Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.


Photohalogenation
allylic halogenation, 497–501, 507, 543
butane, 494–95
chlorination of methane, 490–93
fluorine reactions, 495
iodine reactions, 495
mechanism, 491
methane, 490–93
2-methylpropane, 494–95
photobromination, 493, 494–95, 497–501
selectivity of allylic bromination, 497–501
selectivity of reactions with alkanes, 494–97,
506
thermochemistry, 491–92
transition states for hydrogen abstraction, 495,
496–97
See also Halogenation
Phthalic acid, 856, 881, 882, 894
Phthalic anhydride, 856, 881, 894
Phthalimide, 882
Pinacolone, 408
-Pinene, 558
-Pinene, 214, 413, 558
Piperidine (azacyclohexane), 242, 601, 1019
Pi orbitals, general
alkenes, 98, 104, 125
alkynes, 125
carbonyl compounds, 764–66
ethylene, 104
overlap, in alkenes, 98, 104, 125
Pi stacking, 598, 615
pK
a
acetaldehyde,936
acetic acid, 1179
alcohols, 236, 249, 316, 834
aldehydes, 933
alkanes, 976
ammonium ions, 246–48, 1179
benzoic acid, 760
tert-butyl alcohol, 987
carboxylic acids, 834, 836
cycloheptatriene, 591
1,3-cyclopentadiene, 589, 591, 601, 975
definition, 235, 256, 257
-dicarbonyl compounds, 958
and hydration of alkenes,381
ketones, 933
oxonium ions,236, 248
propane, 589
propene, 589, 936
See also Acid–base chemistry
pK
b
, 248, 259, 601
Planck’s constant (h), 526, 527
Plane-polarized light, 155–59, 168–69, 762, 1128
-pleated sheets, 1191
Poe, Edgar Allan, 148, 148n1
Polar covalent bonds, 14–16, 132
Polarimeters, 159
Polaroid sunglasses, 157–58
Polyamides (nylon), 852, 898
Polyaromatic hydrocarbons (PAHs), 615
See also Polynuclear aromatic compounds
Polycyclic compounds
cycloalkanes, 85–86
cycloalkenes, 121
Diels–Alder reaction, 552–53
structure, 186, 216–17
See also Polynuclear aromatic compounds; Ring
compounds
Polyenes, 110, 118, 511, 529, 533
Polyesters, 852–53
Polyethylene terephthalate (PET), 853
Polyisoprene, 554, 557
Polynuclear aromatic compounds
aromaticity, 592
carcinogens, 571, 605, 614–16
definition, 602
and DNA alkylation, 614–16
fused benzenes, stereochemistry, 179–80
structures, 602–5
See also Aromatic compounds
Polysaccharide,1161, 1166, 1168
Polystyrene, 1208–10
Potassium benzoate, 1004
Potassium chloride, 6, 132
Potassium 2-chlorobutanoate, 832
Potassium permanganate, 255, 443, 806, 840
Ppm scale, 716–17
Prephenate, 684, 1078, 1083
Primary carbon, 76
Primary ozonides,437–39
Primary structure, 1190
Prismane (Ladenburg benzene), 216, 573–74
Product-determining step, 294–95
Progesterone, 86, 562
Proline, 1177, 1178, 1188, 1218
Propagation of chain reactions, 482, 483
Propanal (propionaldehyde), 767, 779, 936
Propane
13
C NMR spectroscopy, 88
boiling point, 770
bond dissociation energy, 475
conformational analysis, 72
dihedral angle, 72
heat of combustion 194
methylene group, 72
Newman projection, 71, 72
pK
a
, 589
rotational energy barrier, 72, 78
sp
3
hybrid orbitals, 72
structure, 51, 71
Propanedial,767
Propane-2-thiol,314
2-Propanol (isopropyl alcohol). See Isopropyl
alcohol
1-Propanol (propyl alcohol), 230, 748
Propargyl compounds, 127
[1.1.1]Propellane (tricyclo[1.1.1.0
1,3
]pentane), 86,
216
Propene
addition of HCl, 138
allyl compounds, 108
boiling point, 770
derivatives and isomers, 108–9
formation, 107
pK
a
, 589, 936
protonation, 378–79
Propionic acid (propianoic acid), 830, 849, 879, 880
Propionitrile (propanenitrile, ethyl cyanide), 803
Propionyl bromide (propianoyl bromide), 880
Propylamine (1-aminopropane), 241
Propylbenzene (1-phenylpropane),641
Propyl bromide, 314
Propyl compounds, 73
Propylene. See Propene
Propylene glycol (1,2-propanediol), 240
Propyl fluoride,268
3-Propyl-1-nonene, 110
Propyl radicals, 471–72, 479
Propyne (methylacetylene), 126–27, 446,489
1-Propynyl compounds, 127
3-Propynyl compounds, 127
Prostaglandins,569, 838
Protecting groups
acetals as protecting groups for aldehydes and
ketones, 788–89
carbohydrate chemistry, 1151–52
Cbz (benzyl chloroformate), 1205, 1207–8,
1217
definition, 788
peptide and protein synthesis, 1205, 1207–10,
1217
tBoc (di-tert-butyl dicarbonate),1205–10,
1217
tetrahydropyranyl (THP) ethers as protecting
groups for alcohols, 789–90
trialkylsilyl ethers as protecting groups for
alcohols, 789–90
Proteins
-helix, 1191, 1192
binding sites, 1193
chromatographic separation and analysis,
1195–96
crystallization, 1192, 1192n3, 1194
definition, 1175
denaturing, 1193–94
disulfide bridges, 1190, 1193, 1194–95, 1216
Edman degradation, 1198–1200, 1202, 1218
fibrous proteins,1192
globular proteins, 1192, 1193
hemoglobin, 1194
hydrogen bonds, 1190–91, 1192, 1194
lactate dehydrogenase, 1192
nitrogen-to-carbon link, 224
NMR structure determination, 1192n3
-pleated sheets, 1191
primary structure, 1190
quaternary structure, 1194
Sanger degradation, 1196–97, 1202
secondary structure, 1191–92, 1193
superhelix, 1192
tertiary structure, 1192–94
X-ray crystallography structure determination,
1192, 1192n3, 1194
See also Peptide and protein synthesis
Protic solvents, 238–39, 256, 280, 417–18, 421
Pseudo-first-order reactions, 340
Pseudomauvine, 648
Putrescine (1,4-butanediamine), 246
Pynchon,Thomas, 364, 364n1, 763
Pyranoses, 1132–33
Pyranosides, 1148
Pyrethroids, 456
Pyridine (azabenzene)
acid–base properties, 601, 620
Chichibabin reaction, 676–77
dipole moment, 600
electron orbitals, 599
electrophilic substitution reactions, 652–53
heterobenzene, 598–99
N-oxides, 678–79
as nucleophile, 602
pyridine N-oxide, 602
resonance, 600
S
N
Ar reactions, 676–79
structure, 242, 592, 598, 652, 804
Pyridine N-oxide, 678
(¢H°
c
