 Alkanes
Lewis base–Lewis acid reactions, even the very simple ones, form the basis for
understanding much of organic chemistry. Essentially all polar reactions can be
looked at in exactly these terms—the ultimately stabilizing reaction of a filled orbital
with an empty orbital.Figure 2.58 really does describe much of the next 1000 pages
or so! But,of course, the details will change and there are surely devils in those details.
Those all-too-prolific details will be easier to manage if you keep Figure 2.58 in mind
at all times—search for the Lewis base and the Lewis acid in every reaction, and
you will be able to generalize.
2.16 Special Topic: Alkanes as Biomolecules
In truth, there really isn’t very much biochemistry of alkanes.The carbon–carbon
and carbon–hydrogen bonds of alkanes are just too strong to enter easily into
chemical reactions. These molecules are not totally inert, as we shall see shortly,
but saturated alkane chains serve more as frameworks in our bodies, holding less
strongly bonded atoms in the proper place for reactions to occur, than as reactive
species themselves. However, as we have seen already (p. 68), in the presence of
oxygen and activating energy (a spark or a lighted match, for example), combus-
tion occurs and heat is given off. We use that energy, that exothermicity of
reaction, to warm our homes and propel our automobiles. Gasoline is largely a
mixture of saturated hydrocarbons. Combustion is a very biological process. Life
depends on it.
In the movie Mad Max: Beyond Thunderdome, the trading village Bartertown
is powered by the methane produced by herds of pigs kept in the depths of
Undertown. How did those piggies produce that methane? Bacteria in the stom-
achs of both pigs and cattle are capable of producing methane from plant material,
and the belching and flatulence of those animals is a major source of planetary
methane. Contrary to conventional wisdom, it is mostly belching that produces
the methane.
Methane may also have been essential to the formation of life.The early atmos-
phere on Earth was relatively rich in methane and ammonia (as are the current
atmospheres of most of the outer planets). Given an energy source such as ultraviolet
..
..
..
–
OH
H
B
H
H
..
..
OH
H
H
H
–
B

radiation or lightning, methane and ammonia react to form hydrogen cyanide
(HCN), a molecule that polymerizes to give adenine, a common component of
ribonucleic acid (RNA) and other molecules of biological importance. In the pres-
ence of water and an energy source, methane and ammonia react to give amino acids,
the building blocks of proteins.
2.17 Summary 93
In this chapter, a bonding scheme for the alkanes is developed.
We continue, as in Chapter 1, to form bonds through the over-
lap of atomic orbitals. We describe a model in which the atomic
orbitals of carbon are combined to form new, hybrid atomic
orbitals. The four orbitals resulting from a combination of three
2p orbitals and one 2s orbital are called sp
3
hybrid atomic
orbitals, reflecting the 75% p character and 25% s character in
the hybrid. These hybrid orbitals solve the problems encoun-
tered in forming bonds between pure atomic orbitals.The sp
3
hybrids are asymmetric, and have a fat and a thin lobe. Overlap
between a hydrogen 1s orbital and the fat lobe provides a
stronger bond than that between hydrogen 1s and carbon 2p
orbitals. In addition, these hybrid orbitals are directed toward
the corners of a tetrahedron, which keeps the electrons in the
bonds as far apart as possible, thus minimizing destabilizing
interactions. Other hybridization schemes are sp
2
, in which the
central atom is bonded to three other atoms, and sp, in which
the central atom is bonded to two other atoms. Simple mol-
ecules in which a central carbon is hybridized sp
2
are planar,
with the three attached groups at the corners of an equilateral
triangle. Molecules with sp hybridized carbons are linear.
Even simple molecules have complicated structures.
Methane is a perfect tetrahedron, but one need only substitute a
methyl group for one hydrogen of methane for complexity to
arise. In ethane, for example, we must consider the consequences
of rotation about the carbon–carbon bond.The minimum ener-
gy conformation for ethane is the arrangement in which all car-
bon–hydrogen bonds are staggered. About 3 kcal/mol above this
energy minimum form is the eclipsed form, the transition state,
the high-energy point (not an energy minimum, but an energy
maximum) separating two staggered forms of ethane. The
3 kcal/mol constitutes the rotational barrier between the two stag-
gered forms.This barrier is small compared to the available ther-
mal energy at room temperature, and rotation in ethane is fast.
