Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.

23.4 Peptide Chemistry 1199
A peptide will react with isothiocyanate at the amino terminus to form a
thiourea. The thiourea can be cleaved in acid without breaking the other amide
bonds in the peptide. As we saw in Chapter 21 (p. 1088), sulfur is an excellent
neighboring group, and the introduction of the thiourea places a sulfur atom
in just the right position to aid in cleavage of the amino terminus amino acid
from the rest of the peptide (Fig. 23.36). The process involves initial protona-
tion of the amino terminus carbonyl. The neighboring sulfur then adds to
the carbonyl carbon, triggering the cleavage reaction to give a molecule called
a thiazolinone.
sulfur acts as
neighboring
group
Side chain of
amino terminus
amino acid
Good leaving
group
Intact peptide chain,
one amino acid shorter,
can be used for
further analysis
S
N
OH
..
..
H
N
..
..
+
+
O
Ph NH
proton
transfers
cleavage
OH
..
..
..
..
..
..
..
..
..
..
..
H
2
N
..
..
SN
..
Ph NH
Rest of
peptide
chain
..
H
2
N
SN
..
..
..
Ph NH
deprotonation
A thiazolinone
A phenylthiohydantoin
R
R R
N
O
H
3
O/H
2
O
+
S
N
Ph
R
+
Rest of
peptide
chain
OH
..
..
S
..
Ph NH
R
..
H
N
HN
..
Rest of
peptide
chain
Rest of
peptide
chain
..
..
..
..
H
H
WEB 3D
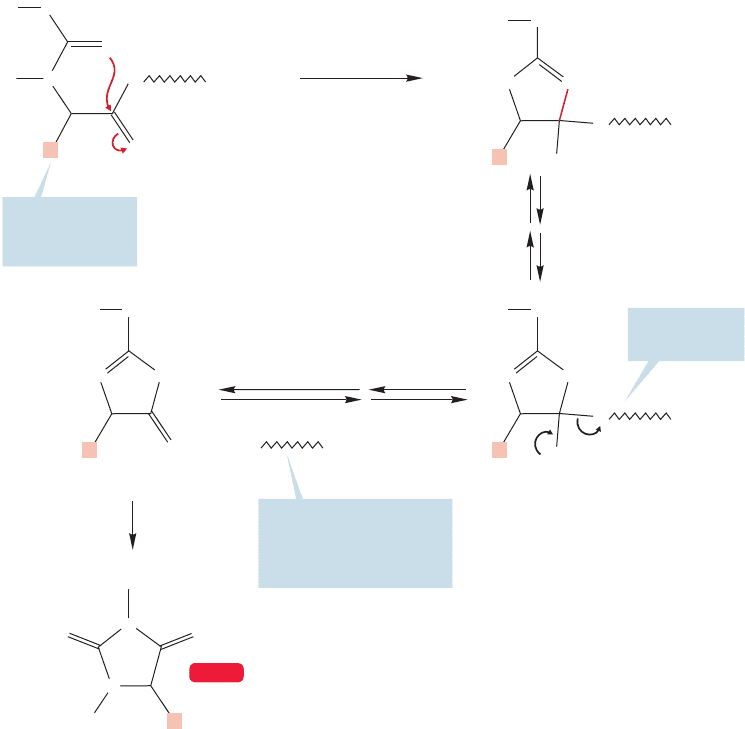
FIGURE 23.36 The Edman degradation procedure for sequencing polyamino acids uses
phenylisothiocyanate.The amino terminus is identified by the structure of the
phenylthiohydantoin formed. Notice that this method does not destroy the peptide
chain as the Sanger procedure does.
Further acid treatment converts the thiazolinone into a phenylthiohydantoin
containing the R group of the amino terminus amino acid, which can now be
determined based on the identity of the R group. The critical point is that
the rest of the peptide remains intact and the Edman procedure can now be

1200 CHAPTER 23 Amino Acids and Polyamino Acids (Peptides and Proteins)
N
H
3
N
N
O
O
..
..
..
..
..
..
..
..
..
–
+
+
Edman
..
..
..
..
..
Edman
R
R
R
H
H
N
H
O
S
N
Ph
R
O
..
..
O
H
3
N
..
..
+
+
+
O
H
3
N
N
O
O
O
..
..
..
..
..
..
..
..
–
R
R
H
..
