Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.

3.3 Derivatives and Isomers of Alkenes 109
to show that the two hydrogens on the end (called terminal) methylene group are
not the same. One, H
a
, is on the same side of the double bond as the methyl group,
whereas the other, H
a′
, is on the opposite side from the methyl. Replacing hydro-
gens H
a
and H
a′
with X gives two different compounds.
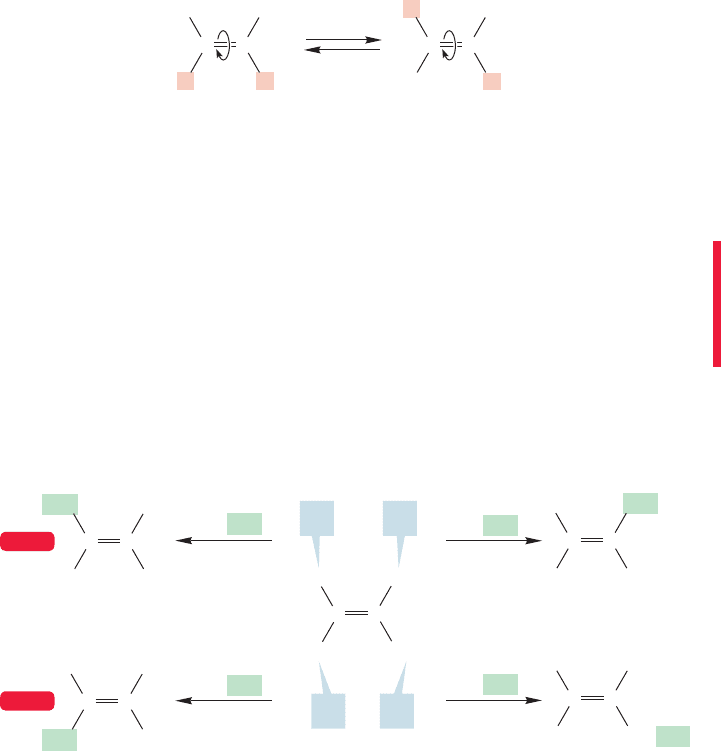
The two molecules on the right of Figure 3.20 are interconvertible only by rota-
tion about the double bond (Fig. 3.21). We know the rotation shown in Figure 3.21
Z
usammen ( Z ):
Hydrogens
on same side
Entgegen (E ):
Hydrogens
on opposite sides
X
H
H
rotate
X
H
CC
CH
3
H
CC
CH
3
FIGURE 3.21 The (hypothetical)
interconversion of two isomers of
by rotation
about the carbon–carbon
double bond.
CH
3
CH
P
CH
O
X
requires about 66 kcal/mol, too high an amount of energy for interconversion to be
common.The molecule with the two hydrogens on the same side of the double bond
is designated as cis or (Z) (for zusammen, German for “together”). The compound
with the hydrogens on the opposite sides is called trans or (E) (for entgegen, German
for “opposite”). Recall the use of cis and trans in our discussion of ring compounds
(p. 84).
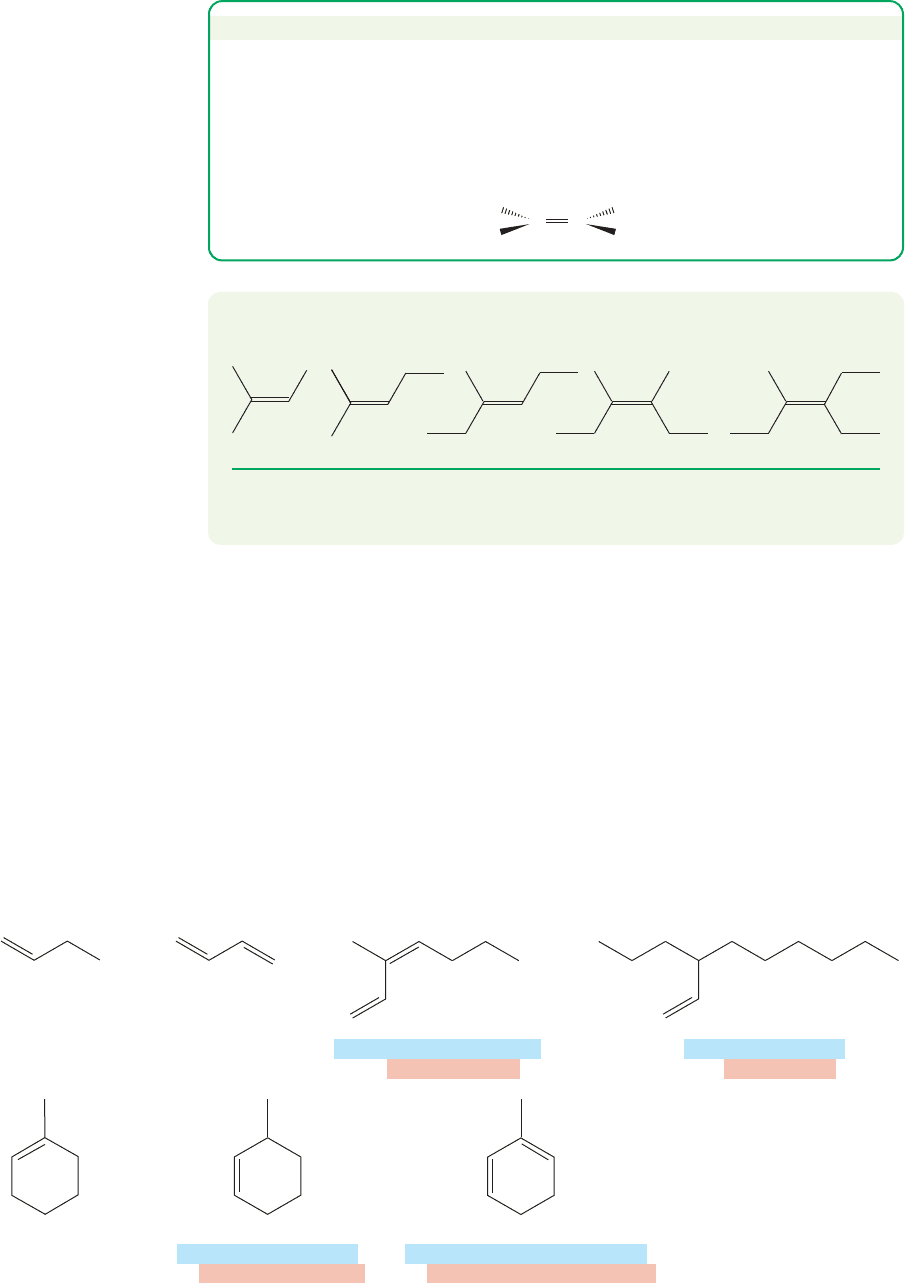
If we let X methyl we get the butenes (C
4
H
8
).The four isomeric butenes are
1-butene,cis-2-butene,trans-2-butene, and 2-methylpropene (or isobutene, but also
sometimes called isobutylene).These are shown in Figure 3.22.The numbers in the
H
H
H
C
C
CH
3
H
H
CC
CH
3
CH
3
H
H
HH
CC
CH
3
CC
CH
3
H
H
H
CC
CH
2
CH
3
H
3
C
H
3
C
H
a
'
H
c
replace H
a
with CH
3
replace H
a'
with CH
3
replace H
b
with CH
3
replace H
c
with CH
3
trans-2-Butene
(E )-2-butene
cis-2-Butene
( Z )-2-butene
2-Methylpropene
isobutene
1-Butene
H
a
H
b
WEB 3D
WEB 3D
FIGURE 3.22 Replacement of the
four different hydrogens in propene
by a methyl group leads to the four
butene isomers.