),
()
()
INDEX I-21

Pyridinium ion, 601–2, 653, 679, 814
Pyrolysis,470–74, 507, 852, 856, 913–14, 923
Pyrophosphate esters, 555–59
Pyrophosphoric acid, 555
Pyrrole
acid–base properties, 601, 621
aromaticity, 599–600, 601
Diels–Alder reaction, 630
dipole moment, 600
electrophilic substitution reactions, 652,
653–54
resonance, 600
structure, 242, 592, 599, 652
Pyrrolidine (azacyclopentane), 242, 963–64
Pyruvate, 1028, 1140
Quantum mechanics and babies,42–43
Quantum model of atoms, 1,2
Quantum numbers
possible combinations of quantum numbers, 7
principal quantum number (n), 6
relationship between n,l, and m
l
, 7
relationship to atomic orbitals, 6–8
second quantum number (l), 6–7, 10
spin quantum number (s), 7–8
third quantum number (m
l
), 7
Quaternary carbon, 76
Quaternary structure, 1194
Quinine, 255, 256, 531, 532
Quinoline, 602, 892
Racemic mixture (racemate), 156–57
Radical anions, 453–54, 609
Radical formation
from azo compounds, 476, 507
carboxyl radicals from acyl peroxides, 476
delocalization of electrons, 475–76, 480, 498
hydrocarbon cracking, 473–74
methyl radical, 63, 469–70
pyrolysis, 470–74, 507
radical production from alkyl peroxides, 476
thermolysis, definition, 470
Radical properties
delocalization of electrons, 475–76, 480, 498
resonance forms, 478, 480, 499
stability, 475, 477–81
structure, 63–64, 100–101, 477
Radical reactions
aging process, 504–5
anti-Markovnikov HBr addition to alkenes,
481–82, 484–86, 506
anti-Markovnikov HBr addition to alkynes,
489–90, 507
benzyl radical,612–13, 618
cleavage, 472, 473, 474
dimerization and polymerization, 472, 473,
474, 488–89
disproportionation reaction,471–72, 473–74,
506
formation of H
2
, methane, and ethane from
radicals, 31–32, 68–69, 83, 103, 468
Hunsdiecker reaction, 861, 869
hydrocarbon cracking, 473–74
hydrogen abstraction, 471, 473
inhibitors, 484
mechanism of radical additions to alkenes,
481–87
methyl ions and radicals as reactive
intermediates, 62
overview, 228, 468, 505–7
rearrangements, 501–4
regiospecificity of radical additions to alkenes,
481–82, 484–86
steps of chain reactions, 482, 483
summary, 473
tetrahalide addition to alkenes, 487–89
thermochemistry of radical HX additions to
alkenes, 486–87
triphenylmethyl radical dimerization,480
Random coil, 1191
Raney nickel, 253, 257, 1002
Rates of chemical reactions. See Kinetics
Reaction coordinate,37
Reaction mechanisms
tert-butyl compounds and reaction mechanism
studies, 203–4
definition and overview, 263–64, 349–51
E2 reactions, 301
hydration of alkenes,138–39, 140, 365–66,
380–84
impossibility of mechanistic hypotheses, 139,
382–83
inversion of configuration, 270–71, 350
microscopic reversibility, 346, 359
problem solving techniques, 999–1001,
1014–17
rectangular hypothesis, 383
S
N
1 reactions, 289–91, 350–51
S
N
2 reactions, 270–71, 350
stereochemistry and, 148–49, 155, 173, 270–71
use of stereochemistry in determining, 396, 425
Reaction progress graphs, 37
Reactive intermediates, definition, 62
See also Intermediates
Rearrangements
alkyl shifts, 387–88, 642
Beckmann rearrangement, 909–11, 923, 924
in biological processes, 401–2
carbocation rearrangements, 389
Claisen rearrangement, 1063, 1077–78
as clue to neighboring group effect, 1086–87,
1089, 1108, 1111, 1113
concerted rearrangements of acyl compounds,
914–20
Curtius rearrangement, 918–19, 920, 924
definition, 387
Friedel–Crafts alkylation, 642–43, 645, 646,
686
Hofmann rearrangement, 919–20, 923
during HX addition to alkenes, 386–89, 501
hydride shifts, 387–88, 401–2, 502, 642
ozonide formation from primary ozonides,
437–39
by phosphorus halides, 285
radicals, 501–4
transition states for 1,2-shifts, 502–4
Wagner–Meerwein rearrangements,387
Wolff rearrangement, 916–18, 924
See also Cope rearrangement; Sigmatropic shift
reactions
Rectangular hypothesis, 383
Reducing sugars, 1133, 1145, 1166
Rees, George Owen, 512, 512n1
Regiochemistry
in addition reactions of alkenes,366–72
additions to alkenes, 137–39, 141, 366–72,
481–82, 484–86
definition, 137, 307
hydroboration, 392–93, 399–400
regiochemistry of epoxide ring opening,
427–29
regioselective reactions, 307
Reserpine, 532, 605
Resolution of enantiomers, 169–72
Resonance and resonance forms
acetate ion resonance stabilization, 421
acid chlorides, 885
in addition reactions of alkenes,366–72
aldehydes, 885
allyl anion, 27–28
allyl cation, 27, 28, 369–71, 535
amides (carbonyl-nitrogen compounds),
884–85, 903, 943
arrow formalism, 23, 26
benzene, 580–82
benzene (overlap of 2p orbitals), 575–78
benzyl radical, 480
1,3-butadiene, 26–27, 28, 519, 521, 535
2-butyne carbanion, 516
and carbocation stability, 374–78
conjugated dienes, 519, 521
curved arrow formalism, 23, 24, 44
cycloheptatrienyl anion, 590
cyclopentadienyl anion, 590
definition, 22, 44
electronegativity, 27–28, 373
electron pushing, 23, 24, 25
enolates, 27–28, 373, 933–34, 955
equilibrium versus resonance, 24–25, 29, 44,
372, 404, 617
esters, 885
formaldehyde, 25
ketones, 885
nitromethane, 22–25, 28
overview, 372–74
paired electron spins, 28
resonance arrow, 23, 25, 44
resonance energy, 581, 629
resonance hybrids, 22, 25–27, 28, 30, 372
stabilizing effect of delocalization of electrons,
27, 102
tropylium (cycloheptatrienylium) cation, 590
weighting factor (c), 372–73
weighting factors, 25, 26–30
Resonance arrow (double-headed arrow), 23, 25, 44
Resorcinol (m-dihydroxybenzene), 597
Retention of configuration, in S
N
2 reactions, 269,
270, 271–72
cis-Retinal, 533
trans-Retinal, 533, 762
Retinal isomerase, 533
