Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.

3.7 Double Bonds in Rings 119
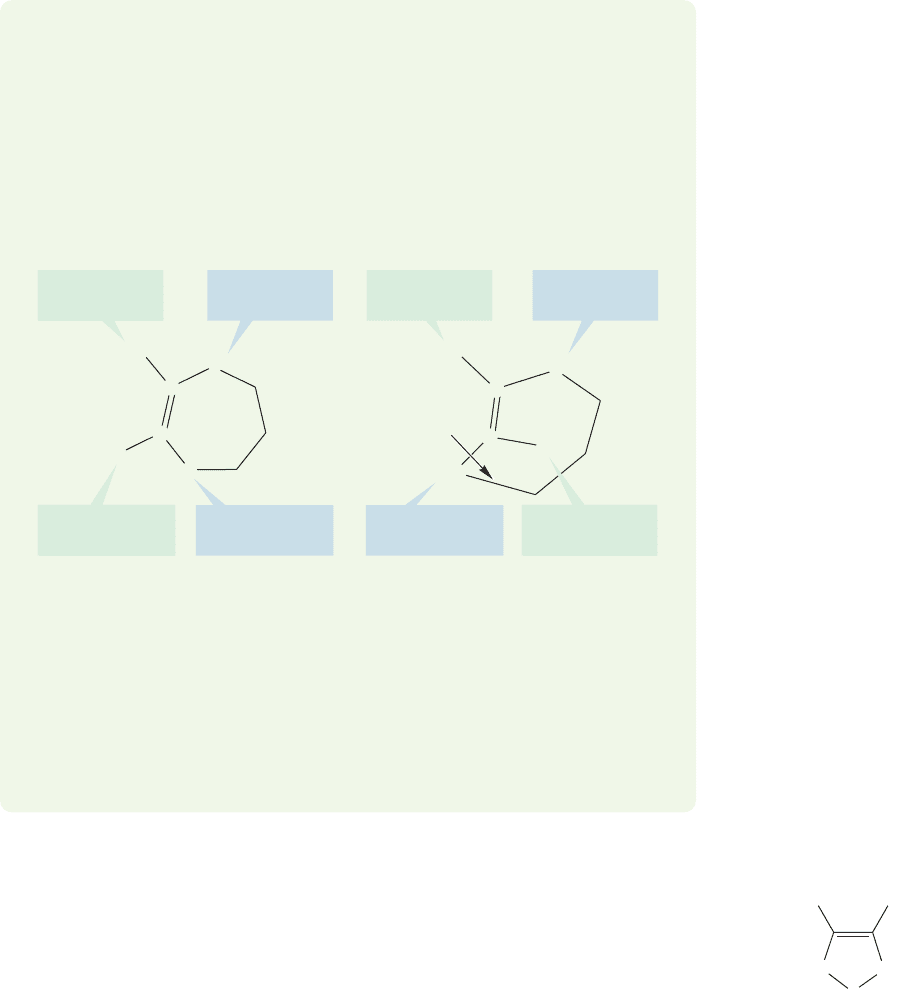
WORKED PROBLEM 3.18 Draw (E)- and (Z)-1-methylcycloheptene. Which isomer
would you expect to be more stable? Explain.
ANSWER In (Z)-1-methylcycloheptene,the two higher priority groups are on the
same side of the double bond, whereas in (E)-1-methylcycloheptene they are on
opposite sides. On carbon 2, hydrogen is lower priority than CH
2
, and on carbon 1,
CH
3
is lower priority than The compound with the higher priority
groups on the same side is (Z), and the compound with the higher priority groups
on opposite sides is (E).
In this example, even the drawings give a clue to the relative stabilities. It is triv-
ial to draw the (Z) form, but the (E) form requires you to stretch bonds (arrow)
in order to make the necessary connections. If you made a model, you saw that
the (E) isomer contains a badly twisted double bond, with poor overlap between
the 2p orbitals making up the π bond.There are no such problems in the (Z) iso-
mer, which is therefore much more stable.
Lower priority
(bottom carbon)
( Z)-1-Methylcycloheptene ( E)-1-Methylcycloheptene
Lower priority
(top carbon)
Lower priority
(top carbon)
Higher priority
(top carbon)
Higher priority
(top carbon)
Higher priority
(bottom carbon)
Higher priority
(bottom carbon)
CH
2
H
3
C
H
2
C
CH
2
H
H
2
C
Note long
bond
Lower priority
(bottom carbon)
H
H
3
C
C
C
C
C
CH
2
O
C.
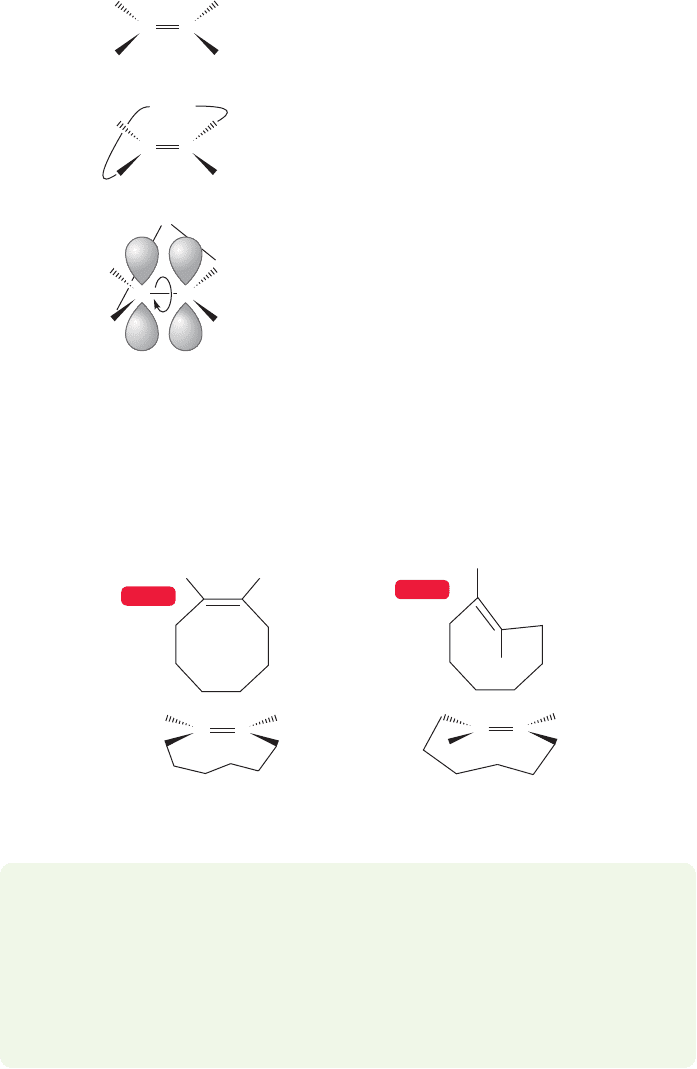
All the double bonds in Figures 3.38 and 3.39 are cis. For some reason this
is often a sticky point for students. Make sure you understand why all the dou-
ble bonds shown in the figures are appropriately called cis. To make the point
absolutely clear we will use cyclopentene as an example. Note that the two
hydrogens on the double bond are on the same side. The two alkyl groups (part
of the ring) are on the other side (Fig. 3.40). The molecule is properly called
cis, or (Z).