names 1-butene, cis-2-butene, and trans-2-butene are used to locate the position of
the double bond.We will shortly discuss the naming convention for alkenes, but you
might try to figure it out here from the names and structures of the butenes.
Not every alkene is capable of cis/trans (Z/E) isomerism! Ethylene and propene
are not, and of the butenes only 2-butene has such isomers. Dealing with cis and
trans isomers is an essential skill that can be “cemented” forever right now. Future
difficulties can be avoided if you try Problem 3.4. It is simple but worthwhile.
Once we get to alkenes containing more than four carbons, the systematic naming
protocol takes over. Five-carbon compounds (C
5
) are called pentenes, six-carbon
compounds (C
6
) are called hexenes,and so on. Be alert for isomerism of the cis/trans
(Z/E) kind. It takes some practice to find it.
CONVENTION ALERT
110 CHAPTER 3 Alkenes and Alkynes
PROBLEM SOLVING
Alkenes are flat! In the minimum energy form of an alkene, the two carbon
atoms making up the double bond and the four atoms directly attached to it
(atoms 1, 2, 3, and 4) are coplanar. It is very easy to forget that fact, or ignore it.
Don’t, because it leads immediately to the idea that alkenes have discernable
sides.Thus, cis and trans isomers become possible for many alkenes.
C
C
1
4
3
2
PROBLEM 3.4 Which of the following alkenes is capable of cis/trans (Z/E)
isomerism?
PROBLEM 3.5 Write all the isomers of the pentenes (C
5
H
10
) and hexenes
(C
6
H
12
). Be alert for isomerism of the cis/trans (Z/E) kind in these molecules.
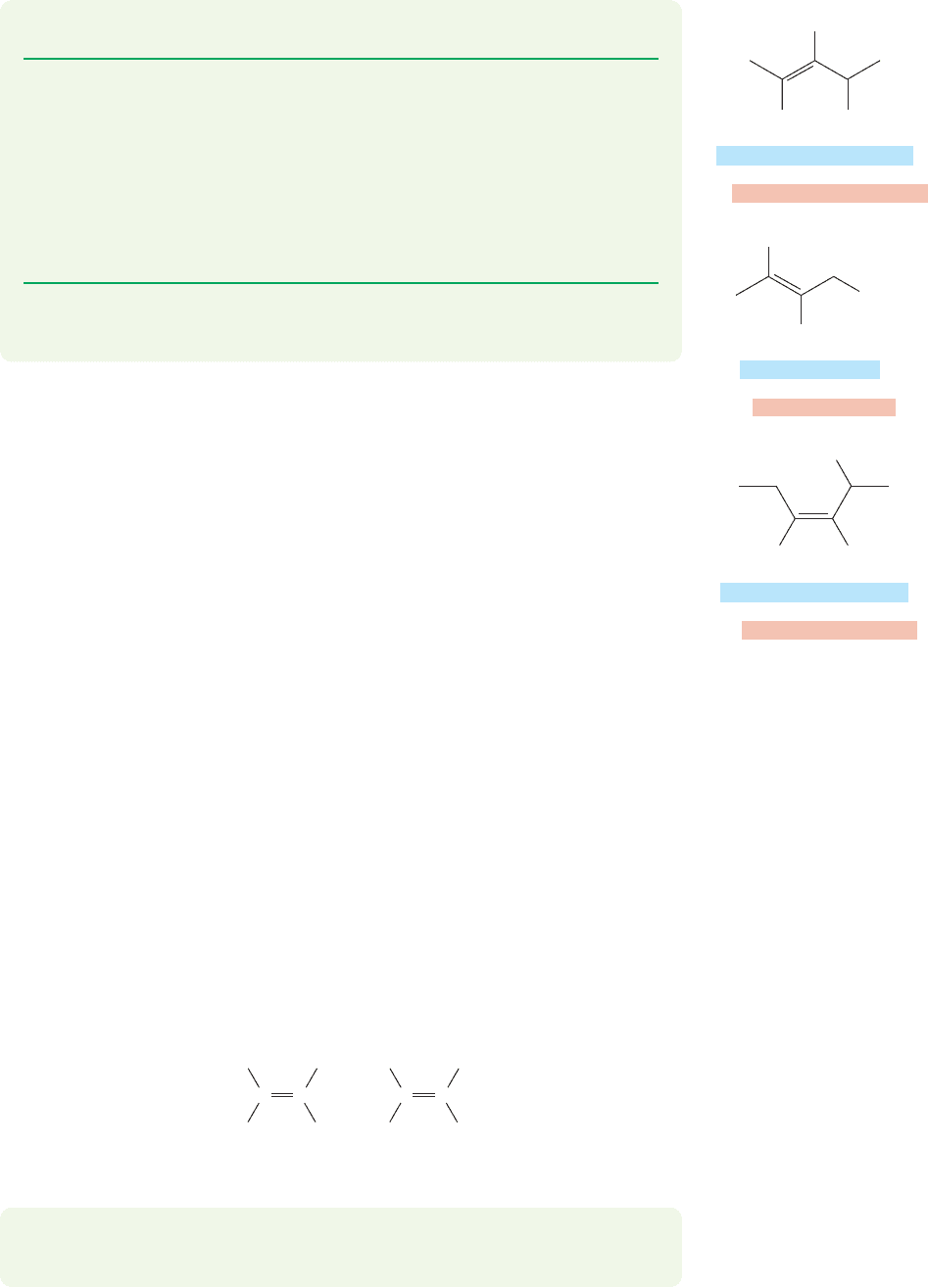
3.4 Nomenclature
Most of the rules for naming alkenes are similar to those used for alkanes, with a
number attached to indicate the position of the double bond. Chains are numbered
so as to give the double bond the smallest possible number. One important rule that
is different in the alkenes is that the name is based on the longest chain containing
a double bond whether or not it is also the longest chain in the molecule. When
molecules contain more than one double bond, they are called polyenes, and are
named as dienes, trienes, and so on. In these cases, the longest straight chain is
defined as the one with the greatest number of double bonds. Cyclic molecules are
named as cycloalkenes.These rules are illustrated with the molecules in Figure 3.23.