..
..
..
..
..
N
H
O
S
N
Ph
R
..
O
..
..
..
–
R
FIGURE 23.37 The
primary structures of
polyamino acids can be
determined through
repeated applications of
the Edman degradation
procedure, which is one
method of sequencing.
repeated, in principle, as many times as are needed to sequence the entire pep-
tide (Fig. 23.37).
N
O
..
..
..
..
BrCN
Methionine Methionine
R
H
..
H
O
N
..
..
R
H
..
..
..
..
H
O
SCH
3
..
..
N
O
..
..
..
..
H
N
..
..
H
O
..
..
N
..
H
++
O
CH
3
S
Lactone
..
..
O
N
..
..
H
H
2
N
..
..
..
..
O
H
2
N
..
O
N
N
..
R
R
R
R
O
..
..
O
..
..
O
..
..
Lactone
FIGURE 23.38 The carboxy
end of each methionine is
cleaved on treatment of a
polypeptide with cyanogen
bromide. A lactone is formed
in place of a methionine.
PROBLEM 23.20 Write a mechanism for the formation of the phenylthiohydan-
toin from the thiazolinone (Fig. 23.36).
But the practical world intrudes, and even the Edman degradation procedure,
good as it is, becomes ineffective for polypeptides longer than about 20–30 amino
acids. Impurities build up at each stage in the sequence of determinations and even-
tually the reaction mixture becomes too complex to yield unequivocal results.
Accordingly, methods have been developed to cut proteins into smaller pieces at
known positions in the amino acid sequence.These methods take advantage of both
biological reactions in which enzymes break peptides only at certain amino acids,
and a very few specific cleavages induced by small-molecule laboratory reagents.
Perhaps the best of these “chemical”(enzymes are chemicals, too) cleavages uses
cyanogen bromide (BrCN) and takes advantage of the nucleophilicity of sulfur and
the neighboring group effect to induce cleavage at the carboxy side of each methio-
nine in a polypeptide (Fig. 23.38).
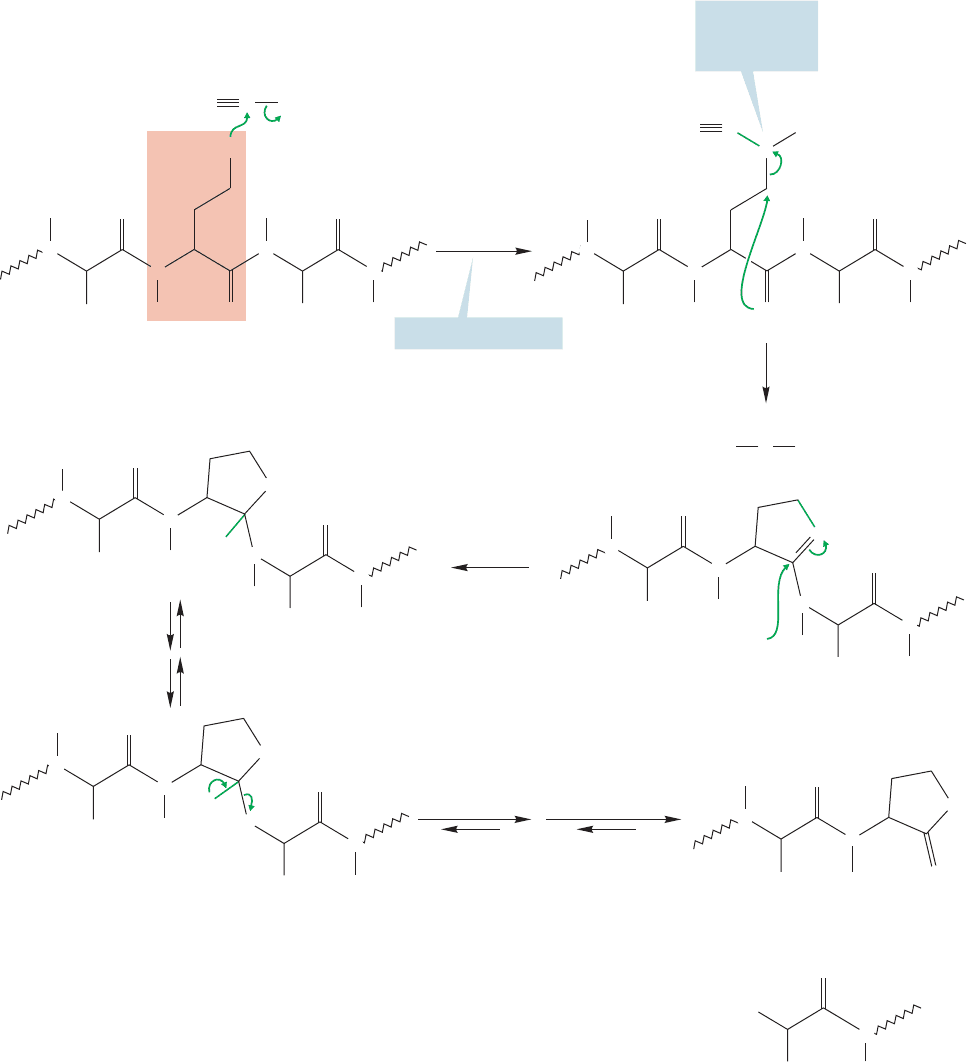
23.4 Peptide Chemistry 1201