It is extremely difficult to put a trans double bond in a small ring,and it’s worth
our time to see why. As we saw in Section 3.2, double bonds depend on overlap of
p orbitals for their stability. There is no great problem in a ring containing a cis
double bond (Fig. 3.40), but if the cycloalkene is trans, the double bond becomes
severely twisted and highly destabilized (see Worked Problem 3.18). Remember
that π bonds derive their stability from overlap of 2p orbitals, and that a fully twist-
ed (90°) π bond, with its 2p orbitals oriented at 90° to one another, is about
66 kcal/mol higher in energy than the 0° form (p. 106). For a small or medium-sized
ring, it is not possible to bridge opposite sides of the double bond without very
cis-Cyclopentene
The two hydrogens are on
the same side of the double
bond—the double bond in
this molecule is cis, or (Z )
CH
2
CH
2
H
2
C
HH
FIGURE 3.40 The double bond in
cyclopentene is cis.
120 CHAPTER 3 Alkenes and Alkynes
severe strain. Let’s use cyclopentene as an example again. A single CH
2
group
cannot span the two carbons attached to opposite sides of a trans double bond
(Fig. 3.41).This difficulty is easy to see in models. Use your models to try to make
trans-cyclopentene. Hold the double bond planar, and then try to introduce the
three bridging methylene groups.They won’t reach. Next, allow the π bond to relax
as you connect the methylenes. The methylenes will reach this time, but now the
2p orbitals making up the π bond no longer overlap efficiently. Make sure you see
this point.
CH
2
H
2
C
H
H
To start a trans double bond,
put the two hydrogens on
opposite sides of the
π bond
Now connect the carbons of the double bond
with a chain of methylene (CH
2
)
n
groups; if
the ring is large enough (n
–
>
6), there is no
great problem in forming the trans isomer
To make trans-cyclopentene, the double
bond carbons must be bridged by only
three methylenes; this cannot be done
without twisting the 2p orbitals out of overlap;
this twisting is very costly in energy terms
(CH
2
)
n
CH
2
C
C
H
H
C
C
H
H
CC
FIGURE 3.41 It is difficult to
incorporate a trans double bond in a
small ring. Either the bridge of
methylene groups is too small to span
the trans positions or the 2p orbitals
making up the π bond must be
twisted out of overlap.
Of course, the larger the ring, the less problem there is because more atoms
are available to span the trans positions of the double bond. In practice, the
smallest trans cycloalkene stable at room temperature is trans-cyclooctene.
Even here, the trans isomer is 11.4 kcal/mol less stable than its cis counterpart
(Fig. 3.42).
H
H
trans-Cyclooctene
H
H
C
C
WEB 3D
FIGURE 3.42 cis- and trans-Cyclooctene.
PROBLEM 3.19 Calculate the equilibrium distribution of cis- and trans-
cyclooctene at 25 °C given an energy difference of 11.4 kcal/mol between the
two isomers (of course, this question assumes that equilibrium can be reached—
something not generally true for alkenes). The relationship between the energy
difference (ΔG°) and the equilibrium constant (K) is ΔG° RT ln K, or
ΔG° 2.3RT log K, where R is the gas constant (1.986 cal/deg mol) and T
is the absolute temperature.
#
H
H
C
C
cis-Cyclooctene
H
H
WEB 3D
3.7 Double Bonds in Rings 121
It was noticed in 1924 by the German chemist Julius Bredt (1855–1937) that
one structural type was conspicuously absent among the myriad compounds isolat-
ed from sources in Nature. There seemed to be no double bonds attached to the
bridgehead position in what are called “bridged bicyclic molecules.” The bridge-
head position is the point at which the bridges meet (Fig. 3.44). Apparently, great
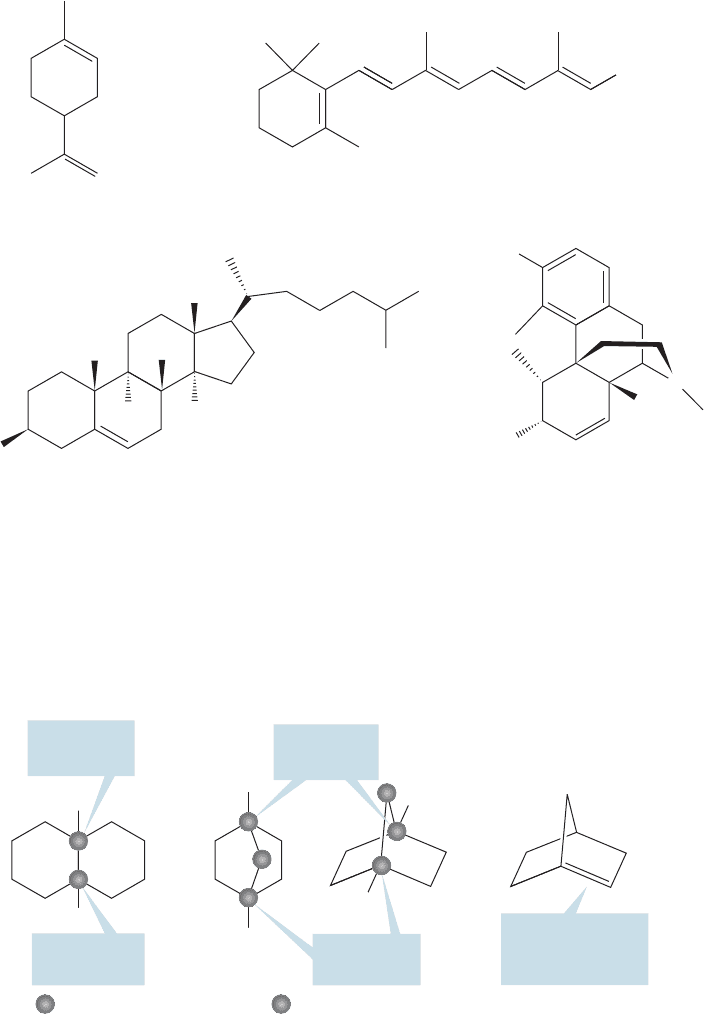
Many cyclic and polycyclic (more than one ring) compounds containing double
bonds are known, including a great many natural products. Figure 3.43 shows some
common structures incorporating cycloalkenes.