2
2
2
2
3
3
3
3
6
79
8
10
7
8
9
6
6
6
6
6
6
6
4
4
4
4
1
1
1
1
5
5
5
5
1-Butene
not 3-butene
1,3-Butadiene
3-Methyl-1,3-heptadiene
not 2-vinyl-2-hexene
3-Propyl-1-nonene
not 4-vinyldecane
1-Methylcyclohexene 3-Methylcyclohexene
not 6-methylcyclohexene
2-Methyl-1,3-cyclohexadiene
not 3-methyl-1,3-cyclohexadiene
2
2
2
2
3
3
3
3
7
4
4
4
4
1
1
1
1
5
5
5
5
FIGURE 3.23 Examples of the naming protocols for alkene nomenclature.
3.4 Nomenclature 111
PROBLEM 3.6 Name the pentenes and hexenes of Problem 3.5.
PROBLEM 3.7 Recall p. 88 in Chapter 2 where the
13
C NMR spectrometer was
described.That machine will find a signal for each different carbon in a molecule.
How many signals will each of the following molecules show in its
13
C NMR
spectrum?
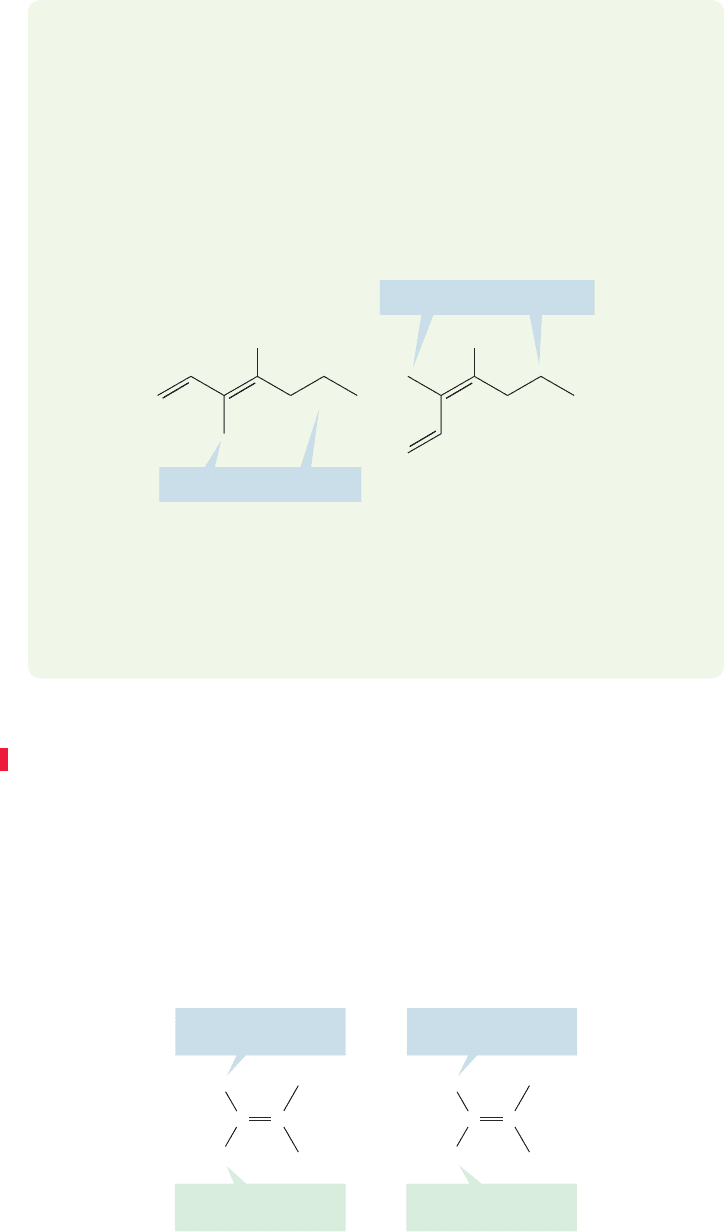
Finally, there are examples in which the numbering of a substituent becomes
important. Usually, it is the double bond numbering that must be considered
first. The double bond is usually given the lowest possible number (Fig. 3.24a).
An important exception is the hydroxyl (OH) group, which has priority
over the double bond, and is given the lower number (Fig. 3.24b). Only if
there are two possible names in which the double bond has the same number
do you have to consider the position of the substituent. In such a case,
give preference to the name with the lower number for the substituent
(Fig. 3.24c).
Usually the cis/trans naming system is adequate to distinguish pairs of iso-
mers, but not always. Problem 3.10 and Figure 3.25 give examples in which
cis/trans naming is inadequate. In the molecule 1-bromo-1-chloropropene there
are clearly two isomers, but it is not obvious which is cis and which is trans. It
was to solve such problems that the old cis/trans system was elaborated into the
(Z/E) nomenclature by three European chemists, R. S. Cahn (1899–1981), C. K.
Ingold (1893–1970), and V. Prelog (1906–1998). The Cahn–Ingold–Prelog
priority system ranks the groups attached to the double bonds, with the higher
priority being 1 and the next 2. The compound with the higher priority groups
on the same side of the double bond is (Z), and the other is (E). This priority
system has other, very important uses to be encountered in Chapter 4, and so we
will spend some time here elaborating it, even though we will see it again in
another context.
5
1
42
3
(E)-4-Chloro-2-pentene
trans-4-Chloro-2-pentene
n
ot trans-2-chloro-3-pentene
24
135
13
24
(E)-2-Buten-1-ol
trans-2-Buten-1-ol
not trans-2-buten-4-ol
4
12 56
65 21
43
34
2
3
1
(Z)-2-Chloro-3-hexene
cis-2-Chloro-3-hexene
not cis-5-chloro-3-hexene
H H
Cl
OH
Cl
H
H
(a)
(b)
(c)
H
H
FIGURE 3.24 The double bond takes
precedence over most substituents,
but when two names put the double
bond at the same numbered position,
we use the name in which the
substituent has the lower number.
PROBLEM 3.9 Draw the following molecules: cis-2-pentene, 2-chloro-1-pentene,
trans-3-penten-2-ol, 4-bromocyclohexene, 1,3,6-cyclooctatriene.
H
Cl
H
3
CBr
CC C
H
Cl
H
3
C
Br
C
FIGURE 3.25 Sometimes the cis/trans
convention is inadequate to
distinguish two isomers. Which of
these two isomers of 1-bromo-
1-chloropropene would you call cis
and which trans?
(a) ethylene
(b) propene
(c) 1-butene
(d) cis-2-butene
(e) trans-2-butene
(f) isobutene (2-methylpropene)
PROBLEM 3.8 How many signals will each of the molecules in Problem 3.4 show
in its
13
C NMR spectrum?