The sulfur atom of methionine first displaces bromide from cyanogen bro-
mide to form a sulfonium ion (Fig. 23.39).Then attack by the proximate carbonyl
group of methionine displaces the excellent leaving group, methylthiocyanate, and
forms a five-membered ring called a homoserine lactone. Hydrolysis steps follow
to produce two smaller peptide fragments, one containing a lactone at its car-
boxy terminus.
C
N
O
..
..
..
..
BrCN
HCl
Methionine
R
H
N
NBr
..
..
H
O
H
3
CS
..
..
..
..
N
..
..
..
..
R
H
N
..
..
H
O
+
intramolecular
S
N
2
addition
elimination deprotonation
proton
transfers
Sulfonium ion
(an excellent
leaving group)
addition–elimination
Homoserine lactone
N
O
..
..
..
..
R
H
H
N
N
..
..
O
..
N
..
..
..
Br
..
..
..
..
R
H
N
..
..
H
O
CH
3
S
+
–
C
N
O
..
..
R
H
H
N
..
..
O
..
..
N
..
..
..
R
H
..
N
H
O
..
CH
3
S
+
NC
..
..
H
2
O
of H
2
O
..
N
..
..
..
R
H
H
N
..
..
O
N
..
..
R
H
..
N
H
O
+
..
H
2
O
..
N
..
..
R
H
H
N
..
..
O
N
..
..
R
H
..
N
H
2
O
+
..
..
HO
N
O
..
..
..
..
R
N
..
..
H
H
O
N
..
..
R
H
..
..
H
2
N
O
..
..
O
+
O
..
..
O
..
FIGURE 23.39 The mechanism of cleavage using BrCN.
1202 CHAPTER 23 Amino Acids and Polyamino Acids (Peptides and Proteins)
Most methods used to chop large peptides into smaller fragments take advan-
tage of enzymatic reactions. Chymotrypsin, for example, is an enzyme that
cleaves peptides at the carboxyl groups of amino acids containing aromatic side
chains (phenylalanine, tryptophan, or tyrosine).Trypsin cleaves only at the car-
boxy end of lysine and arginine. There are several other examples of this kind
of specificity. Long peptide chains can be degraded into shorter fragments that
can be effectively sequenced by the Edman procedure. A little detective work suf-
fices to put together the entire sequence. Let’s look at a simple example and then
do a real problem.