HO
H
H
CH
2
OH
Vitamin ALimonene
Cholesterol
H
Morphine
H
HO
O
N
CH
3
HO
FIGURE 3.43 Some natural products
incorporating cycloalkenes.
Bridged bicyclic system
Bridgehead
position
Bridgehead
position
This double bond
is at the bridge-
head position
Bridgehead
position
Fused bicyclic system
Three shared
carbons
Two shared
adjacent carbons
=
H
H
H
H
H
H
Bridgehead
position
FIGURE 3.44 Fused and bridged
bicyclic molecules.
instability is caused by the incorporation of a double bond at the bridgehead
position. You have met such structures before in Chapter 2 (p. 85), and they will
reappear in some detail in Chapter 5, but you might make a model now of a simple
bridged bicyclic structure. Figure 3.44 shows two kinds of bicyclic molecules,
“bridged” and “fused.” In fused compounds, two rings share a pair of adjacent car-
bons. In bridged bicyclic compounds, more than two carbons are shared.
122 CHAPTER 3 Alkenes and Alkynes
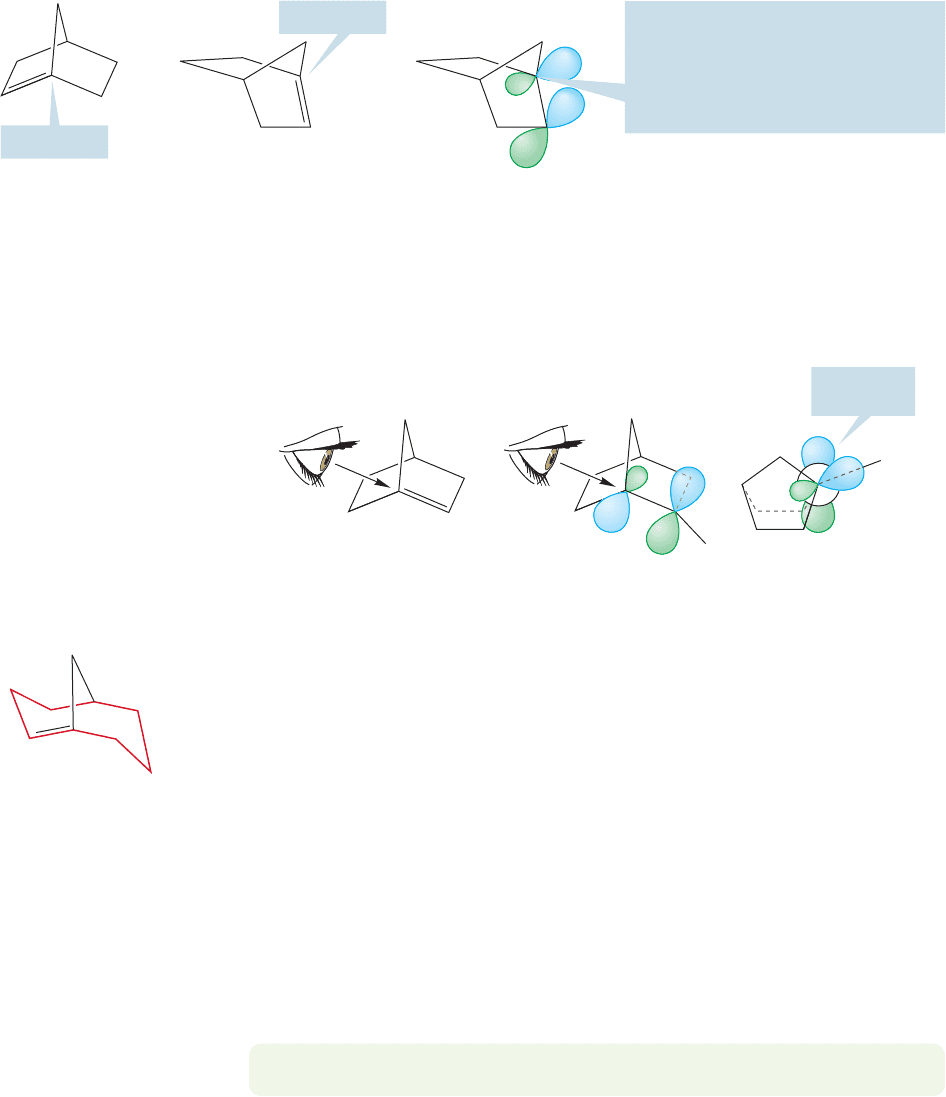
Bredt was unable to explain this absence of double bonds at the bridgehead
position, but was alert enough to note the phenomenon—the absence of molecules
with double bonds at the bridgehead.That there could not be such compounds has
become deservedly known as Bredt’s rule.What is the reason behind this rule? First
of all, the rigid cage structure with its pyramidal bridgehead carbons (Fig. 3.44)
requires that the π bond, the “double”part of the alkene, be formed not from 2p/2p
overlap, but by 2p/hybrid orbital overlap. Overlap is not as good as in a normal
alkene π system (Fig. 3.45). There is even more to this question, however, and a
PROBLEM 3.20 Is the bicyclo[3.3.1]non-1-ene shown in Figure 3.47 (Z) or (E)?
Bridgehead
Bridgehead
==
This bridgehead carbon cannot become
planar
—flatten out—the rigid cage
prevents this. The orbital on this carbon
cannot be a pure p orbital; it must
be a hybrid, and will therefore not
overlap well with an adjacent p orbital
FIGURE 3.45 Three views of a bridged bicyclic molecule containing a double bond at the bridgehead.