112 CHAPTER 3 Alkenes and Alkynes
WORKED PROBLEM 3.10 In Figure 3.23, the structure of 3-methyl-1,3-heptadiene
is not completely specified by that name. Even if the cis/trans nomenclature is
applied, problems remain. What’s the difficulty? Can you devise a system for
resolving the ambiguity?
ANSWER In describing fully the structure of 3-methyl-1,3-heptadiene, we
need to specify how groups are oriented in space (the stereochemistry), in
order to distinguish the two possible forms. In one of the structures, the two
saturated alkyl groups (methyl and propyl) are cis while in the other they
are trans.
Often, cis/trans will do the job, but here these terms fail.There is but one H, so
you cannot find two hydrogens “on the same side” (cis), and two hydrogens “on
opposite sides” (trans). That is the problem; it is up to you to devise a protocol
for resolving it. For more about the device used by organic chemists, the Z/E
system, read on in the text.
cis CH
3
and CH
2
CH
2
CH
3
trans CH
3
and CH
2
CH
2
CH
3
H H
3.5 The Cahn–Ingold–Prelog Priority System
1. The first step in using the priority system is to distinguish atoms on the basis of
atomic number. The atom of higher atomic number has the higher priority
(Fig. 3.26). Thus, a methyl group, attached to the double bond through a carbon
atom (atomic number 6), has a higher priority than the hydrogen attached to the
same carbon (atomic number 1). Similarly, in the second compound, oxygen (atomic
number 8) has a higher priority than the boron attached to the same carbon
(atomic number 5).
Higher priority
(atomic number = 8)
Lower priority
(atomic number = 5)
H
2
B
H
3
CO
C
H
Higher priority
(atomic number = 6)
Lower priority
(atomic number = 1)
H
3
C
C
C C
FIGURE 3.26 The substituent with
the higher atomic number gets the
higher priority.
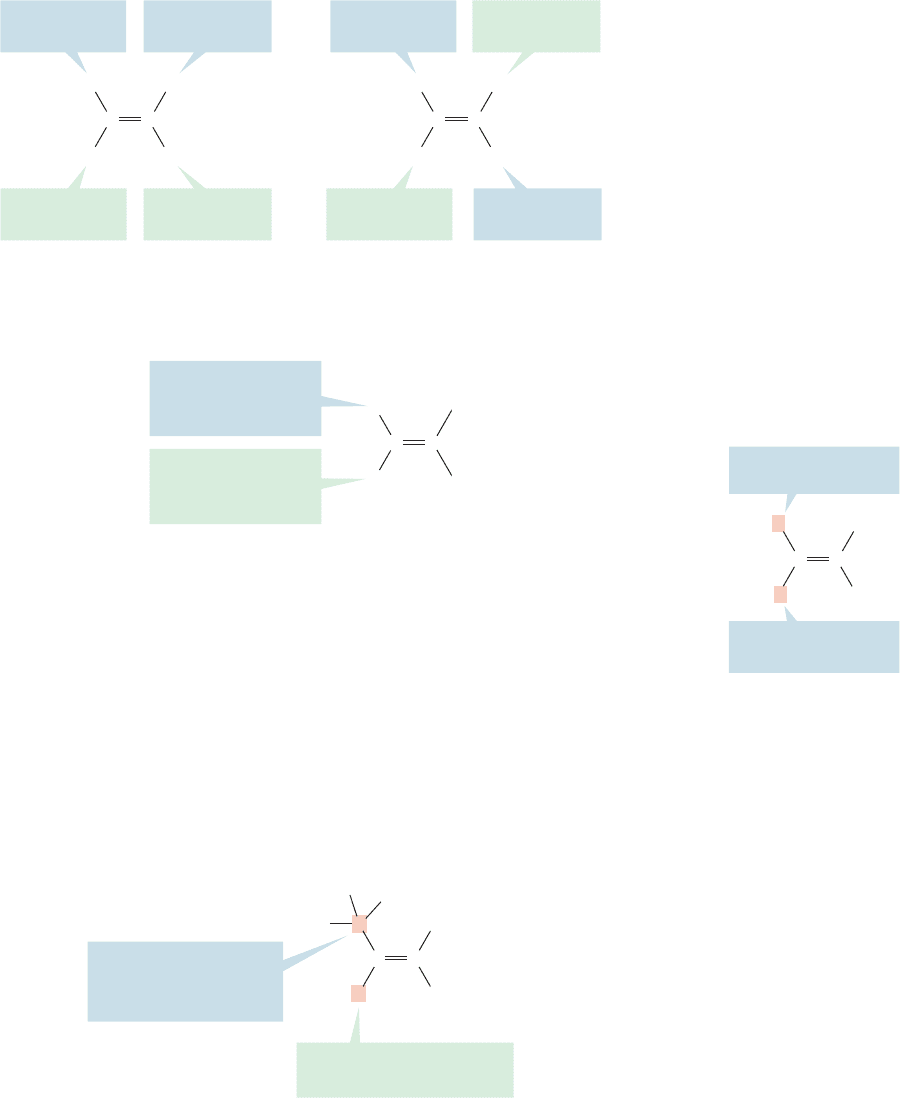
How is this first rule used in practice? Consider the two isomers in Figure 3.25.
Bromine has a higher atomic number than chlorine, so the isomer in which the
CONVENTION ALERT
3.5 The Cahn–Ingold–Prelog Priority System 113
higher-priority methyl group and the higher-priority bromine are on the same side
is (Z), and the isomer in which the lower-priority hydrogen is on the same side as
the higher-priority bromine is (E) (Fig. 3.27).
H
( Z )-Isomer: The high-priority
groups are on the same side
( E )-Isomer: The high-priority
groups are on opposite sides
Cl
H
3
CBr
CC C
H
Cl
H
3
C
Br
C
Higher priority
(right C)
Higher priority
(left C)
Higher priority
(left C)
Lower priority
(right C)
Lower priority
(right C)
Lower priority
(left C)
Lower priority
(left C)
Higher priority
(right C)
FIGURE 3.27 Use of the
Cahn–Ingold–Prelog priority system
in naming alkenes.
2. For isotopes, atomic mass is used to break the tie in atomic number. Thus deuteri-
um (atomic number 1, atomic mass 2) has a higher priority than hydrogen (atomic
number 1, atomic mass 1) (Fig. 3.28).
H
D
C
Higher priority
(atomic number = 1,
atomic mass = 2)
Lower priority
(atomic number = 1,
atomic mass = 1)
C
FIGURE 3.28 When the atomic numbers are the same, the
heavier isotope gets the higher priority.