Retinol dehydrogenase, 533
trans-Retinol (vitamin A), 121, 533
Retrosynthetic analysis, 807–9, 973, 982
Reverse Diels–Alder reactions, 554, 564, 630–31
Rhodium (Wilkinson’s catalyst), 411
Rhodopsin, 533
Ribonucleic acid (RNA)
codons for amino acids, 1214–15
messenger RNA (mRNA), 1214–15
structure and composition, 93, 614–15, 1149,
1211–12, 1214
synthesis, 1214
transfer RNA (tRNA), 1215
See also Nucleic acids
Ribonucleosides, 1211
I-22 INDEX

D-Ribose
chain lengthening, 1138–39, 1160
D-ribofuranose, 1136
in NAD
, 814
in nucleic acids, 1211, 1214
structure, 1129, 1158, 1170, 1211
Rilke, Rainer Maria, 932, 932n1
Ring compounds
bicyclic compounds, from Diels–Alder
reaction, 551–54
bicyclic compounds, nomenclature, 214–15,
551–52
bicyclic compounds, structure, 211–17
bridged substitution, 121, 211
bridgehead position, 121–22, 212, 213–16
chlorocyclopropane, stereochemistry, 173–74
cyclic polyenes, 110, 118
dichlorocyclopropane, stereochemistry, 174–76,
204
disubstituted ring compounds, structure,
204–10
endo and exo isomers, 551–52
fused benzenes, stereochemistry, 179–80
fused bicyclic molecules, definition, 121, 211
fused bicyclic molecules, structure, 212–13
fused substitution, 211
heterocyclic five-membered rings, 436, 459
heterocyclic five-membered rings from 1,3
dipolar reagents, 436
monosubstituted cyclohexanes, structure,
194–204
polycyclic compounds, structure, 186, 216–17
polycyclic cycloalkanes, 85–86
polycyclic cycloalkenes, 121
ring formation through additions to alkenes,
457
S
N
2 reactions, 276
spiro substitution, 211
stereochemical analysis, 173–76
stereochemistry of ring compounds, 214–16
strain, 187–93
structural properties, overview, 186, 219–20
three-membered rings in biochemistry, 455–56
triple bonds in rings, 128
See also specific types
RNA. See Ribonucleic acid (RNA)
Robbins,Thomas Eugene, 98, 98n1
Robinson, Robert, 172, 998
Robinson annulation, 998–99
Rosenmund reduction, 892, 923
Rotational energy barriers
alkenes, 105–6, 107, 144, 522
1,3-butadiene s-trans and s-cis conformations,
523–24
cyclobutane, 189
cyclohexane, 198–99
ethane, 67, 70, 74–75, 93, 105–6, 188, 273
ethylene, 105–6, 107, 144
propane, 72, 78
Roth, W. R, 1056, 1057, 1063, 1066
(R/S) convention, 152–55, 181, 1178
Rubisco (ribulose-1,5-bisphosphate
carboxylase/oxygenase), 1138
Ruff degradation, 1139–40
Rutherford, Ernest, 2, 43
Saccharide. See Carbohydrates
Saccharin, 1164
Sandmeyer reaction, 649–51, 686, 687
Sanger degradation, 1196–97, 1202
Saponification, 862, 869, 895
Saturated hydrocarbons. See Alkanes
Saunders, Martin, 696n2
Saytzeff elimination, 300, 306, 308, 310, 322
Schiemann reaction, 649, 687
Schiff base. See Imines
Schröder, Gerhard, 1070–71
Schrödinger, Erwin, 6
s-cis conformation, 523–24, 545–46, 550
Secondary amines, 240, 241
Secondary carbon, 76
Secondary structure, 1191–92, 1193
Second-order reactions, 268, 273, 340, 341
Semibullvalene, 1068
Semicarbazones, 793
Serine, 1176, 1189
Seth, Vikram, 572, 572n1
Sharpless, Barry, 426
Shikimate-3P, 683
Side chains of amino acids, 1175, 1176–77
Sigma bonds definition, 54
Sigmatropic shift reactions
antarafacial motion, 1055–58
and bond dissociation energy, 1049–50
definition, 1032, 1050
degenerate reactions,1050–51
formal description and nomenclature, 1050
homolytic bond breaking, 1053
1,3-pentadiene, 1048–52
photochemical reactions, 1048, 1051–52, 1053,
1054, 1057–58
problem solving techniques, 1072–73
rules for reactions, 1058
suprafacial motion, 1055–59
symmetry effects, 1055
transition state model, 1053–55
See also Cope rearrangement; Pericyclic
reactions
Silyl ethers, 789–90
Singlet carbenes,433–34, 436, 458
Smalley, R. E., 603–4
S
N
1 reactions
activation energy ( G°), 342–43
benzylic compounds, 610–11, 618
tert-butyl bromide S
N
1 reactions, 289, 294–95
competition between S
N
1 and E1 reactions,
298–99
effect of leaving group, 281, 295, 343, 347
effect of nucleophile on structure of product,
290, 294–95
effect of solvent polarity, 295
effect of substrate structure, 292–94, 296–97
equilibrium, 333, 337, 350
as first-order reactions, 268, 289, 294, 340, 341
incomplete racemization, 291–92
inversion of configuration, 291–92, 297
mechanism, 289–91, 350–51
problem solving techniques, 293
product-determining step, 290, 294–95
rate-determining step,294–95
rate law, 289–91, 341
relative nucleophilicity of common species, 279
relative rates of S
N
1 solvolysis of different
substrates, 542
resonance-stabilized allylic cation formation,
538, 541–42
solvolysis reactions, 290, 538, 541–42
stereochemistry, 291–92
steric effects, 329
summary and overview, 296–98, 322
transition states, 333, 343, 346–49
S
N
2 reactions
activation energy, 273–74, 277, 287
alkylation in the position of carbonyl
compounds, 954–64
allyl halides, 542–43, 564,612
benzylic compounds, 612, 618
in biochemistry, 288–89
Cahn–Ingold–Prelog priority system, 270
competition between S
N
2 and E2 reactions,
301, 321
deoxyribonucleic acid (DNA) substitution
reactions, 262, 288–89
effect of nucleophile, 277–81
effect of solvent polarity, 279–81, 286–87, 295
effect of substrate branching on E2–S
N
2 mix,
301–2
effect of substrate (R group) structure, 275–77,
296–97, 301–2
equilibrium, 267–68, 274, 332
ether cleavage by haloacids, 282–83
first-order reactions of substrate and
nucleophile, 268, 340
HOMO–LUMO interactions, 271–72
intramolecular S
N
2 reactions, 316–17, 917,
1081–88, 1091, 1095, 1101
inversion of configuration, 269, 270–75, 284,
288, 297
mechanisms, 270–71, 282–85, 350