Poor orbital
overlap
H
H
FIGURE 3.46 A Newman projection
shows that in these bridgehead
alkenes the orbitals making up the
“π bond” cannot overlap well.
FIGURE 3.47 The smallest bridgehead
alkene stable under normal conditions
is bicyclo[3.3.1]non-1-ene. This
molecule contains a trans-cyclooctene,
shown in color.
careful three-dimensional drawing of the molecule best reveals the story. As is so
often the case, a Newman projection is the most informative way to look at the mol-
ecule (Fig. 3.46). Note that the orbitals making up the (hypothetical) double bond
do not overlap at all well. Just as trans cycloalkenes are severely twisted, so are
bridgehead alkenes. Moreover, the rigid structure of these compounds (make a
model!) allows for no relief. This is a good example of how the two dimensions
of the paper can fool you. There is no difficulty in drawing the lines making up the
double bond on the paper, and unless you can see the structure, you will almost
certainly be fooled.
As the bridges in bicyclic molecules get longer, flexibility returns and bridgehead
“anti-Bredt” alkenes become stable (Fig. 3.47). Clever syntheses have been devised so
that even quite unstable compounds can be made and studied, and old Bredt’s rule
violated. In simple bridgehead alkenes, the limits of room temperature stability are
reached with bicyclo[3.3.1]non-1-ene (do not worry yet about the naming system;
we will deal with it in Chapter 5), which contains a trans double bond in an eight-
membered ring.As with the simple trans cycloalkenes, it is the eight-carbon compound
that is the first molecule stable at room temperature. It doesn’t seem unreasonable that
there should be a rough correspondence between the trans cycloalkenes and the bridge-
head alkenes, which also contain a trans double bond in a ring.
3.9 Alkynes: Structure and Bonding 123
PROBLEM 3.21 Draw the other form of bicyclo[3.3.1]non-1-ene (Z or E).Why is
it less stable than the one in Figure 3.47? Hint: Focus on the rings in which the
double bonds are contained.
TABLE 3.2 Some Simple Alkenes
Name Formula mp (°C) bp (°C)
Ethene (ethylene) 169 103.7
Propene (propylene) 185.2 47.4
1-Butene 185 6.3
cis-2-Butene 138.9 3.7
trans-2-Butene 105.5 0.9
2-Methylpropene (isobutene) 40.7 6.6
1-Pentene 165 30.1
1-Hexene 139.8 63.3
1-Heptene 119 93.6
1-Octene 101.7 121.3
1-Nonene 83 146
1-Decene 66.3 170.5H
2
C
P
CH
O
(CH
2
)
7
O
CH
3
H
2
C
P
CH
O
(CH
2
)
6
O
CH
3
H
2
C
P
CH
O
(CH
2
)
5
O
CH
3
H
2
C
P
CH
O
(CH
2
)
4
O
CH
3
H
2
C
P
CH
O
(CH
2
)
3
O
CH
3
H
2
C
P
CH
O
(CH
2
)
2
O
CH
3
(CH
3
)
2
CH
P
CH
2
CH
3
O
CH
P
CH
O
CH
3
CH
3
O
CH
P
CH
O
CH
3
H
2
C
P
CH
O
CH
2
O
CH
3
H
2
C
P
CH
O
CH
3
H
2
C
P
CH
2
TABLE 3.3 Some Simple Cycloalkenes
Name mp (°C) bp (°C)
Cyclobutene 2
Cyclopentene 135 44.2
Cyclohexene 103.5 83
Cycloheptene 56 115
cis-Cyclooctene 12 138
trans-Cyclooctene 59 143
cis-Cyclononene 167–169
trans-Cyclononene 94–96 (at 30 mmHg)
3.8 Physical Properties of Alkenes
There is little difference in physical properties between the alkenes and their saturated
relatives, the alkanes. The odors of the alkenes are a bit more pungent and perhaps jus-
tify being called “evil-smelling.” In fact, the old trivial name for alkenes, olefins, readily
evokes the sense of smell.Tables 3.2 and 3.3 list some data for alkenes and cycloalkenes.
3.9 Alkynes: Structure and Bonding
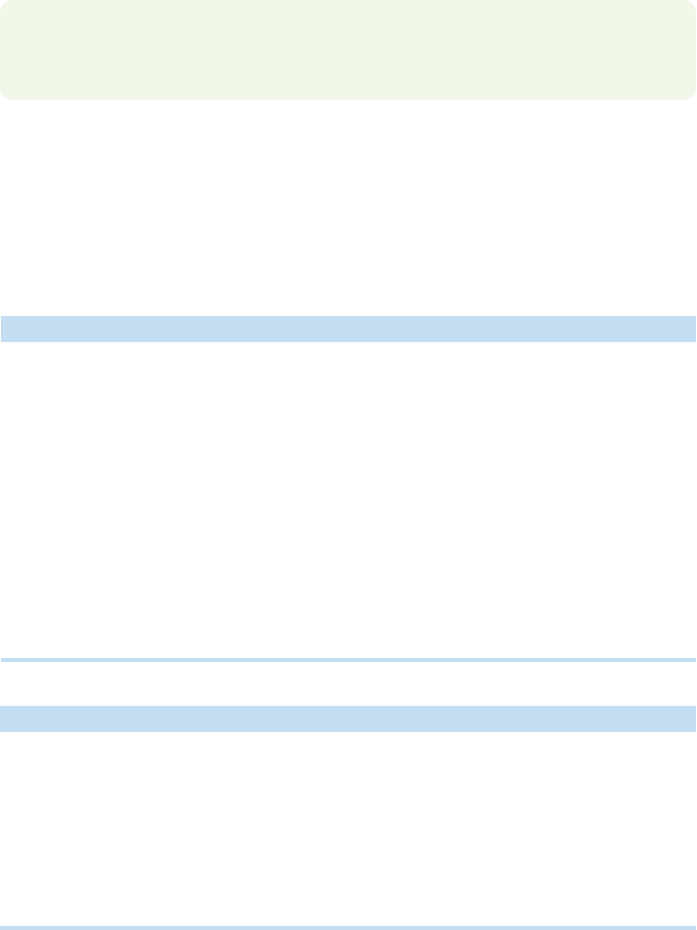
Like the simplest alkene,ethylene,the simplest alkyne is generally known by its triv-
ial name, acetylene, not the systematic name, ethyne. Recall that more complicated,
substituted alkynes are often named as derivatives of acetylene. Acetylene itself is a
symmetrical compound of the formula HCCH in which the two carbon atoms are
attached to each other by a triple bond. Each carbon is attached to two other atoms:
one hydrogen and the other carbon.Accordingly, a reasonable bonding scheme must
yield only two hybrid orbitals, one to be used in bonding to the hydrogen, and one
to be used in bonding to the carbon. As in the discussion of alkanes and alkenes
(p. 64; p. 99), the atomic orbitals of carbon are combined to yield hybrid orbitals,
which do a better job of bonding than the unchanged atomic orbitals. Because only
two bonds are needed, one for the bond to hydrogen, and another for the bond to
carbon, we need to combine only two of carbon’s atomic orbitals to give us our
hybrids. A combination of the 2s and 2p
x
orbitals will yield a pair of sp hybrids.