This chapter covers again the concept of Lewis acids and
bases. The familiar Brønsted bases compete for a proton, but
Lewis bases are far more versatile. A Lewis base is defined as
anything with a reactive pair of electrons, and a Lewis acid is
anything that reacts with a Lewis base. We are paid back for
these very general definitions with an ability to see as similar all
manner of seemingly different reactions. These concepts will
run through the entire book.
2.17 Summary
New Concepts
alkanes (p. 51)
alkyl compounds (p. 69)
Brønsted acid (p. 90)
Brønsted base (p. 90)
butyl group (p. 75)
sec-butyl group (p. 75)
tert-butyl group (p. 75)
carbanion (p. 62)
carbocation (p. 62)
cis (p. 84)
conformation (p. 65)
conformational analysis (p. 73)
conformational isomers (p. 70)
cycloalkanes (p. 51)
dihedral angle (p. 65)
eclipsed ethane (p. 65)
ethyl compounds (p. 69)
hybridization (p. 52)
hybrid orbitals (p. 53)
hydride (p. 62)
hydrocarbon (p. 51)
isobutyl group (p. 75)
isomers (p. 70)
isopropyl group (p. 73)
methane (p. 51)
methine group (p. 75)
methyl anion (p. 62)
methyl cation (p. 62)
methyl compounds (p. 60)
methylene group (p. 72)
methyl radical (p. 63)
natural product (p. 86)
Newman projection (p. 65)
NMR spectrum (p. 88)
nuclear magnetic resonance (NMR)
spectroscopy (p. 88)
primary carbon (p. 76)
propyl group (p. 73)
quaternary carbon (p. 76)
reactive intermediates (p. 62)
saturated hydrocarbons (p. 83)
secondary carbon (p. 76)
sigma bond (p. 54)
spectroscopy (p. 88)
sp hybrid (p. 53)
sp
2
hybrid (p. 56)
sp
3
hybrid (p. 56)
staggered ethane (p. 65)
steric requirements (p. 78)
structural isomers (p. 73)
substituent (p. 60)
tertiary carbon (p. 76)
trans (p. 84)
transition state (TS) (p. 67)
unsaturated hydrocarbons (p. 83)
van der Waals forces (p. 87)
Key Terms
94 CHAPTER 2 Alkanes
In this chapter, new molecules are built up by first constructing
a generic substituted molecule such as ( ).
The new hydrocarbon is then generated by letting X CH
3
.In
principle, each different carbon–hydrogen bond in a molecule
could yield a new hydrocarbon when X CH
3
. In practice, this
procedure is not so simple. The problem lies in seeing which
hydrogens are really different. The two-dimensional drawings
are deceptive. One really must see the molecule in three dimen-
sions before the different carbon–hydrogen bonds can be identi-
fied with certainty.
The coding or drawing procedures can get quite abstract. It
is vital to be able to keep in mind the real, three-dimensional
structures of molecules even as you write them in two-dimen-
CH
3
O
Xmethyl
O
X
sional code. The Newman projection, an enormously useful
device for representing molecules three-dimensionally, is
described in this chapter.
The naming convention for alkanes is introduced. There
are several common or trivial names that are often used and
which, therefore, must be learned.
Both
1
H and
13
C NMR spectroscopy are introduced
in this chapter. At this point, the NMR spectrometer
functions basically as a machine to determine the numbers
of different hydrogens or carbons in a molecule. That function
is important, however, both in these early stages of your
study of chemistry and in the “real world” of structure
determination.
Reactions, Mechanisms, and Tools
It is easy to become confused by the abstractions used to repre-
sent molecules on paper or blackboards. Do not trust the flat
surface! It is easy to be fooled by a “carbon” that does not really
exist, or to be bamboozled by the complexity introduced by ring
structures. One good way to minimize such problems (none of
us ever really becomes free of the necessity to think about struc-
tures presented on the flat surface) is to work lots of isomer
problems, and play with models!