methyl compounds, 249, 275
nucleophilicity, 278–81
problem solving techniques, 284
rate law, 268, 341, 350
rates in methyl, primary, and secondary
substrates, 275, 288
relative reaction rates various substrates,543,
612
replacement of
I with
I*, 270–73
retention of configuration, 269, 270, 271–72
in ring compounds, 276
as second-order reactions, 268, 273, 340
stereochemistry, 268–75
steric hindrance with tertiary substrates, 275,
296
sulfonate reactions,281–86, 322, 427, 555
summary and overview, 296–98, 322
transition states, 272–74, 276–77, 332, 344–46,
543
See also Leaving groups
S
N
Ar reactions
addition–elimination pathway, 675–76, 677
benzyne as intermediate, 681–82
Chichibabin reaction, 676–77, 761
elimination–addition pathway, 681
ipso attack, 677–78
Meisenheimer complex, 675
nitro groups, 674–75, 679, 680
N-oxides, 678–79
nucleophilic additions to benzenes, 674–76
nucleophilic aromatic substitution to
heteroaromatics, 676–79
organolithium reagents, 677
pyridine, 676–79
Soaps, 832, 864, 866–67
Sodamide, 986
Sodium bicarbonate, 859
Sodium borohydride, 421, 423
¢
(),
INDEX I-23

Sodium carbonate, 859
Sodium chloride, solubility, 239
Sodium chromate, 805
Sodium fluoride, 5–6, 132
Sodium propanoate, 832
Solvated electrons, 453–54
Solvation, definition, 237
Solvents
aprotic solvents, 238–39, 240, 280
effect on S
N
1 reactions, 295
effect on S
N
2 reactions, 279–81, 286–87, 295
hydrogen bonding, 238–39, 256, 280
“like dissolves like,”239–40, 256
polar solvents, 238–39
protic solvents, 238–39, 256, 280, 417–18, 421
solvation, definition, 237
Solvolysis reactions
tert-butyl iodide in water, 333, 343–44, 346–51,
383
definition, 290
exo- and endo-2-norbornyl tosylate, 1110–16
methyl iodide in water, 340
neopentyl iodide (1-iodo-2,2-
dimethylpropane) in water, 389
relative rates of S
N
1 solvolysis of different
substrates, 542
resonance-stabilized allylic cation formation,
538, 541–42
steric effects, 329
Soxhlet extractors, 972–73, 974
Spaldeens, 159–60, 160n4
Spearmint [(R)-(–)-carvone], 163
Specific rotation, 159
Spectroscopy
definition, 88, 699
determining structure by using spectroscopy,
742–45
overview and history, 525–26, 696, 696n2,
750–51
See also Mass spectrometry (MS); Nuclear
magnetic resonance spectroscopy (NMR);
Ultraviolet/visible (UV/vis) spectroscopy
sp hybrid orbitals, 53–55
sp
2
hybrid orbitals, 55–56
sp
3
hybrid orbitals, 56–57
Spin–spin coupling
chemical exchange of OH and NH hydrogens,
733–34, 739, 746
cis and trans coupling constant (J
cis
, J
trans
) for
alkenes, 732
coupling constant (J), definition, 718, 728
coupling constant (J) for aromatic compounds,
732
dihedral angle, effect on coupling constant,
731
Karplus curve, 731–32
long-range coupling, 731, 736
magnitude of J for two- and three-bond
coupling, 731
multiple couplings, 730
n 1 rule, 727–29, 735–36
overview, 727
tree diagrams, 730
See also Hydrogen nuclear magnetic resonance
spectroscopy (
1
H NMR)
Spiropentane, 211, 509
Spiro substitution, 211
Squalene, 559
Staggered conformation, 65–67
Starch, 1161, 1168
Stephenson, Neal, 410, 410n1
Stereochemistry
absolute configuration, 152–55, 172–73, 181
achiral molecules, definition, 150, 150n2
bridged bicyclic molecules, 214–16
1-bromo-2-chlorocyclopropane, 204
Cahn–Ingold–Prelog priority system, 152–55
chemical differences between enantiomers,
159–63
chirality, overview, 148–51
chirality without four different groups per
carbon atom, 177–80
chlorocyclopropane, 173–74
cyclohexane, 190–92, 197–99
dichlorocyclopropane, 174–76, 204
E2 reactions, 302–6
fused benzenes, 179–80
molecules with more than one stereogenic
atom, 164–69
neighboring group effect, 1083–85, 1089–91,
1093–94, 1097, 1111–14
overview, 148–49, 181
ring compounds, 173–76, 214–16
(R/S) convention, 152–55, 181, 1178
S
N
1 reactions, 291–92
S
N
2 reactions, 268–75
stereochemical analysis of cycloalkanes, 173–76
stereogenic atoms, 152–55, 167–69, 173, 180
stereoisomers, definition, 148, 176
use in determining reaction mechanisms, 396,
425
Stereogenic atoms, 152–55, 167–69, 173, 180
Steric requirements, definition, 78
Steroids, 456, 530, 559–62
Strain
angle strain, 128, 187, 190, 276, 474
and heat of combustion 194–97
and heat of formation 193–94
quantitative evaluation of strain energy, 193–97
in ring compounds, 187–93
S
N
2 reactions in ring compounds, 276–77
torsional strain, 105, 188–89, 190
van der Waals strain, 198
s-trans conformation, 523–24
Strecker synthesis, 1184, 1216
Structural isomers, definition, 73, 174, 176
Structure determination, 88, 525–26, 696
See also Nuclear magnetic resonance
spectroscopy (NMR); Spectroscopy;
Ultraviolet/visible (UV/vis) spectroscopy
Structure drawings
alkanes, overview, 70–71
ball-and-stick models, 78
line drawings, 71–72, 77–78
Newman projection, 65–66, 94
space-filling models, 78
Strychnine, 171, 172, 256, 532
Styrene (vinylbenzene or ethenylbenzene), 489,
595
Substituents, definition, 60
Substituted benzenes
aromatic substitution reactions, 606–8
Birch reduction,609
disubstituted benzenes, 596–97
formation of disubstituted benzenes, 655–66
meta (m-) substitution, definition, 596, 656
monosubstituted benzenes, 595–96
ortho (o-) substitution, definition, 596, 656
para (p-) substitution, definition, 596, 656
pattern of further reactions, 656
physical properties, 598
polysubstituted benzenes, 597, 668–73
See also Benzene; Substitution reactions of
aromatic compounds
Substitution, nucleophilic, bimolecular reactions.
See S
N
2 reactions
Substitution, nucleophilic, unimolecular reactions.