124 CHAPTER 3 Alkenes and Alkynes
We can anticipate that these new sp hybrids will be directed so as to keep the bonds,
and the electrons in them, as far apart as possible, which produces 180° angles
(Fig. 3.48). (Recall our discussion of BeH
2
, p. 53.)
C
2s 2p
z
2p
x
2p
y
Used to make two
sp h
y
brid orbitals
An sp hybrid
orbital
Two sp hybrids
Not used
C
180⬚
FIGURE 3.48 To make an sp hybrid we combine the wave functions for the carbon 2s and
2p
x
orbitals. The 2p
y
and 2p
z
orbitals are not used in the hybridization scheme.
C
2p
y
2p
y
sp
s
p
2p
z
2p
z
FIGURE 3.49 An sp-hybridized
carbon atom. Note the leftover,
unhybridized 2p
y
and 2p
z
orbitals.
sp/1s Overlapsp/1s Overlap
C C
sp/sp Overlap
H
H
FIGURE 3.50 The σ bonding system
of acetylene: two bonds and
one bond.C
O
C
C
O
H
These new sp hybrid orbitals generally resemble the sp
2
and sp
3
hybrids we made
before (compare Figure 3.48 with Figures 2.6 and 2.23). Because we used only two of
carbon’s four available atomic orbitals, the 2p
y
and 2p
z
orbitals are left over. Figure 3.49
shows an sp-hybridized carbon atom.(From now on, we will not show the small lobes.)
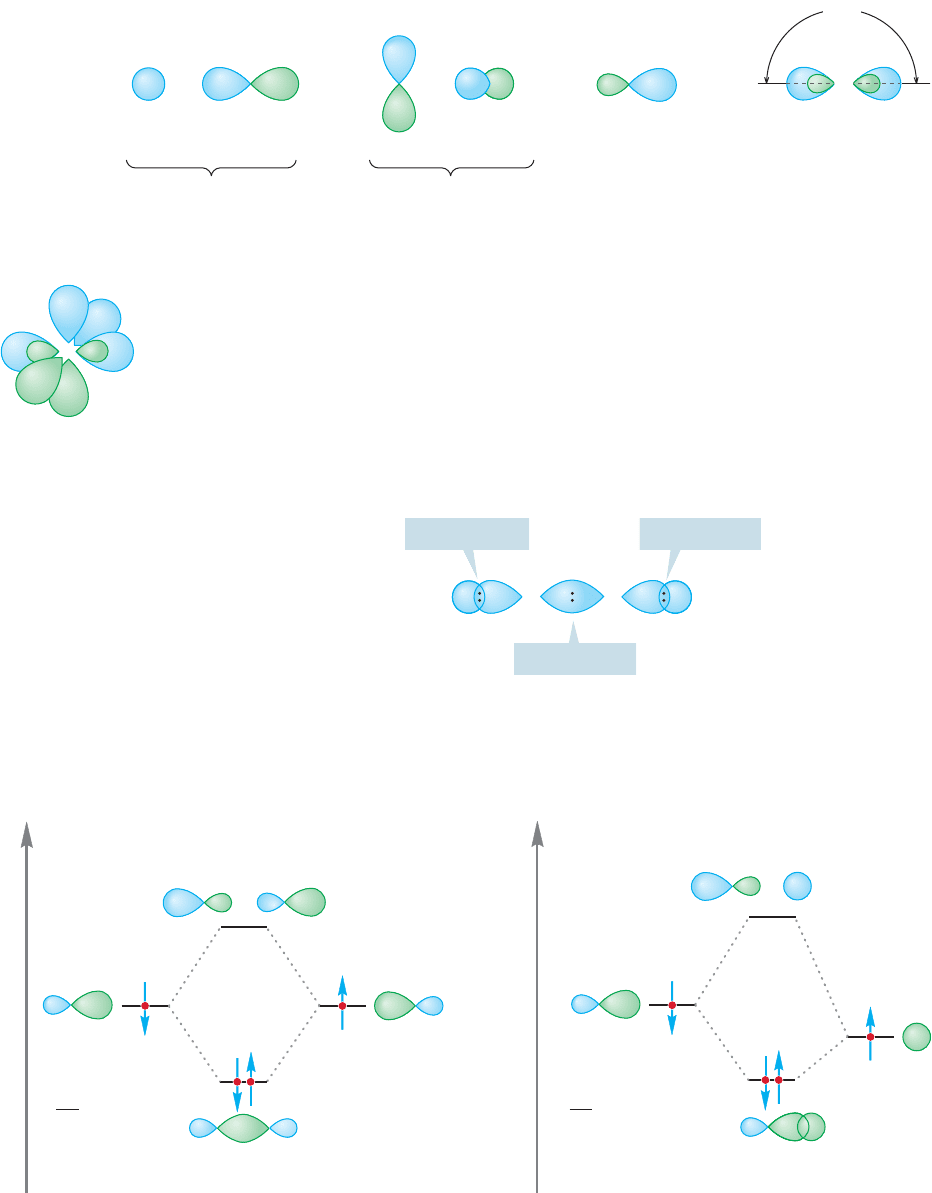
Each carbon–hydrogen bond in acetylene is formed by overlap of one sp hybrid
with the hydrogen 1s orbital, and the carbon–carbon bond is formed by the overlap
of two sp hybrids. This process forms the σ bonds of the molecule (Fig. 3.50).