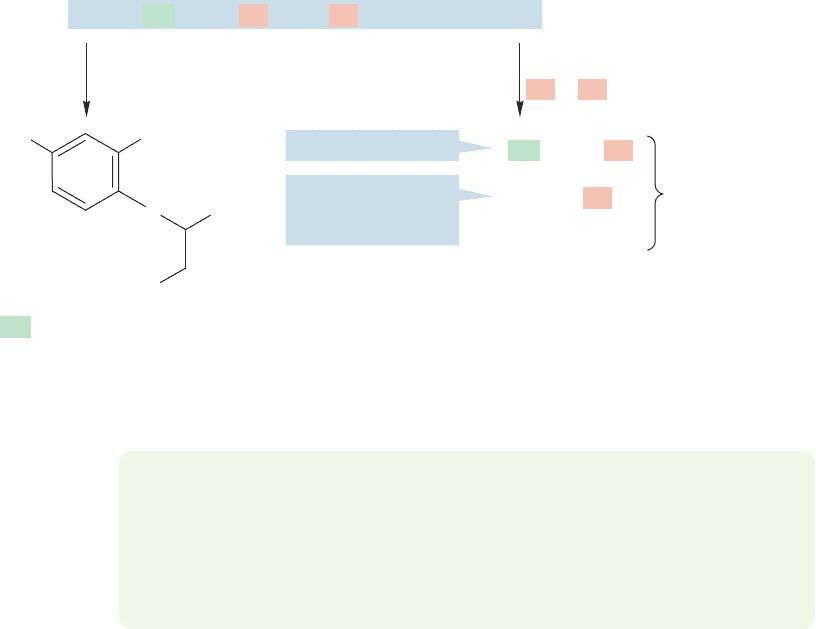
Consider the decapeptide in Figure 23.40. We can determine the amino termi-
nus through the Sanger procedure to be Phe. Next, we use the enzyme trypsin in a
separate experiment to cleave the decapeptide into three fragments.As we know the
peptide must start with Phe, the first four amino acids are . The
only other cleavage point is at Arg, so the fragment must be the mid-
dle piece. If this tripeptide were at the end, with Arg as the carboxy terminus, no
cleavage would be possible. The sequence must be
.Arg
.
Glu
.
Leu
.
Val
Phe
.
Tyr
.
Trp
.
Lys
.
Asp
.
Ile
.
Asp
.
Ile
.
Arg
Phe
.
Tyr
.
Trp
.
Lys
Sanger
Phe must be the amino terminus
trypsin
(cleaves only after
Lys or Arg)
Can be sequenced
using Edman
Must start with Phe
Contains a cleavage
point (Arg)—must be
middle fragment
N
H
Ph
COOH
Phe
.
Tyr
.
Trp
.
Lys
.
Asp
.
Ile
.
Arg
.
Glu
.
Leu
.
Val
Phe
.
Tyr
.
Trp
.
Lys
Asp
.
Ile
.
Arg
+
+
Glu
.
Leu
.
Val
O
2
N
NO
2
FIGURE 23.40 An example of peptide sequencing using Sanger and Edman procedures as well as enzymatic
cleavage reactions.
PROBLEM 23.21 The nonapeptide bradykinin can be completely hydrolyzed in
acid to give three molecules of Pro, two molecules each of Arg and Phe, one mol-
ecule of Ser, and one molecule of Gly. Treatment with chymotrypsin gives the
pentapeptide , the tripeptide . End-
group analysis shows that the amino acids on both the amino and carboxy termini
are the same. Provide the sequence for bradykinin.
23.4c Synthesis of Peptides Now that we know how to sequence proteins,
how about the reverse process—how can we put amino acids together in any
sequence we want? Can we synthesize proteins? It is simply a matter of forming
amide bonds in the proper order, and this task might seem easy. However, two dif-
ficulties surface as soon as we begin to think hard about the problem. First, there’s
a lot of work to do in order to make even a small protein. Imagine that we only want
Ser
.
Pro
.
Phe, and ArgArg
.
Pro
.
Pro
.
Gly
.
Phe
23.4 Peptide Chemistry 1203
COO
–
Ala
Ala
.
Ala
Ala
.
Leu
Further
reactions
Leu
.
Ala
Leu
.
Leu
H
3
N
+
COO
–
Leu
H
3
N
+
H
3
N
+
O
..
..
COO
–
N
..
H
H
3
N
+
O
..
..
COO
–
N
..
H
H
3
N
+
O
..
..
COO
–
N
..
H
H
3
N
+
O
..
..
COO
–
N
..