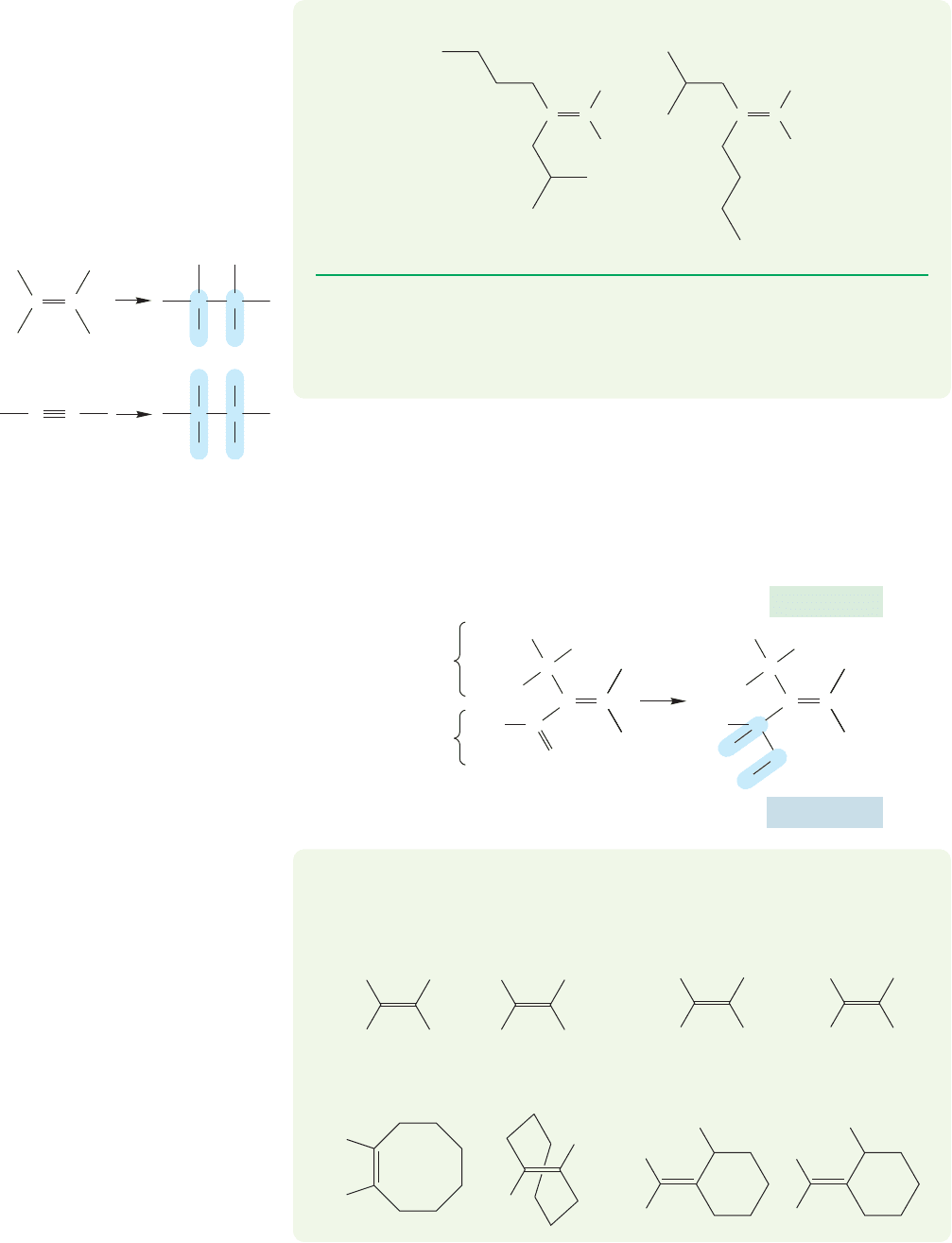
3. Nonisotopic ties are broken by looking at the groups attached to the tied atoms. For
example, 1-chloro-2-methyl-1-butene has a double bond to which a methyl and an
ethyl group are attached. Both these groups are connected to the double bond
through carbons, so Rule 1 does not differentiate them (Fig. 3.29).To break the tie,
we look at the groups attached to these carbons. The carbon of the methyl group is
attached to three hydrogens, whereas the “first”carbon of the ethyl group is attached
to two hydrogens and a carbon. Therefore the ethyl group gets the higher priority
(Fig. 3.30). If the groups are still tied after this procedure, one simply looks farther
out along the chain to break the tie, as in Problem 3.11.
C
H
3
C
Same priority
(atomic number = 6)
Same priority
(atomic number = 6)
1-Chloro-2-methyl-1-butene
H
H
3
CCH
2
Cl
C
FIGURE 3.29 In this molecule,
the two C atoms attached to
the double bond of course have the
same atomic number and, therefore,
the same priority according to
Cahn–Ingold–Prelog Rule 1.This
isomer is Z.
Cl
H
H
3
C
H
3
C
C
H
H
This C is attached to
two H atoms and
one C = higher priority
This C is attached to three
H atoms = lower priority
C
C
FIGURE 3.30 The red methyl C is attached to three H atoms.The red ethyl C is attached
to C, H, and H. The ethyl C gets the higher priority. Because the two higher priority
groups, ethyl and Cl, are on the same side of the double bond, this isomer is Z.
114 CHAPTER 3 Alkenes and Alkynes
PROBLEM 3.11 Determine which of the following molecules is (Z) and which is (E).