Common Errors
PROBLEM 2.41 Provide the IUPAC name for the following
compounds:
2.18 Additional Problems
(a) (b) (c) (d)
(e) (g)(f) (h) (i)
F
Cl
Br
Br
Br
F
I
Cl
Cl
Cl
Br
Cl
Cl
PROBLEM 2.42 Write Lewis structures for ethane, ethylene,
acetylene, ethanol, ethanethiol, tetraethylammonium ion,
diethylphosphine, the imine of diethyl ketone, diethylborane,
tetraethylborate ion, diethyl ether, diethyl sulfide, acetalde-
hyde, acetone, acetic acid, ethyl acetate, acetamide, acetyl
chloride, propanenitrile, ethyl fluoride, and ethyl chloride.
Show nonbonding electrons as dots and electrons in bonds
as lines.
PROBLEM 2.43 Draw all the isomers of “bromopentane,”
C
5
H
11
Br. Give systematic names to all of them.
PROBLEM 2.44 How many signals would a
13
C NMR spectrome-
ter “see” for each of the molecules in your answer to Problem 2.43?
PROBLEM 2.45 Draw all the isomers of “chlorohexane,”
C
6
H
13
Cl. Give proper systematic names to all of them. Hint:
There are 17 isomers.
2.18 Additional Problems 95
PROBLEM 2.46
Use your model set to look down the
bond of 2-chlorobutane. Draw the Newman
projection for all possible staggered conformations. Circle the
one you think is the most stable and explain why you’ve chosen
that one.
PROBLEM 2.47 Use your model set to look down the
bond of 2-bromo-3-methylbutane. Draw the
Newman projection for all possible staggered conformations.
Circle the one you think is the most stable and explain why
you’ve chosen that one. Determine the number of gauche
interactions in each projection.
PROBLEM 2.48 Draw the Newman projections for the differ-
ent eclipsed and staggered conformations of 2,3-dichlorobutane.
Label each projection as either eclipsed or staggered. In each
staggered projection, determine the number and type of gauche
interactions.
PROBLEM 2.49 Draw Newman projections of the eclipsed
and staggered conformations of 1,2-dichloropropane by looking
down the bond.C(1)
O
C(2)
C(2)
O
C(3)
C(2)
O
C(3)
C
H
Cl
CH
3
ClCH
2
(e)
(f)
(a)
CH
2
(d)
HC C
(b)
C(CH
3
)
4
(c)
H H
C
HC
H
C
PROBLEM 2.50 Draw Newman projections constructed by
looking down the bond of 2-methylpentane.
Repeat this process looking down the bond. In
each case, indicate which conformations will be the most stable.
PROBLEM 2.51 Although a chlorine is about the same size as
a methyl group, the conformation of 1,2-dichloroethane in
which the two chlorines are eclipsed is higher in energy than
the conformation of butane in which the two methyl groups are
eclipsed. Explain. Hint: Size isn’t everything!
PROBLEM 2.52 What is the approximate hybridization of the
indicated carbon in the following compounds?
C(2)
O
C(3)
C(1)
O
C(2)
PROBLEM 2.54 Use the boiling point data in Table 2.4 to
estimate the boiling point of pentadecane, C
15
H
32
.
PROBLEM 2.55 Rationalize the differences in the melting
points for the isomeric pentanes shown below.
(a) Xanturil is a diuretic
H
3
C
O
N
O
O
N
CH
3
N
N
(b) Viquidil is a vasodilator and
antiarrhythmic found in cinchona bark
HN
O
O
N
N
Pentane
mp –129.7 ⬚C
Isopentane
mp –159.9 ⬚C
Neopentan
e
mp –16.5 ⬚C
PROBLEM 2.53 Indicate the hybridization for each carbon,
nitrogen, and oxygen in the molecules shown below.