See S
N
1 reactions
Substitution reactions of aromatic compounds
benzene, resistance to substitution reactions,
606–8, 631–32
benzene deuteration, 606–8, 618
blocking groups, 671
diazonium ions, 647–51, 686, 687
disubstituted benzenes, formation of, 655–66
electrophilic aromatic substitution, general
reaction, 685
electrophilic aromatic substitution, overview,
635, 685
electrophilic substitution of furan and
thiophene, 654–55
electrophilic substitution of pyridine and
pyrrole, 652–54
energy barriers to reactions, 624–25, 627–28
Friedel–Crafts acylation, 643–46, 686, 687, 893
Friedel–Crafts alkylation, 639–43, 646, 655
halogenation, 638–39, 670
inductive effects of groups, 666–68
meta (m-) substitution, definition, 596, 656
meta substitution of anisole, 657
meta substitution of ethyl benzoate, 664–65
meta substitution of halobenzenes, 667–68
meta substitution of nitrobenzene, 663–64
meta substitution of toluene, 660–61
meta substitution of trimethylanilinium ion,
662–63, 666
nitration, 637–38, 664, 668–69, 673, 687
nucleophilic addition to benzenes (S
N
Ar
reaction), 674–76
nucleophilic addition to heteroaromatics (S
N
Ar
reaction), 676–79
ortho (o-) substitution, definition, 596, 656
ortho substitution of anisole, 658
overview, 606–8, 624–26, 646, 685–88
para ( p-) substitution, definition, 596, 656
para substitution of anisole, 657–60
para substitution of ethyl benzoate, 665
para substitution of halobenzenes, 667–68
para substitution of nitrobenzene, 664
para substitution of toluene, 660–61
para substitution of trimethylanilinium ion,
662–63
pattern of further reactions of substituted
benzenes, 656
polysubstituted benzenes,formation of, 668–73
Sandmeyer reaction, 649–51, 686, 687
sulfonation, 636–37, 686
Substitution reactions of nonaromatic compounds
alkyl halides, 267–68
equilibrium, 267–68, 274, 332–33
overview, 262–63
synthetic potential of substitution reactions,
312–13
See also Leaving groups; S
N
1 reactions; S
N
2
reactions
Succinic acid, 830, 881
Succinic anhydride, 881
(¢H°
f
),
(¢H°
c
),
I-24 INDEX

Sucralose, 1164
Sucrose, 239, 1125, 1161, 1166
Sugars. See Carbohydrates
Sugar substitutes, 1164
Sulfate ion, 45
Sulfides. See Thioethers
Sulfonates
benzene sulfonation, 636–37, 686
brosylate (p-bromobenzenesulfonate), 1085–86
Coates’cation, 1117
formation from alcohols,281–86, 322, 427, 555
sulfonation of phenols, 671
Sulfones, 810, 819
Sulfonic acids, 636–37, 686, 809, 819, 866–67
Sulfonium ions, 313, 314
Sulfoxides, 810, 819
Sulfur compounds as nucleophiles, 313–14
Sulfuric acid, 45
Superacid, 679–80
Suprafacial motion, 1055–59
Syn addition, 394–95
Syn elimination, 305–6
Synthesis
building a map as problem-solving technique,
999–1001, 1014–17
keeping track of methods, 320
“real world”difficulties, 318–20, 323
synthetic potential of substitution reactions,
312–13
See also specific substances
Table sugar (sucrose), 239, 1125, 1161, 1166
D-Talose, 1129, 1160
Tautomers, 29, 939–40, 944, 961, 966
Taxol, 183
tBoc (di-tert-butyl dicarbonate),1205–10, 1217
Terephthalic acid, 853
Termination of chain reactions, 483
Terpenes, 554–58
See also Isoprene
Tertiary carbon, 76
Tertiary structure, 1192–94
Testosterone, 562
Tetracycline, 531
Tetraethylammonium bromide, 242
Tetrahedral intermediate in addition–elimination
reactions
acid anhydride formation, 856
acid chloride formation, 854
amide formation, 851
amide hydrolysis, 901
Claisen condensation, 987
definition, 842
ester hydrolysis, 895
ester reaction with Grignard reagent, 898
Fischer esterification reaction, 842–44
halogenation of carbonyl compounds, 949
overview, 868, 877, 878, 890
See also Intermediates
Tetrahydrofuran (THF), 250
13
C NMR spectroscopy, 89
dipole moment, 620
ether–borane complex, 391
and Grignard reagent formation, 228, 251, 254
as Lewis base, 391
and organolithium reagent formation, 228
solvation of water, 240
structure, 89, 250, 254
Tetrahydropyran (pentamethylene oxide,
oxacyclohexane), 254
Tetrahydropyranyl (THP) ethers, 789–90
2,2,3,3-Tetramethylbutane, 195–96, 195n2
Tetramethylsilane (TMS), 716–17, 720–21
Tetra-tert-butyltetrahedrane, 216
Tetroses, 1127, 1129