PROBLEM 3.12 Make drawings of the following molecules:
(a) (E)-3-fluoro-3-hexene
(b) (E)-4-ethyl-3-heptene
(c) (Z)-1-bromo-2-chloro-2-fluoro-1-iodoethylene
CC
Cl
H
CC
Cl
H
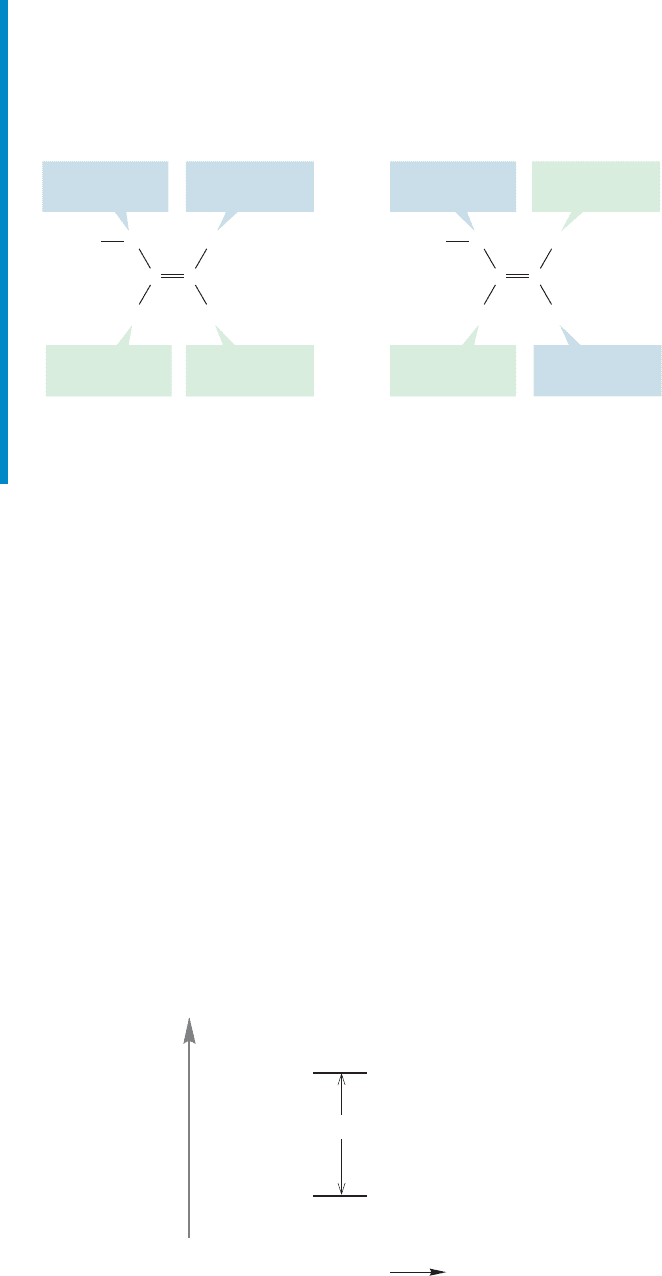
4. Multiple bonds attached to alkenes are treated as multiplied single bonds. A dou-
ble bond to carbon is considered to be two single bonds to the carbons in the double
bond, as shown in Figure 3.31. This convention results in an isopropenyl group
being of higher priority than a tert-butyl group, for example (Fig. 3.32). As we will
shortly see, it is also possible to connect two carbon atoms through a
triple bond
(see Section 3.9). The priority system treats triple bonds in a similar fashion.
C C
C
C
C
C
C C
C
C
C
C C
C
FIGURE 3.31 The priority of doubly
bonded carbons is determined by
adding two single carbon bonds as
shown. A triple bond is treated
similarly.
H
3
C
CH
2
tert-Butyl
group
Isopropenyl
group
Higher priority
Lower priority
C
C
C
CH
3
H
3
C
H
3
C
C
H
3
C
CH
2
C
C
C
C
C
CH
3
H
3
C
H
3
C
C
FIGURE 3.32 An isopropenyl group
has a higher priority than a tert-butyl
group.
PROBLEM 3.13 In each of the following pairs of isomers, which is (Z), and which
is (E)?
(c)
(b)
(a)
(d)
H
3
C
H
D
H
H
H
3
C
H
D
H
H
H
H
3
C
H
3
C
NH
2
NH
2
CH
3
CH
2
CH
3
CH
2
H
F
HH
H
3
C
F
H
H
H
3
C
3.6 Relative Stability of Alkenes: Heats of Formation 115
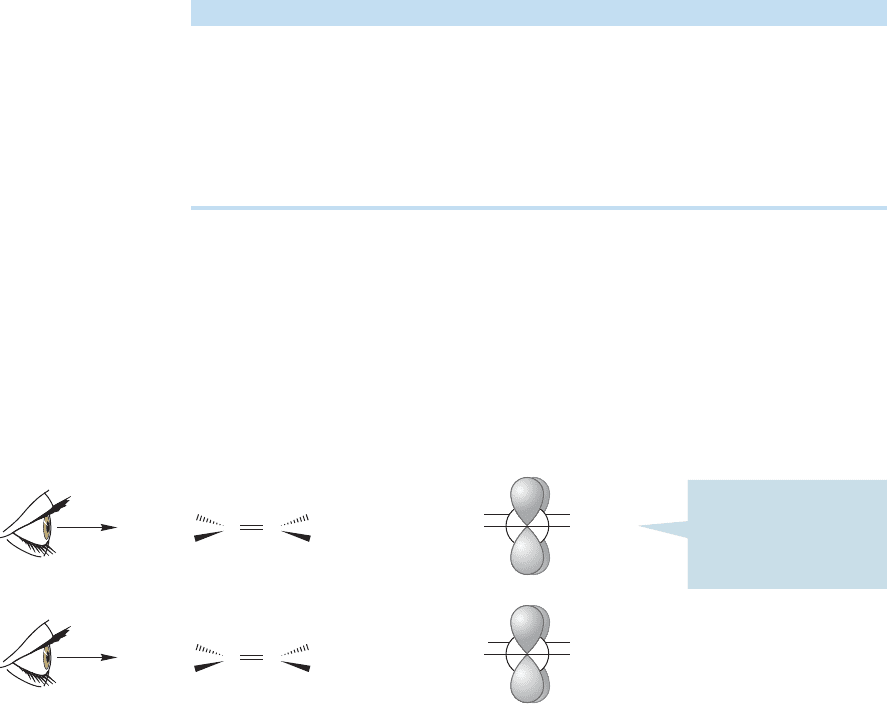
Summary
The technique of assigning (Z) and (E) is to determine the priority (high or low)
at each carbon of the double bond.The isomer with the two high-priority groups
on the same side of the double bond is (Z).The isomer with the two high-priority
groups on opposite sides is (E). Two examples are given in Figure 3.33.
FIGURE 3.33 Two examples of the
Cahn–Ingold–Prelog priority system
at work.
3.6 Relative Stability of Alkenes: Heats of Formation
The heat of formation of a compound is the enthalpy of formation from its
constituent elements in their standard states.The standard state of an element is the
most stable form of the element at 25 °C and 1 atm pressure. For an element in its
standard state, is taken as zero.For carbon,the standard state is graphite.Thus,
for graphite, as well as simple gases (e.g., H
2
,O
2
, and N
2
), is 0 kcal/mol.The
more negative—or less positive—a compound’s is, the more stable the com-
pound is. A negative for a compound means that its formation from its con-
stituent elements is exothermic—heat is liberated in the reaction.In contrast,a positive
means that the constituent elements are more stable than the compound and
its formation is endothermic—energy must be applied.
Remember: Bonding is an energy-releasing process. We expect the formation of
a molecule from its constituent atoms to be an exothermic process.For example, con-
sider the simple formation of methane from carbon and hydrogen. Carbon in its
standard state (graphite) and gaseous H
2
have , whereas for
methane . The formation of methane from graphite and
hydrogen releases 17.8 kcal/mol. In other words, this reaction is exothermic by
17.8 kcal/mol (Fig. 3.34).
=-17.8 kcal>mol¢H °
f
= 0 kcal>mol¢H °
f
¢H °
f
¢H °
f
¢H °
f
¢H °
f
¢H °
f
(≤H °
f
)
Energy
C (Graphite) + H
2
(Gas)
ΔH
f
⬚ = 0 kcal/mol ΔH
f
⬚ = 0 kcal/mol ΔH
f
⬚ = –17.8 kcal/mol
CH
4
(Gas)
17.8 kcal/mol
CH
4
C + 2 H
2
FIGURE 3.34 The formation of
methane from graphite and gaseous
hydrogen is exothermic.