Figure 3.50 shows the bonds formed by sp/sp and sp/1s overlap, but don’t forget
that these overlapping hybrid and atomic orbitals create both bonding and antibond-
ing molecular orbitals.The empty antibonding orbitals are not shown in Figure 3.50,
but are shown in the schematic construction of Figure 3.51.
Energy
*
Energy
*
sp
sp
sp
– sp (antibonding)
sp
+ sp (bonding)
sp
1s
sp
–1s (antibonding)
sp
+ 1s (bonding)
C
C σ Bond C
H σ Bond
FIGURE 3.51 Orbital interaction diagrams showing the bonding and antibonding orbitals formed by sp/sp
and sp/1s overlap.
3.9 Alkynes: Structure and Bonding 125
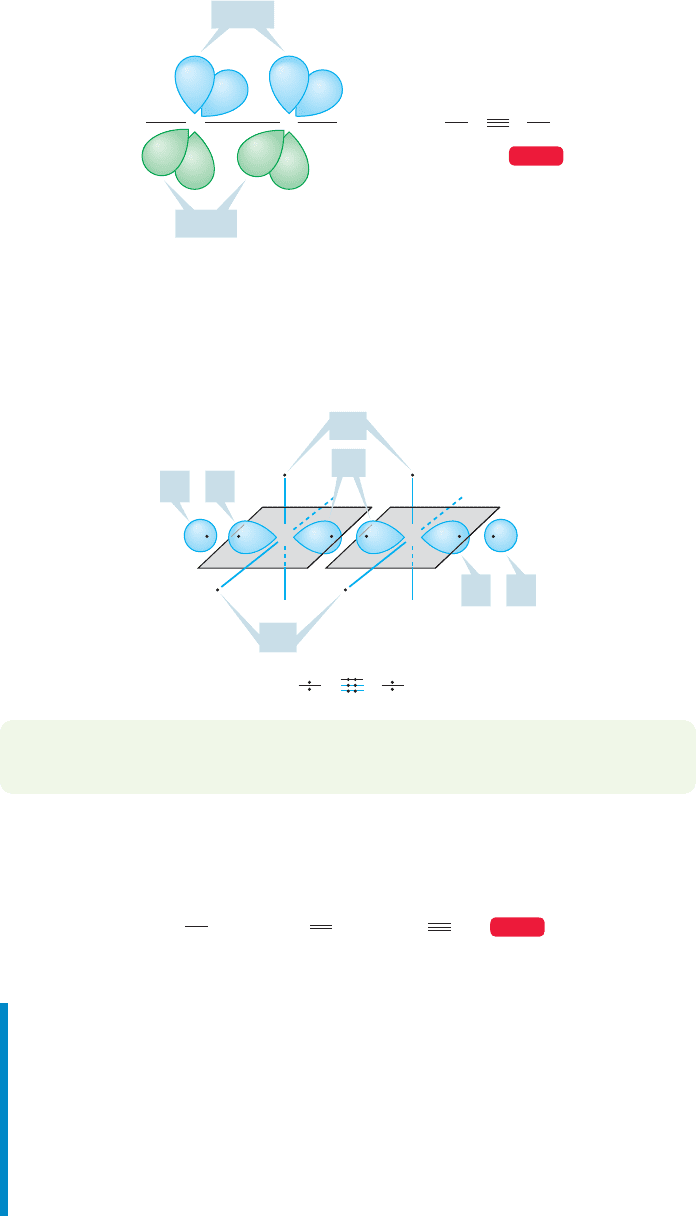
As in the alkenes, the remaining unhybridized 2p orbitals in the alkynes overlap
to form π bonds. This time there are two p orbitals remaining on each carbon of
acetylene, and we can form a pair of π bonds that are directed, as are the 2p orbitals
making up the π bonds, at 90° to each other (Fig. 3.52).
HHH
=
HCC
CC
2p
z
2p
y
WEB 3D
FIGURE 3.52 Overlap of two 2p
y
and
two 2p
z
orbitals forms a pair of π
bonds in alkynes.
Both carbons in acetylene have four valence electrons (Remember: All carbons
have four bonding electrons because we ignore the two very low energy 1s electrons)
and each can participate in four two-electron bonds.In acetylene, these are the σ bond
to hydrogen and the carbon–carbon triple bond composed of one carbon–carbon
σ bond and two carbon–carbon π bonds (Fig. 3.53).
HHC
C
sp1s
1ssp
CH H
2p
z
2p
y
C
sp
FIGURE 3.53 A highly schematic
construction of the π and σ bonds in
acetylene.
1.54 A
⬚
1.33 A
⬚
1.20 A
⬚
H
3
CH
2
C
HC
CH
3
CH
2
CH
WEB 3D
FIGURE 3.54 Bond lengths in
angstroms (Å) of simple two-carbon
hydrocarbons.
PROBLEM 3.22 Draw the orbital interaction diagrams for construction of the π
bonds of acetylene from the 2p orbitals.
One of the structural consequences of triple bonding is an especially short
carbon–carbon bond distance (⬃1.2 Å), considerably shorter than either
carbon–carbon single or double bonds (Fig. 3.54).
Summary
The σ bonding schemes we used to construct alkanes and cycloalkanes are not
the only way that atoms can be held together to form molecules. In alkenes and
alkynes, there is π bonding as well as σ bonding. In the construction of a π bond,
2p orbitals overlap side-to-side to form a bonding and antibonding pair of π
molecular orbitals. Alkenes and cycloalkenes contain one π bond,whereas alkynes
have two π bonds. Ring compounds can also contain double (and sometimes
triple) bonds.
126 CHAPTER 3 Alkenes and Alkynes
We can start constructing the family of alkynes by replacing X in Figure 3.55
with a methyl group, CH
3
.This process produces propyne (or methylacetylene), the
lone three-carbon alkyne (Fig. 3.56).
3.10 Relative Stability of Alkynes:
Heats of Formation
Heats of formation show that alkynes are very much less stable than their con-
stituent elements.Table 3.4 lists heats of formation for some alkynes, alkenes, and
alkanes. The virtues of using hybrid orbitals for σ bonding rather than p orbitals
for π bonding are apparent. The more π bonds in a molecule, the more positive
the heat of formation, and the more endothermic the formation of the compound
from its constituent elements.The very positive heats of formation for alkynes have
their practical consequences. When a very high heat is desired, as for many weld-
ing applications, acetylene is the fuel of choice. Acetylenes are high-energy com-
pounds (with correspondingly high heats of formation) and lots of energy is given
off when they react with oxygen to form the much more stable molecules water
and carbon dioxide.