Thalidomide, 180, 180n6
Thermodynamic control, 353, 360, 537–41
Thermodynamic enolates, 1008
Thermodynamics
definition, 351
entropy change 335, 337–39, 349
Gibbs free energy 120, 334–39, 349
and position of equilibrium, 333–34
thermodynamics versus kinetics effect on rates,
351–57, 359–60
See also Enthalpy change
Thermolysis, definition, 470
Thiazolium ion, 1028
Thioethers (sulfides)
formation of sulfonium ions, 313
mustard gas [bis(2-chloroethyl)thioether], 1089
neighboring group effect in intramolecular
reactions, 1088–89
nomenclature, 252–53
odor, 251–52
oxidation to sulfoxides, 810
reduction with Raney nickel, 253, 257
sulfur-containing compounds in garlic, 252
synthesis by mercaptide S
N
2 reactions, 313
thioester A and cholesterol formation, 358
See also Ethers
Thiolate ions, 284, 314, 324
Thiols (mercaptans)
acidity, 253
mercaptide formation, 253
nomenclature, 252
odor, 251–52
overview, 251–52
oxidation reactions, 809–11
reduction with Raney nickel, 253, 257
sulfur-containing compounds in garlic, 252
synthesis by mercaptide S
N
2 reactions, 313
synthesis from alkene, 314
synthesis from alkyl halide, 314
Thionyl chloride, 284–85, 643, 853–54, 868
Thiophene, 599, 599–600, 654–55
Thioureas, 1198–99, 1206, 1218
Thomson, J. J., 2
Three-center, two-electron bonding, 1102, 1116,
1116n4
Three-dimensional structures, drawing, 16
Threonine, 1176
Threose, 1129
Thujone, 558
Thurber, James Grove, 332, 332n1
Thymine, 615, 1212, 1214
Tolkien, John Ronald Reuel, 186, 186n1
p-Tolualdehyde (4-methylbenzaldehyde),768
Toluene (methylbenzene or phenylmethane)
Friedel–Craft alkylation, 641, 655–56
mass spectrometry (MS), 702–3
meta substitution, 660–61
para substitution, 660–61
radical bromination, 613
structure, 329, 592, 595
substitution reactions, 655, 660–62, 663
synthesis, 641, 655–56
Torsional strain, 105, 188–89, 190
Tosylates
as leaving group, 283–84, 305, 555, 798, 1093
3-methyl-2-butyl tosylate reaction with acetic
acid, 1108–9
7-norbornenyl tosylates reaction with acetic
acid, 1099–1108
3-phenyl-2-butyl tosylate conversion to 3-
phenyl-2-butyl acetate, 1096–99
solvolysis reaction of exo- and endo-2-
norbornyl tosylate, 1110–16
Transesterification, 896–98, 924, 987
Transfer RNA (tRNA), 1215
trans isomers, 84, 85, 144
Transition states
additions to alkenes, 136
comparison to intermediates, 186
cyclohexane conformations, 197–98
definition, 67, 72, 186
effect of solvent polarity, 286–87
Hammond postulate, 355–57, 377, 496
parallelism between transition state and
product energies, 353–57
photohalogenation, 495, 496–97
rate-determining step,294–95, 350–51, 359
relationship to reaction rate, 339
S
N
1 reactions, 333, 343, 346–49
S
N
2 reactions, 272–74, 276–77, 332, 344–46,
543
thermodynamics versus kinetics, 351–57
transition state theory, 349
See also Activation energy ( G°)
Trialkylsilyl ethers, 789–90
Trialkylsilyl halides, 789–90
Triamantane, 218, 219
A [5]Triangulane, 211
Triangular hydrogen (H
3
,H
3
), 102, 217, 1103
Tribromophenol, 671
2,4,6-Tribromophenol, 670, 671
1,3,5-Trichlorobenzene, 597
Tricyclo[3.3.1.1
3,7
]decane (adamantane), 217–19
Tricyclo[4.1.0.0
4,6
]heptane, 211
Tricycloillinone, 217
Triethylamine, 241, 277–78
Trifluoroacetic acid, 424
Trifluoroperacetic acid, 424,672
2,3,3-Trimethyl-1-butene, 419
Trimethylammonium group, 666
Trimethylanilinium ion, 662–64
2,2,3-Trimethylbutane, 83
Trimethylene oxide (oxacyclobutane, oxetane), 254
Trimethyloxonium fluoborate, 249
1,3,5-Trinitrobenzene, 668
1,3,5-Trioxane (1,3,5-trioxacyclohexane,
paraldehyde), 254
Triphenylmethyl radical, 480
Triphenylphosphine, 285, 811
Triple bonds
Cahn–Ingold–Prelog priority system, 114,
153–54
Lewis structure, 18–19
pi ( ) orbitals, 125
in rings, 128
sigma σ bonding,123–25, 128
See also specific compounds
Triplet carbenes, 433–36, 468
Triptycene, 692
Triptycenyl chloride, 611
Triquinacene, 532
¢
(¢H°)
(¢G°),
(¢S°),
INDEX I-25

Trityl cation, 587, 611, 618
Tropylium fluoborate, 587, 618
Tropylium ion (cycloheptatrienylium ion), 587–89,
590, 618
Trypsin, 1202
Tryptophan, 1176
Twistoflex, 604
Tyrosine, 684, 1176
Ultraviolet/visible (UV/vis) spectroscopy
Beer–Lambert law, 527