H
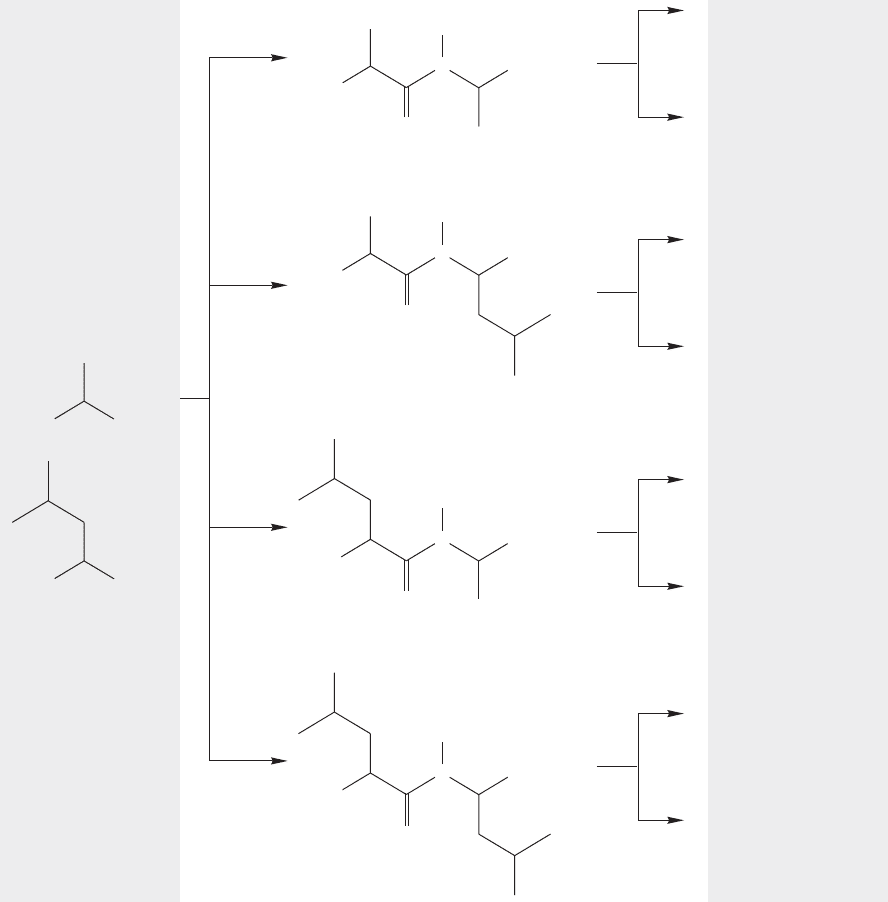
FIGURE 23.41 The possible combinations of two unprotected amino acids.
to create the nine amide linkages of the decapeptide in Figure 23.40. Even if we can
make each amide bond in 95% yield, we are in trouble because a series of nine
reactions each proceeding in 95% yield, which probably seems quite good to any-
one who has spent some time in an organic lab, gives an overall yield of only
(0.95)
9
63%. Obviously, to get anywhere in the real world of proteins, in which
chain lengths of hundreds of amino acids are common, we are going to have to
do much better than this.
Second, there is a problem of specificity. Suppose we want to make the triv-
ial dipeptide . Even if we can avoid simple acid–base chemistry, ran-
dom reaction between these two amino acids will give us at least the four
possible dimeric molecules, and in practice, other larger peptides will be pro-
duced as well (Fig. 23.41).
Ala
.
Leu
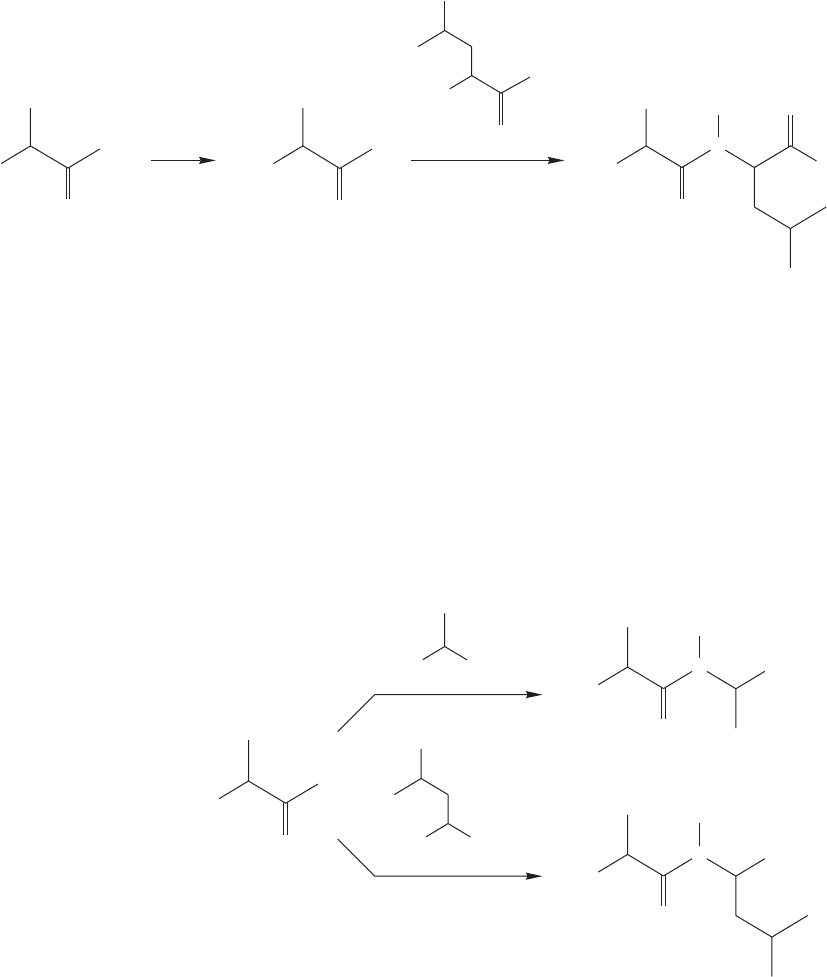
1204 CHAPTER 23 Amino Acids and Polyamino Acids (Peptides and Proteins)
Ala
SOCl
2
H
3
N
+
–
Leu
H
3
N
+
O
O
O
–
..
..
O
..
..
..
–
O
..
..
..
Acid chloride
of Ala
H
3
N
+
O
..
..
..
..
O
..
..
Cl
..
..
..
Ala
•
Leu
H
3
N
+
O
..
..
N
..
H
..
..
..
FIGURE 23.42 Some specificity in the reaction between two amino acids might be obtained by allowing the
acid chloride of one amino acid to react with the other amino acid.
COO
H
3
N
+
COO
–
H
3
N
+
–
H
3
N
+
O
..
..
Cl
..
..
..
H
3
N
+
O
..
..
COO
–
N