Put a circle around the sp
3
-hybridized atoms, a triangle
around sp
2
-hybridized atoms and a box around the
sp-hybridized atoms.
PROBLEM 2.56 Imagine replacing two hydrogens of a mol-
ecule with some group X. For example, methane (CH
4
) would
become CH
2
X
2
. (a) What compounds would be produced by
replacing two hydrogens of ethane? (b) What compounds
would be produced by replacing two hydrogens of propane?
(c) What compounds would be produced by replacing two
hydrogens of butane?
PROBLEM 2.57 Write systematic names for all the pentanes
and hexanes.
PROBLEM 2.58 How many signals would a
13
C NMR spec-
trometer “see” for each of the molecules in your answer to
Problem 2.57?
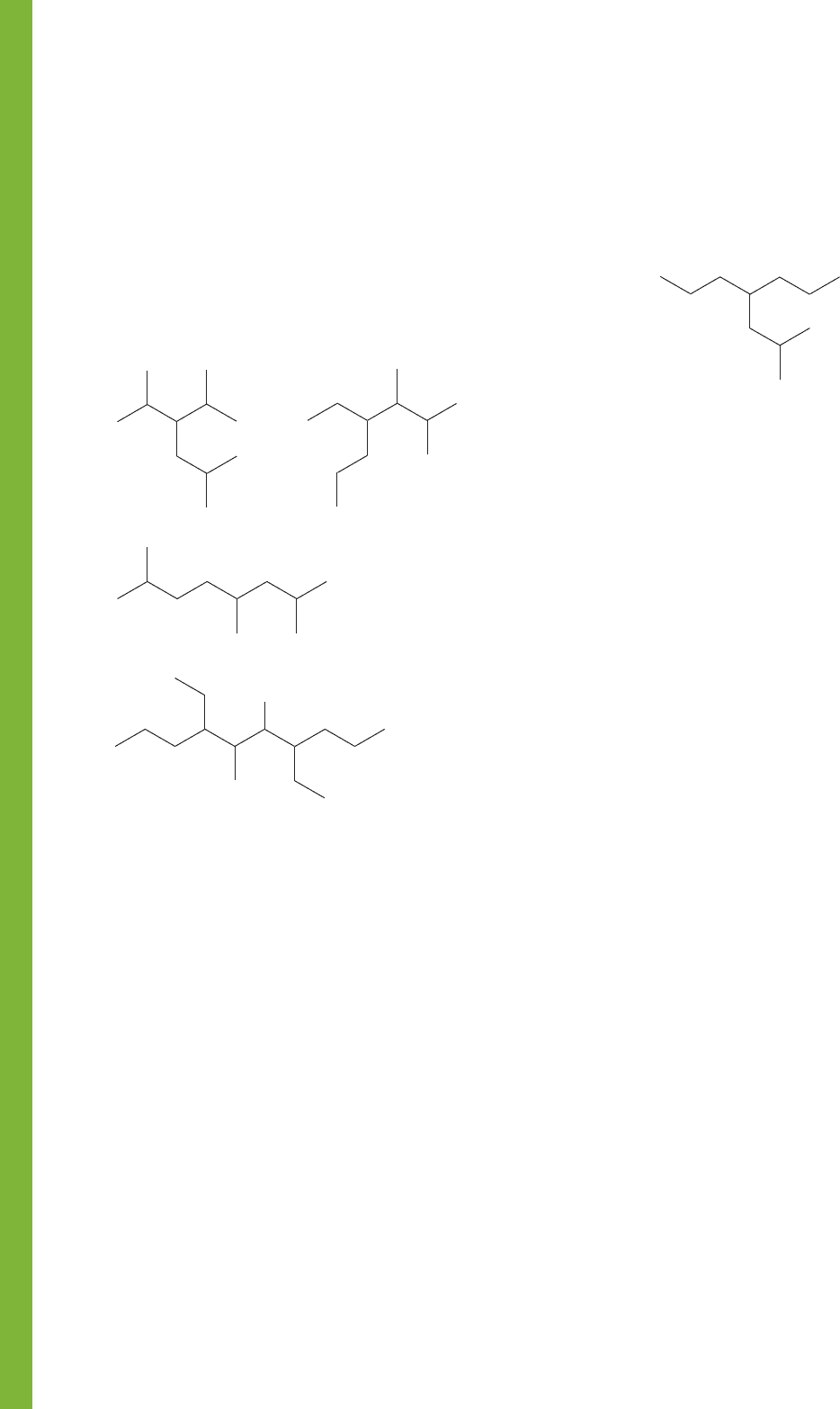
96 CHAPTER 2 Alkanes
PROBLEM 2.59 Draw and write systematic names for all the
isomers of nonane (C
9
H
20
). (Hint: There is a rumor that
there are 35 isomers!) This problem is hard to get completely
right.