1,3-butadiene, 528–29
-carotene, 529
complex spectra from conjugated molecules,
530
conjugated dienes, 528–32
electron transitions and energies, 527–29
ethylene (ethene), 527–29
extinction coefficient, 527
Fiesers’ rules, 530
history and background, 525–26, 696
HOMO–LUMO gap, 528–29
spectrometers, 527
Woodward’s rules,530–31
Unimolecular elimination reactions. See E1
reactions
Unsaturated hydrocarbons, 83
See also Alkenes; Alkynes
Uracil, 1212, 1214
Urea, 860, 1194
substituted, 1198, 1206, 1218
Valence electrons, 3–6, 16
Valine, 955, 1175, 1176, 1178, 1189
van der Waals forces, 87
van der Waals strain, 198
Vanillin, 582
van’t Hoff, Jacobus Henricus, 1153
Vicinal groups
definition, 414, 447, 471
dihalides, 414, 448, 459, 490, 499, 507
vicinal diols, oxidative cleavage, 807, 817, 818,
845
vicinal diols from alkene oxidation, 443, 459,
840
Vilsmeier reagent, 873, 928
Vinyl alcohol (hydroxyethene), 939–40
Vinyl ammonium ion, 45
Vinylboranes,450–51
Vinyl carbocations, 444–45
Vinyl chloride,45, 367, 372
Vinyl compounds, 107
Vinyl halides,444, 459
Vinylic hydrogens, 724–25, 739
Vinyl iodide (iodoethene),226
Vinyl radicals,454, 489
Viquidil, 95
Vitamin A (trans-retinol), 121, 533
Vitamin B
12
, 532
Vitamin E, 505
Vogel, Emanuel, 594, 1034–35, 1038
von Baeyer, Adolf, 187
Voodoo lily, 838
Wagner–Meerwein rearrangement,387
Walden, Paul, 274
Watson, James D., 1213
Wave functions
antibonding molecular orbitals (
antibonding
,
A
), 32–33
bonding molecular orbitals (
bonding
,
B
),
32–33
definition, 6
electron density in atomic orbitals, 10, 11–12,
32–33
nodes in atomic orbitals, 11–12
probability of finding electron in a volume of
space 6, 10, 11–12
Schrödinger’s wave equation, 6
See also Orbitals
Wavenumber, 708
Weighting factors, 26–30, 372–73, 419–20
Wiberg, Kenneth B., 216–17
Wilkins, Maurice,1213
Wilkinson’s catalyst (rhodium), 411
Williamson ether synthesis,254, 315–17, 322,
1147, 1150, 1163
Wittig reaction, 811–13, 817
Wöhler, Friedrich, 30
Wolff–Kishner reduction, 645, 686,802
Wolff rearrangement, 916–18, 924
Woodward, Robert Burns, 172, 530–31, 532,
1031–32, 1034
Woodward–Hoffmann theory, 1031–32, 1034,
1035, 1056
Wurtz, Charles Adolphe, 966n2
Xanthate esters, 914, 923, 925
Xanturil, 95
X-ray crystallography, 173, 1192, 1192n3, 1194,
1213
Xylene (dimethylbenzene), isomers, 597,598
D-Xylose, 1129, 1159
Yeats, William Butler,1031, 1031n1
Ylides, 811–12
Zusammen (Z) isomers, 109, 111, 112–15
See also cis isomers
Zwitterions, 1179–80
(°
2
),
££
£
£
(°)
I-26 INDEX
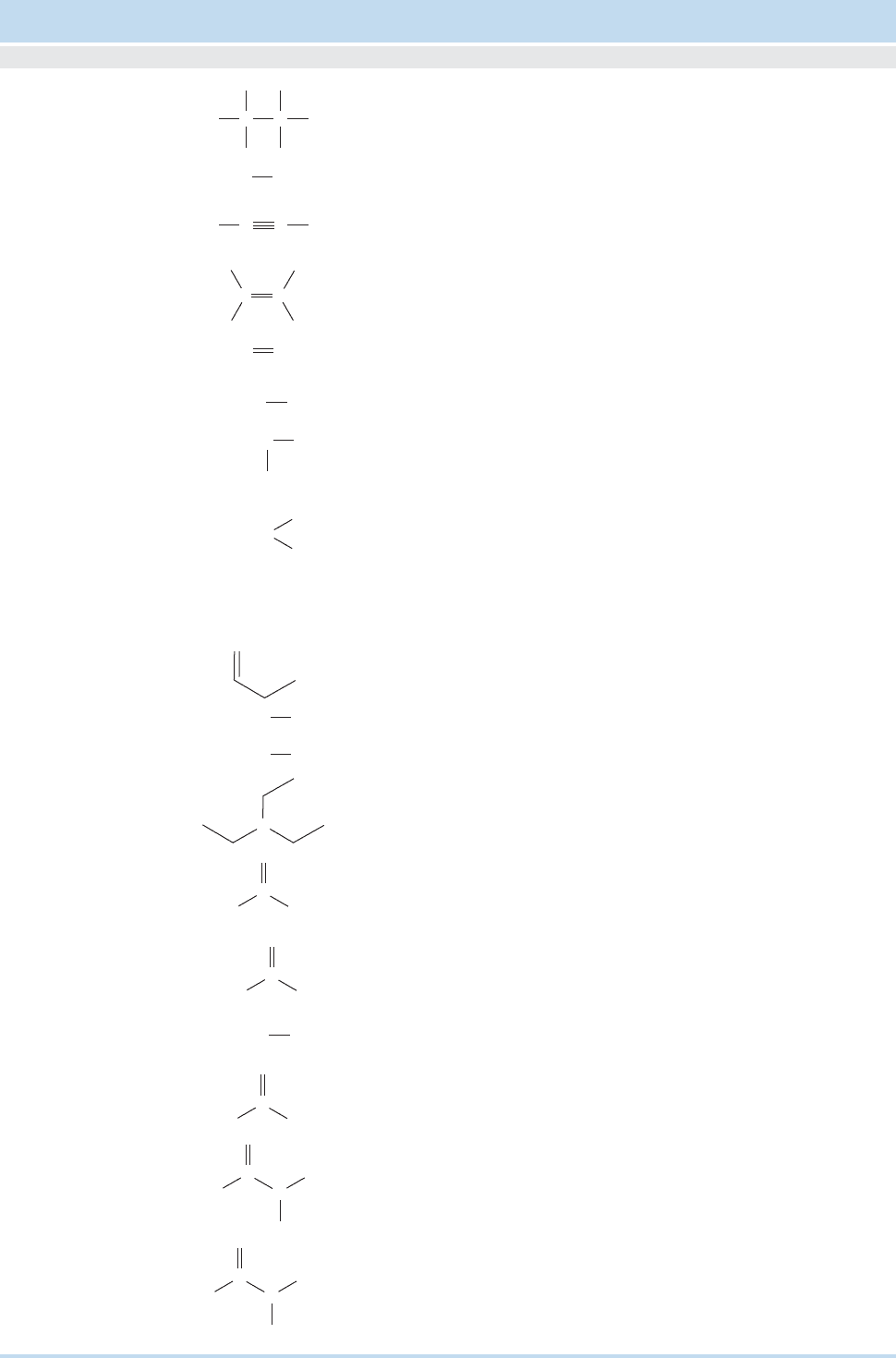
Commonly Encountered Structural Types of Organic Chemistry
Functional
Group Example
Common Name
(if applicable)
—
Suffix Prefix
IUPAC
Name
Ethane
Principal Groups
a
Alkane
AcetyleneEthyne
Alkyne
EthyleneEthene
Alkene
Amines
EthylamineEthanamine
Primary amine
EthylmethylamineN-Methylethanamine
Secondary amine
EthyldimethylamineN,N-Dimethylethanamine
Tertiary amine
Amides
AcetamideEthanamide
Primary amide
—N-Methylethanamide
Secondary amide
Dimethylformamide
(DMF)
N,N-Dimethyl-
methanamide
Tertiary amide
TetraethylammoniumN,N,N-Triethylethanammonium
Quaternary amine
Propanal imine1-Propanimine
Imine
Ethyl mercaptanEthanethiol
iol
Ethyl alcoholEthanol
Alcohol
—Triethylborane
Borane
AcetonePropanone
Ketone
AcetaldehydeEthanal
Aldehyde
Ethyl cyanidePropanenitrile
Nitrile
H
HH
H
CC
H
H
CH
3
CH
2
N
CH
3
H
CH
3
CH
2
N
CH
3
CH
3
(CH
3
CH
2
)
4
N
+
NH
CH
3
CH
2
SH
CH
3
CH
2
OH
CH
3
CH
2
CN
B
C
H
3
C
CH
3
O
C
H
3
C
H
O
C
H
3
CNH
2
O
—
—
—
amino-
amino-
amino-
amido-
amido-
amido-
—
imino-
mercapto-
hydroxy-
boro-
oxo-
oxo-
cyano-
-ane
-yne
-ene
-amine
-amine
-amine
-amide
-amide
-amide
-ammonium
-imine
-thiol
-ol
-borane
-one
-al
-nitrile
CCH
3
H
3
C
N
H
O
CCH
3
H
N
CH
3
O
H
2
CCH
2
CC
H
H
H
H
CCHH
CH
3
CH
3
CH
3
CH
2
NH
2
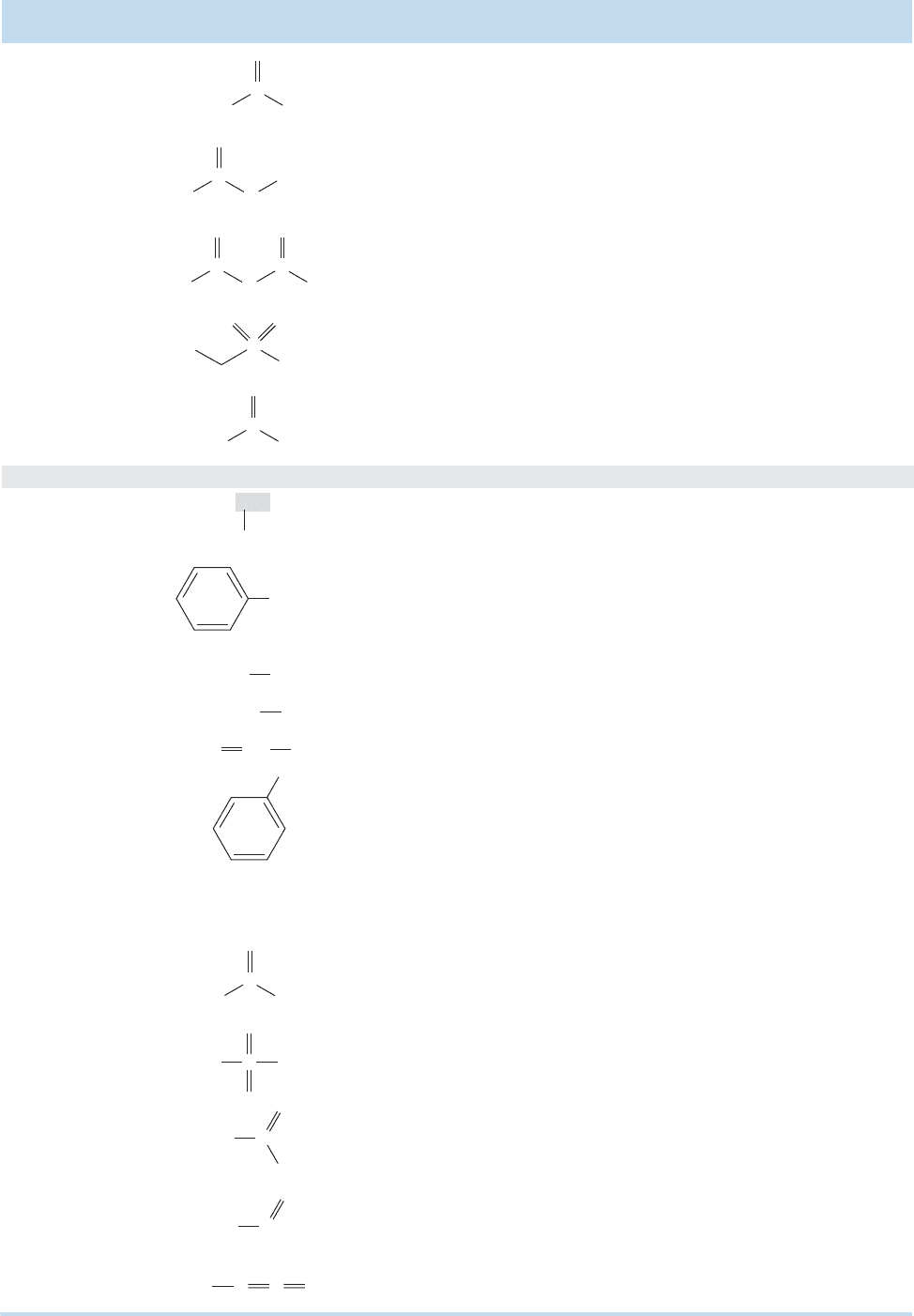
Commonly Encountered Structural Types of Organic Chemistry (continued)
Functional
Group Example
Common Name
(if applicable) Suffix Prefix
IUPAC
Name
a
e principal groups are listed in increasing order of priority. e subordinate groups have no established priority and can only be referenced as prefixes.