..
H
H
3
N
+
O
..
..
COO
–
N
..
H
Ala
.
Ala
Ala
Leu
Ala
.
Leu
Acid chloride
of Ala
(activated acid)
FIGURE 23.43 Here, too, more than
one product is inevitable, because as
the acid chloride is formed it will
react with any amine in solution.
However, even this procedure is hopeless! What is to prevent the amino group
of the original alanine from reacting with the acid chloride? Nothing. Again, a mix-
ture is certain to result (Fig. 23.43). Remember, we ultimately will have to make
dozens, perhaps hundreds of amide bonds. We can tolerate no mediocre yields or
even worse, mixtures, if we hope to make useful amounts of product. We need to
find the most efficient way of making the amide bond specifically at the point we
want it, and at the same time avoiding side reactions.
One simple strategy for avoiding undesired reactions between alanine and
leucine would be to activate the carbonyl group of alanine by transforming the
acid group of alanine into the acid chloride, and then treating it with leucine
(Fig. 23.42).
In this trivial case, not only do we need to activate the carboxyl group of ala-
nine, but we need to block the reaction at the amino group of alanine and at the
carboxyl group of leucine. Three positions must be modified (the carboxyl groups
of alanine and leucine and the amino group of alanine). Left free for reaction are
the two positions we hope to join, the activated carboxyl group of alanine and the
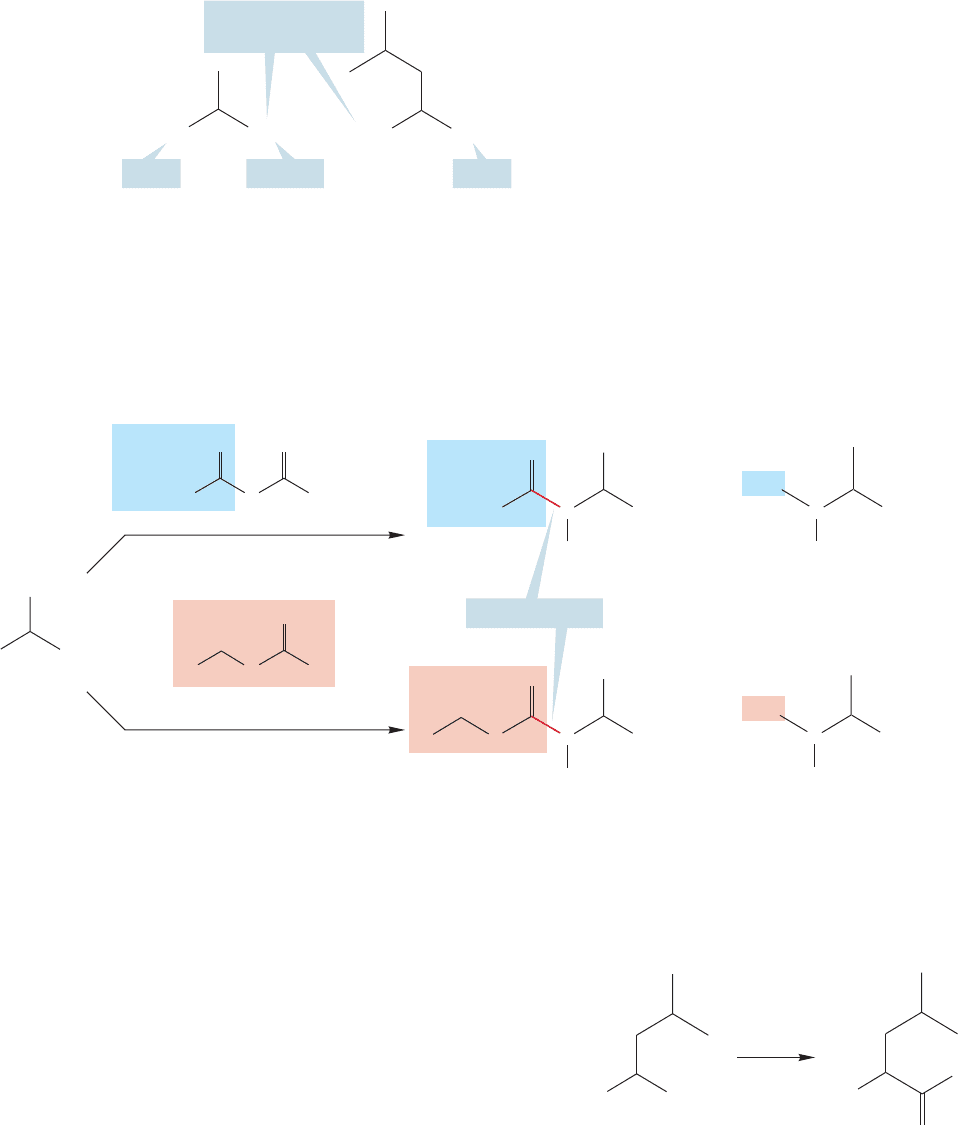
23.4 Peptide Chemistry 1205