You may want to continue isomer drawing and naming on
your own. But be careful, this is no trivial task. For example,
there are 75 structural isomers of decane and over 6500 for
tetradecane.
PROBLEM 2.60 Write systematic names for the following
compounds:
PROBLEM 2.63 One of the octane isomers of Problem 2.28,
3-ethyl-2-methylpentane, could have been reasonably named
otherwise. Draw this compound and find the other reasonable
name. The answer will tell you why the name given is preferred.
PROBLEM 2.64 Name the following compound:
(a)
(b)
(d)
(c)
Br
PROBLEM 2.61 Draw structures for the following compounds:
(a) 4-ethyl-4-fluoro-2-methylheptane
(b) 2,7-dibromo-4-isopropyloctane
(c) 3,7-diethyl-2,2-dimethyl-4-propylnonane
(d) 2-chloro-7-iodo-5-isobutyldecane
PROBLEM 2.62 Although you should be able to draw struc-
tures for the following compounds, each is named incorrectly
below. Give the correct systematic name for each of the follow-
ing compounds:
(a) 2-methyl-2-ethylpropane
(b) 2,6-diethylheptane
(c) 1-bromo-3-propylpentane
(d) 5-fluoro-8-methyl-3-tert-butylnonane
PROBLEM 2.65 Show the curved arrow formalism (electron
pushing or arrow pushing) for the protonation of water in sul-
furic acid (HOSO
2
OH H
2
SO
4
).
PROBLEM 2.66 Show the curved arrow formalism (electron
pushing or arrow pushing) for the protonation of ammonia
(NH
3
) in hydrochloric acid.
PROBLEM 2.67 Show the curved arrow formalism for the pro-
tonation of an alcohol (ROH) in sulfuric acid.
Use Organic Reaction Animations (ORA) to answer the
following questions:
PROBLEM 2.68 Choose the reaction titled “S
N
2 with cyanide”
and click on the Play button. As you watch the reaction occur,
notice that the methyl group on the left rotates during the
process. Explain why there is rotation.
PROBLEM 2.69 Choose the reaction “Alkene hydrohalogena-
tion” and click on the Play button. Notice that there are two
steps to this reaction. Identify the electrophile and the nucleo-
phile in the first step. Identify the electrophile and the nucleo-
phile in the second step.
PROBLEM 2.70 Choose the reaction “Bimolecular nucleophilic
substitution: S
N
2” and click on the Play button. Notice that the
S
N
2 is a one-step reaction. What is the nucleophile in this
reaction? You can click on the highest occupied molecular
orbital (HOMO) button to observe the electron density of the
nucleophile. What is the electrophile? The electrophile is shown
in the LUMO movie.