The two high-priority groups are
on the same side
—this molecule
is (Z)-2-methoxy-2-pentene
Here the situation is different
—
the two high-priority groups are
on opposite sides; this molecule
is (E )-2-methoxy-2-pentene
O
H
3
C
H
3
C
CH
2
CH
3
H
C
C
O
H
3
C
H
3
C
CH
2
CH
3
H
C
C
Higher priority
(right C)
Higher priority
(left C)
Higher priority
(left C)
Lower priority
(right C)
Lower priority
(right C)
Lower priority
(left C)
Lower priority
(left C)
Higher priority
(right C)
116 CHAPTER 3 Alkenes and Alkynes
Thus, the double bond in 1-hexene is monosubstituted and the double bond in
2-hexene or 3-hexene is disubstituted.The trans isomer is the more stable compound
(more negative ). Figure 3.35 shows the reason; in the cis isomer there is a cis
ethyl–ethyl eclipsing that is absent in the trans compound. In the trans isomer, both
alkyl groups are eclipsed by hydrogen, and the destabilizing ethyl–ethyl repulsion
is missing.
¢H °
f
Table 3.1 gives the heats of formation of a number of hexenes. First look at the
pair of disubstituted alkenes, the isomers cis- and trans-3-hexene. “Substituted”
simply means that an atom other than hydrogen is attached to the double bond.
TABLE 3.1 Heats of Formation for Some Hexenes
Isomer
10.0 Least stable
cis 11.2
trans 12.1
16.0
16.6 Most stable(CH
3
)
2
C
P
C(CH
3
)
2
(CH
3
)
2
C
P
CHCH
2
CH
3
CH
3
CH
2
CH
P
CHCH
2
CH
3
CH
3
CH
2
CH
P
CHCH
2
CH
3
CH
2
P
CHCH
2
CH
2
CH
2
CH
3
(kcal/mol)≤H
°
f
Z———————U
H
H
cis-3-Hexene
=
=
C
C
C
CH
2
CH
3
CH
3
CH
2
H
H
trans-3-Hexene
CH
2
CH
3
Here is the high-energy
destabilizing interaction
in the cis compound
—
the eclipsed ethyl–ethyl
“bumping”
Newman projections
CH
2
CH
3
CH
2
CH
3
H
CH
2
CH
3
CH
3
CH
2
C
CH
3
CH
2
H
H
H
FIGURE 3.35 Newman projections show the unfavorable ethyl–ethyl eclipsing interaction
in cis-3-hexene.
Both disubstituted isomers cis- and trans-3-hexene are more stable than the
monosubstituted 1-hexene, and the trisubstituted isomer is more stable than
either disubstituted molecule. Tetrasubstituted 2,3-dimethyl-2-butene has the
lowest energy of all (lowest heat of formation, most stable). From these obser-
vations, it would appear that the degree of substitution (the number of alkyl
groups attached to the double bond) is important in stabilizing the molecule.
In general, the more substituted the double bond, the more stable it is
(Table 3.1).
The reason for this has been the subject of some controversy. One good way to
look at the question focuses on the different kinds of carbon–carbon bonds present
in isomeric molecules of different substitution patterns.In all the molecules in Table
3.1, three kinds of σ carbon–carbon linkages are present: sp
2
/sp
2
, sp
2
/sp
3
, and sp
3
/sp
3
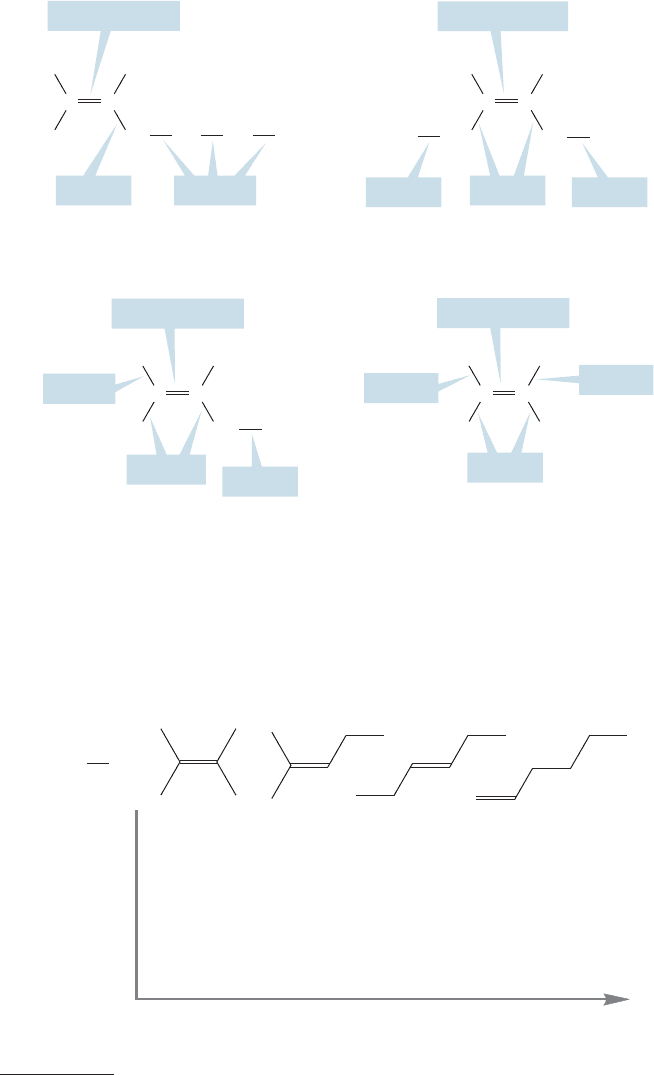
3.6 Relative Stability of Alkenes: Heats of Formation 117
bonds (Fig. 3.36). An electron in a 2s orbital is at lower energy than an electron
in a 2p orbital. The more s character in an orbital, the more an electron in it is
stabilized. In these isomeric hexenes, the more substituted the carbon–carbon
double bond is, the more relatively low-energy (strong) sp
2
/sp
3
bonds are present.
Monosubstituted
CH
2
CH
2
CH
2
CH
3
σ Bond sp
2
/sp
2
sp
3
/sp
3
sp
2
/sp
3
Disubstituted
H
2
CH
3
C
CH
2
CH
3
σ Bond sp
2
/sp
2
H
3
C
H
3
C
Trisubstituted Tetrasubstituted
H
CH
2
CH
3
sp
2
/sp
3
sp
3
/sp
3
sp
2
/sp
3
sp
2
/sp
3
sp
2
/sp
3
sp
3
/sp
3
sp
3
/sp
3
H
H
H
H
3
C
H
3
C
CH
3
CH
3
sp
2
/sp
3
sp
2
/sp
3
σ Bond sp
2
/sp
2
σ Bond sp
2
/sp
2
CC
CC CC
H
H
CC
FIGURE 3.36 Three kinds of
carbon–carbon σ bonds in hexene
isomers: sp
2
/sp
2
overlap, sp
2
/sp
3
overlap, and sp
3
/sp
3
overlap.