Because of their triple bonds, alkynes can be only mono- or disubstituted.
Disubstituted alkynes are more stable than their monosubstituted isomers. For
example, we can see from Table 3.4 that the heat of formation of 1-butyne is about
5 kcal/mol more positive than that for 2-butyne. This phenomenon echoes the
increasing stability of alkenes with increasing substitution (Section 3.6).
TABLE 3.4 Heats of Formation for Some Small Hydrocarbons
Alkanes Alkenes Alkynes
Ethane 20.1 Ethylene 12.5 Acetylene 54.5
Propane 25.0 Propylene 4.8 Propyne 44.6
Butane 30.2 1-Butene 0.1 1-Butyne 39.5
2-Butene 1.9 (cis) 2-Butyne 34.7
2.9 (trans)
(kcal/mol)≤H
°
f
(kcal/mol)≤H
°
f
(kcal/mol)≤H
°
f
3.11 Derivatives and Isomers of Alkynes
We can imagine replacing one of the hydrogens of acetylene with an atom or group X
to give substituted alkynes. Figure 3.55 gives an example of an “ethynyl”compound. In
practice, two naming systems are used here, and the figure shows them. Chloroethyne
can also be called chloroacetylene, and both naming systems are commonly used.
CC
HH
CC
XH
CC
Cl
Chloroethyne
(or chloroacetylene)
X = Cl
H
FIGURE 3.55 An ethynyl compound,
chloroethyne, a monosubstituted
alkyne.
HCCX
X = CH
3
CH
3
HCC
Propyne
(
meth
y
lacet
y
lene
)
WEB 3D
FIGURE 3.56 Replacement of X with
a methyl (CH
3
) group gives propyne.
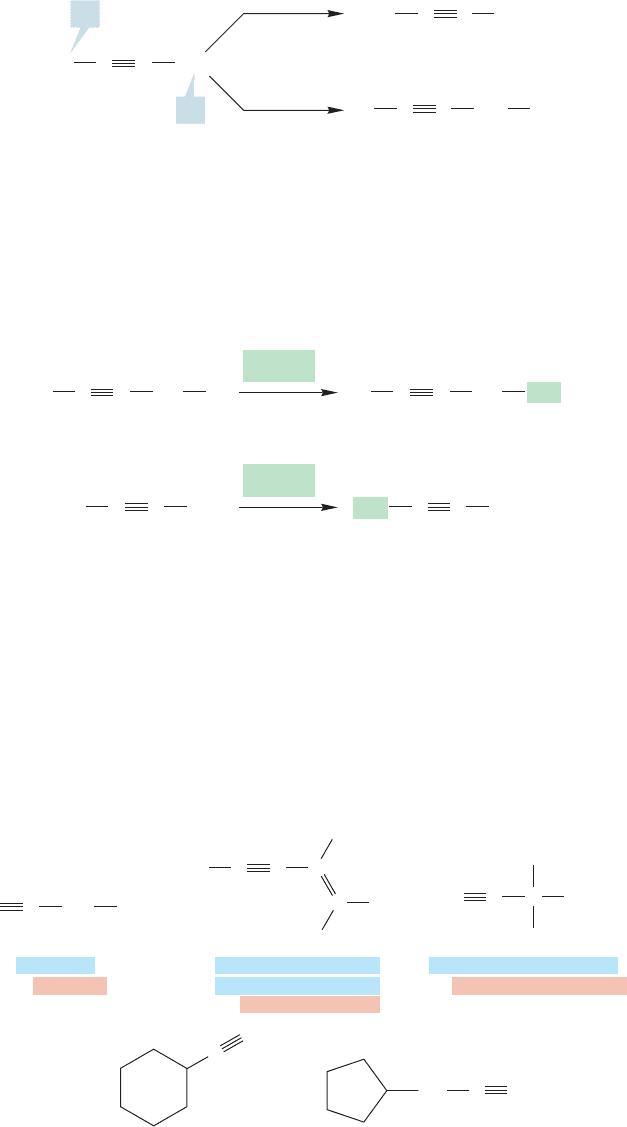
3.11 Derivatives and Isomers of Alkynes 127
HCC
H
a
replace H
a
CH
3
XCCCH
3
HCCCH
2
X
H
b
1-Propynyl compounds
3-Propynyl compounds
(propargyl compounds)
Propyne
—a
terminal alkyne
replace H
b
FIGURE 3.57 Examples of the two
kinds of hydrogen in propyne, each
replaced with X to give derivatives.
There are two different hydrogens in propyne, and therefore two different ways to
produce a substituted compound (Fig.3.57).When the acetylenic hydrogen is replaced,
the compounds can be named either as acetylenes or as 1-propynyl compounds.
3-Propynyl compounds result from replacement of a methyl hydrogen with X.
The common name propargyl is reserved for the group and is often
seen. Note the relationship between the allyl (Fig. 3.19) and propargyl groups.
When X CH
3
we produce the two four-carbon alkynes, which must be
butynes (Fig. 3.58).
HC
q
C
O
CH
2
X = CH
3
X = CH
3
HCCCH
2
X HCCCH
2
1-Butyne
XCCCH
3
H
3
CCCCH
3
CH
3
2-Butyne
FIGURE 3.58 If X CH
3
, we get
the two butynes.
The naming protocol for alkynes is similar to that used for alkenes. As with the
double bond of alkenes,the triple bond position is designated by assigning it a num-
ber, which is kept as low as possible. When both double and triple bonds are pres-
ent,the compounds are named as enynes, and the numbers designating the positions
of the multiple bonds kept as low as possible. If the numbering scheme could
produce two names in which the lower number could go to either the “-ene” or the
“-yne,” the “-ene” gets it (Fig. 3.59).
HC C CH
3
CH
2
C
C
H
H
Ethynylcyclohexane
or cyclohexylacetylene
3-Cyclopentylpropyne
or propargylcyclopentane
CCH
3
C
CH
CH
HC C CH
3
C
1-Butyne
not 3-butyne
3,3-Dimethyl-1-butyne
not 2,2-dimethyl-3-butyne
(E)-2-Hexen-4-yne
trans-2-hexen-4-yne
not (E)-4-hexen-2-yne
CH
3
CH
3
CH
3
65
56
23 41
2341
2
3
41
2341
2
34
1
2341
CH
2
C
C
FIGURE 3.59 Some examples of the
naming convention for alkynes.