Alkenes and Alkynes
97
3.1 Preview
3.2 Alkenes: Structure and
Bonding
3.3 Derivatives and Isomers of
Alkenes
3.4 Nomenclature
3.5 The Cahn–Ingold–Prelog
Priority System
3.6 Relative Stability of Alkenes:
Heats of Formation
3.7 Double Bonds in Rings
3.8 Physical Properties of Alkenes
3.9 Alkynes: Structure and
Bonding
3.10 Relative Stability of Alkynes:
Heats of Formation
3.11 Derivatives and Isomers of
Alkynes
3.12 Triple Bonds in Rings
3.13 Physical Properties of Alkynes
3.14 Acidity of Alkynes
3.15 Molecular Formulas and
Degrees of Unsaturation
3.16 An Introduction to Addition
Reactions of Alkenes and
Alkynes
3.17 Mechanism of the Addition of
Hydrogen Halides to Alkenes
3.18 The Regiochemistry of the
Addition Reaction
3.19 A Catalyzed Addition to
Alkenes: Hydration
3.20 Synthesis: A Beginning
3.21 Special Topic: Alkenes and
Biology
3.22 Summary
3.23 Additional Problems
HOT CHEMISTRY The smallest alkyne is acetylene It is a gas and its
most common use is for welding. An acetylene/oxygen mixture burns at the very high
temperature of 3200 °C (5800 °F).
(HC
q
CH).
3

98 CHAPTER 3 Alkenes and Alkynes
CH
3
CH
2
CH
2
CH
3
CH
2
CH
2
H
2
C
H
2
C
Butane
(C
4
H
10
)
a saturated alkane
Cyclobutane
(C
4
H
8
)
a cycloalkane
FIGURE 3.1 The chemical properties
of saturated alkanes, C
n
H
2n2
,are
very similar to those of the
cycloalkanes, C
n
H
2n
.
On the atomic and subatomic levels, weird electrical forces are crackling
and flaring, and amorphous particles ...are spinning simultaneously
forward, backward, sideways, and forever at speeds so uncalculable that
expressions such as “arrival,” “departure,” “duration,” and “have a nice
day” become meaningless. It is on such levels that magic occurs.
—TOM ROBBINS,
1
SKINNY LEGS AND ALL
3.1 Preview
Not all hydrocarbons have the formula C
n
H
2n2
. Indeed, we have already seen some
that do not: the ring compounds, C
n
H
2n
(Fig. 3.1). We noted in Chapter 2 (p. 83)
that the chemical properties of these ring compounds closely resemble those of the
1
Thomas Eugene Robbins is an American author born in 1936 in Blowing Rock, NC.
acyclic saturated hydrocarbons. There is another family of hydrocarbons that also
has the formula C
n
H
2n
,many of whose chemical properties are sharply different from
those of the ring compounds and saturated chains we have seen so far. These com-
pounds are different from other hydrocarbons in that they contain carbon–carbon
double bonds. One bond is a sigma bond similar to the sigma bond of alkanes, the
other is a weaker bond formed by overlapping pi orbitals (see Section 3.2).They are
called alke
nes to distinguish them from the saturated alkanes.
There are also hydrocarbons of the formula C
n
H
2n2
, whose chemical proper-
ties resemble those of the alkenes but not those of the alkanes or cycloalkanes.These
compounds are the acetylenes, or alky
nes.
Alkenes and alkynes contain fewer hydrogen atoms than alkanes with the same
number of carbons. Because they are not “saturated” with hydrogen, they are called
unsaturated hydrocarbons. The structures and some of the properties of the fami-
lies of alkenes and alkynes are discussed in this chapter.
Our study of the structures of alkenes and alkynes allows us to begin to exam-
ine chemical reactivity in a serious way.
ESSENTIAL SKILLS AND DETAILS
1. The hybridization model for sp
2
and sp bonding. It is critical that you be able to use the
hybridization model to derive structures for the alkenes (double bonds) and the alkynes
(triple bonds).
2. Reactivity—the addition reaction. In this chapter, we encounter reactivity, as addition
reactions to alkenes appear. It is most important to see these reactions not as the first of
a near-endless series of different processes, but as the initial exemplars of classes of
reactions. Be sure you can relate what you learn about these addition reactions to the
discussion of acids and bases in Chapters 1 and 2.
3. Priorities! The Cahn–Ingold–Prelog priority system is essential for determining E and
Z, the arrangement of groups around a double bond.
4. Character counts. It is important to see the relationship between the amount of s
character of an orbital and the stability of an electron in that orbital.