By contrast, less substituted alkenes have more relatively high-energy (weaker)
sp
3
/sp
3
bonds. The more strong bonds present, the more stable is the molecule.
Figure 3.37 shows the number of sp
3
/sp
3
, sp
2
/sp
3
, and sp
2
/sp
2
bonds in the isomer-
ic hexenes of Table 3.1. 2,3-Dimethyl-2-butene, the molecule with the strongest
bonds, is the most stable isomer of the set, and 1-hexene, with the weakest set of
bonds, is the least stable.
3
3
This analysis is somewhat primitive because it ignores the carbon–hydrogen bonds, which are also different
in differently substituted alkenes. However, the changes in carbon–carbon bonds dominate. You might try to
do a full comparison using detailed bond energies.
C C
σ Bonds
sp
2
/sp
2
sp
2
/sp
3
sp
3
/sp
3
Energy
Most
stable
1
4
0
1
3
1
1
2
2
Least
stable
1
1
3
FIGURE 3.37 The isomeric hexenes
have different numbers of three types
of carbon–carbon σ bonds.
118 CHAPTER 3 Alkenes and Alkynes
PROBLEM 3.14 Carry out an analysis similar to that of Figure 3.37 for the series
of molecules: 1-pentene, 2-methyl-2-butene, (Z)-2-pentene.
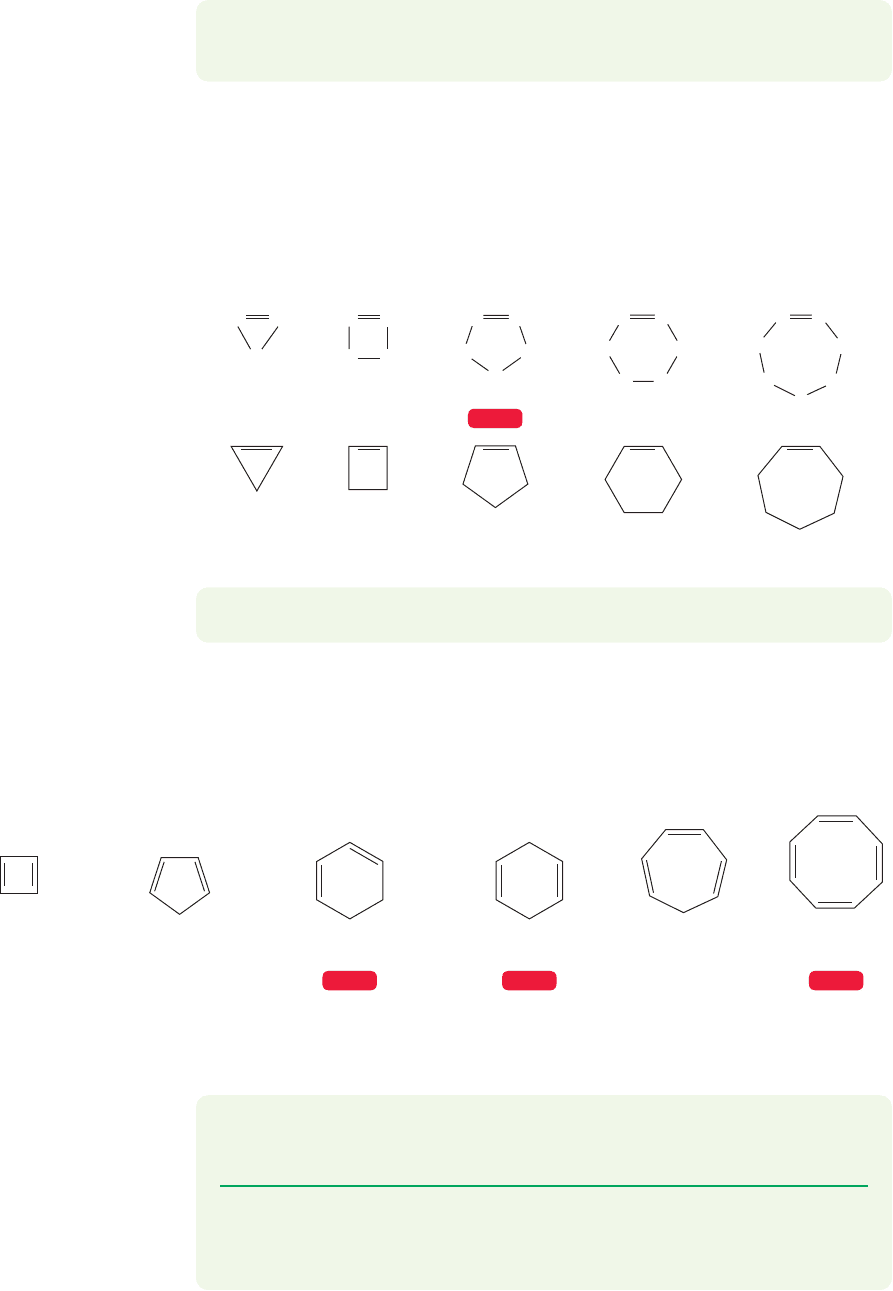
3.7 Double Bonds in Rings
Figure 3.38 shows a number of cycloalkenes, ring compounds containing one or more
double bonds. Note that even small rings can contain double bonds.
HC CH
CH
2
HC
H
2
C
CH
CH
2
HC
H
2
C
CH
HC CH
CH
2
CH
2
CH
2
CH
2
CH
2
CH
2
CH
2
H
2
C
H
2
C
H
2
C
H
2
C
HC
CH
WEB 3D
FIGURE 3.38 Some cyclic alkenes
(cycloalkenes).
1,3,5-Cyclo-
heptatriene
1,3-Cyclohexadiene 1,4-Cyclohexadiene
Cyclopentadiene
Cyclobutadiene
1,3,5,7-Cyclo-
octatetraene
WEB 3DWEB 3D WEB 3D
FIGURE 3.39 Some cyclic polyenes, ring compounds containing more than one double bond.
PROBLEM 3.15 Name all the compounds in Figure 3.38.
Once the ring becomes larger than cyclopropene, it becomes possible for it to
contain more than one double bond. Several such molecules are shown in
Figure 3.39.
PROBLEM 3.16 How many signals will appear in the
13
C NMR spectrum (p. 88)
of the compounds in Figure 3.39?
PROBLEM 3.17 Write the structures for 1,3,5-cyclohexatriene, 1,3,5,7-
cyclooctatetraene, 3-methyl-1,4-cyclohexadiene, 2-fluoro-1,3-cyclohexadiene,
and 2-bromo-1,4-cycloheptadiene.