128 CHAPTER 3 Alkenes and Alkynes
PROBLEM 3.23 Draw all the pentynes, hexynes, and heptynes.
PROBLEM 3.24 Name all the isomers you drew in Problem 3.23.
PROBLEM 3.25 How many signals will appear in the
13
C NMR spectra of the
compounds in Figure 3.59 (see Section 2.14)?
Can alkynes exhibit cis/trans isomerism? It takes only a quick look to see that
there can be no such isomerism in a linear compound (Fig. 3.60). The sp
hybridization requires 180° angles, and therefore there can be no cis/trans iso-
merism in alkynes. The alkynes are simpler to analyze structurally than the
alkenes.
CCH
3
CCH
3
180⬚ 180⬚
FIGURE 3.60 There are 180° angles in
2-butyne.
3.12 Triple Bonds in Rings
Like double bonds, triple bonds can occur in rings,although very small ring alkynes
are not known.The difficulty comes from the angle strain induced by the prefer-
ence for linear, 180° bond angles in an acetylene. When a triple bond is incorpo-
rated in a ring, it becomes difficult to accommodate these 180° angles. Deviation
from 180° reduces the overlap between the p orbitals making up one of the π
bonds of the acetylene and that raises the energy of the compound (Fig. 3.61).
Remember: “R” is shorthand for a general group.
R
C
R
R
R
R
bend
A c
y
clic acet
y
lene
C
C
C
Second
π bond
in the acetylene
Reduced
overlap
FIGURE 3.61 Incorporation of a triple
bond in a ring is difficult because
orbital overlap between the 2p
orbitals of one of the π bonds is
reduced by the bending required by
the ring.
Cycloheptyne
(observable only at
low temperature)
Cyclooctyne
(stable at
room temperature
but very reactive)
Cyclononyne
(a compound of
normal reactivity)
WEB 3D
FIGURE 3.62 Some cycloalkynes.
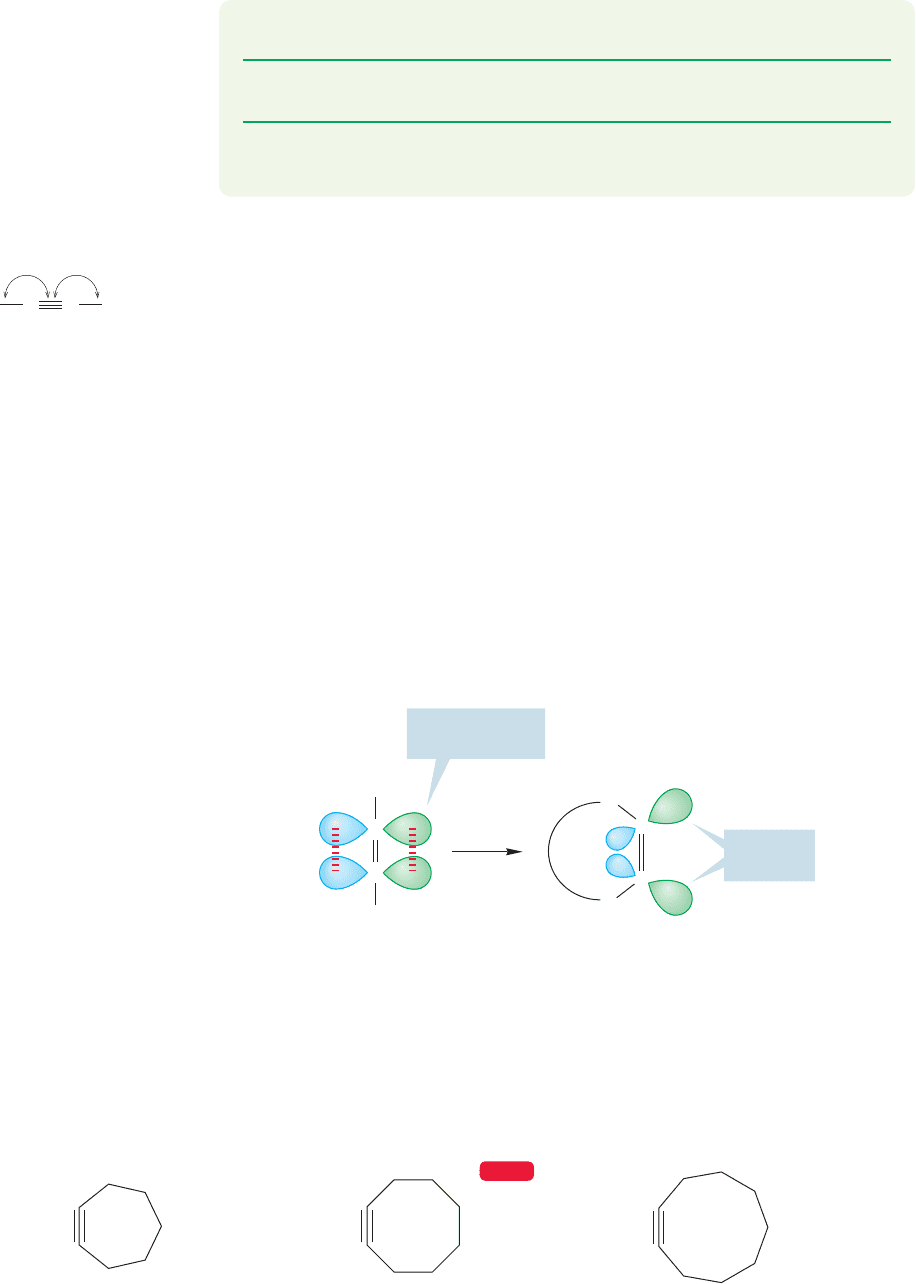
A similar problem with angles exists for the cycloalkenes, but it is less severe. In
practice, the smallest ring in which an alkyne is stable under normal conditions is
cyclooctyne. Thus, it and larger cycloalkynes (cyclononyne, etc.) are known, but
the smaller cycloalkynes are either unknown or have been observed only as fleet-
ing intermediates (Fig. 3.62).