Subordinate Groups
a
Acetyl chloride -oyl
chloride
Ethanoyl chloride
Methyl fluoride — fluoro-Fluoromethane
Fluoride
Ethyl chloride — chloro-Chloroethane
Chloride
Vinyl bromide — bromo-Bromoethene
Bromide
Phenyl iodide — iodo-Iodobenzene
Iodide
Ethyl acetate -oate
chlorocarbonyl-
Ethyl ethanoate
Acetic anhydride -oic
anhydride
alkyoxycarbonyl-
Ethanoic anhydride
— -sulfonic
acid
acyloxycarbonyl-
Ethanesulfonic acid
Acetic acid -oic acid
sulfo-
carboxy-Ethanoic acid
Diethyl ether — alkoxy-Ethoxyethane
Diethyl sulfide — alkylthio-Ethylthioethane
Dimethyl sulfoxide
(DMSO)
— sulfinyl-
Methylsulfinylmethane
Dimethyl sulfone
— sulfonyl-
Methylsulfonylmethane
— — nitro-Nitromethane
— — nitroso-Nitrosomethane
Methyl azide — azido-Azidomethane
— — alkyl-3-Methylpentane
Benzyl alcohol — phenyl-Phenylmethanol
Acid chloride
Halides
Ester
Anhydride
Sulfonic acid
Carboxylic acid
Ether
Sulfide
Sulfoxide
Sulfone
Nitro
Nitroso
Azide
Alkyl
Aryl
C
H
3
CO
O
CH
2
CH
3
C
H
3
CCl
O
CH
2
CH
Br
CH
3
F
CH
3
CH
2
Cl
C
H
3
CO
O
C
CH
3
O
S
OH
O
O
C
H
3
C
OH
O
CH
3
CH
3
CH
2
CHCH
2
CH
3
I
CH
3
CH
2
OCH
2
CH
3
CH
3
CH
2
SCH
2
CH
3
H
3
C
N
O
–
O
+
S
O
O
H
3
C
CH
3
S
H
3
C
CH
3
O
H
3
C
N
O
H
3
C
NNN
+
–
CH
2
OH

4.002602
10.811 12.0107 14.0067 15.9994 18.9984032 20.1797
22.98976928 24.3050 26.9815386 28.0855 30.973762 32.065 35.453 39.948
6.941 9.012182
1.00794
1.00794
39.0983 40.078 44.955912 47.867 50.9415 51.9961 54.938045 55.845 58.933195 58.6934 63.546 65.409 69.723 72.64 74.92160 78.96 79.904 83.798
85.4678 87.62 88.90585 91.224 92.90638 95.94 [98] 101.07 102.90550 106.42 107.8682 112.411 114.818 118.710 121.760 127.60 126.90447 131.293
132.9054519 137.327 138.90547 178.49 180.94788 183.84 186.207 190.23 192.217 195.084 196.966569 200.59 204.3833 207.2 208.98040 [209] [210] [222]
[223] [226] [227] [261] [262] [266] [264] [277] [268] [271] [272] [285]
140.116 140.90765 144.242 [145] 150.36 151.964 157.25 158.92535 162.500 164.93032 167.259 168.93421 173.04
232.03806 231.03588 238.02891 [237] [244] [243] [247] [247] [251] [252] [257] [258] [259]
174.967
[262]
Metals
Nonmetals
Metalloids
Hydrogen
Hydrogen
Lithium Beryllium
Sodium
Potassium
Rubidium
Cesium
Francium
Strontium
Barium
Radium
Yttrium
Lanthanum
Actinium
Zirconium
Hafnium
Rutherfordium
Cerium
orium
Praseodymium
Protactinium
Neodymium
Uranium
Promethium
Neptunium
Samarium
Plutonium
Europium
Americium
Gadolinium
Curium
Terbium
Berkelium
Dysprosium
Californium
Holmium
Einsteinium
Erbium
Fermium
ulium
Mendelevium
Ytterbium
Nobelium
Lutetium
Lawrencium
Niobium
Tantalum
Dubnium
Molybdenum
Tungsten
Seaborgium
Technetium
Rhenium
Bohrium
Ruthenium
Osmium
Hassium
Rhodium
Iridium
Meitnerium
Palladium
Platinum
Darmstadtium
Silver
Gold
Cadmium
Mercury
Indium
allium
Tin
Lead
Antimony
Bismuth
Tellurium
Polonium
Iodine
Astatine
Xenon
Radon
Roentgenium Copernicium
Calcium Scandium Titanium Vanadium Chromium Manganese Iron Cobalt Nickel Copper Zinc Gallium
Aluminum
Boron
Silicon
Carbon
Phosphorus
Nitrogen
Sulfur
Oxygen
Chlorine
Fluorine
Argon
Neon
Helium
Germanium Arsenic Selenium Bromine Krypton
Magnesium
We have used the United States system as well as the system recommended by the International Union of Pure and Applied Chemistry (IUPAC) to label the groups in this periodic table. e system used
in the United States includes a letter and a number (1A, 2A, 3B, 4B, etc.), which is close to the system developed by Mendeleev. e IUPAC system uses numbers 1–18 and has been recommended by the
American Chemical Society (ACS). Elements with higher atomic numbers have been reported but not yet fully authenticated.
He
Na
K
Mg Al Si P S Cl Ar
Li Be B
H
H
CNOFNe
Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr
Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe
Cs Ba La Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn
Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb
Pa U Np Pu Am Cm Bk Cf Es Fm Md No
Lu
Lr
Fr Ra Ac Rf Db Sg Bh Hs Mt Ds Rg Cp
2
11 12 13 14 15 16 17 18
34 5
1
1
678910
19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36
37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54
55 56 57 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86
87 88 89 104 105 106 107 108 109 110 111 112
58 59 60 61 62 63 64 65 66 67 68 69 70
90 91 92 93 94 95 96 97 98 99 100 101 102
71
103
Atomic number
Symbol
Name
Average atomic mass
1
1A
2
2A
13
3A
14
4A
15
5A
16
6A
17
7A
3
2
1
4
5
6
7
6 Lanthanides
7 Actinides
3
3B
4
4B
5
5B
6
6B
7
7B
11
1B
12
2B
89
8B
10
18
8A
PERIODIC TABLE OF THE ELEMENTS

Compound
a
pK
a
Compound
a
pK
a
Alkanes
CHCl
3
CH
3
CN
NH
3
RC CH
H
43
41
29
25
24
50–60
45–50
38
43
25
17
16.5
15.9
H
CH
2
H
24
(CH
3
)
3
COH
(CH
3
)
2
CHOH
CH
3
CH
2
OH
18
16.7
19.3
18.1
18.3
HC C
3
H
H
2
O
Ph CH
2
O
H
NHR
O
H
CH
O
H
H
2
CH
O
H
H
2
C OCH
2
CH
3
O
H
H
2
CNH
2
O
H
CH
2
H
pK
a
Values for Commonly Encountered Structural Types
19.9
O
RCHCH
3
H