Di-tert-butyl dicarbonate
O
..
..
..
..
..
..
O
..
..
O
..
..
Benzyl chloroformate
(benzyloxycarbonyl chloride)
Ph
Cl
O
..
..
O
..
..
(CH
3
)
3
CO OC(CH
3
)
3
H
3
N
+
O
=
=
..
..
..
..
COOH
New amide links
COO
–
N
..
H
O
..
..
..
COOH
H
Cbz
COOH
H
tBoc
COOH
N
..
H
Ph
O
..
N
..
N
..
(CH
3
)
3
CO
..
..
..
FIGURE 23.45 Two protecting or blocking groups for the amine ends of amino acids.
So now we have two ways of blocking one amino group, in this case the amino
group of alanine,so it cannot participate in the formation of the peptide amide bond.
Now we need to block the carboxy end of the other amino acid, leucine. This pro-
tection can be accomplished by simply transforming the acid into an
ester (Fig. 23.46).
We need to do two more things. First, we still have to do the
actual joining of the unprotected amino group of leucine to the unpro-
tected carboxyl group of alanine, and we need to do this very efficient-
ly and under the mildest possible conditions. Second, we need to
deprotect the blocked amino and carboxyl groups after the amide link-
age has been formed.
Dicyclohexylcarbodiimide (DCC) is the reagent of choice for
peptide bond making. Formation of the amide bond is a dehydration
reaction (water is the other product), and DCC is a powerful dehy-
unmodified amino group of leucine. Remember also that eventually the blocking,
or protecting, groups will have to be removed (Fig. 23.44).
COO
–
Ala
Block Activate
Only these two
positions can react
Block
H
3
N
+
COO
–
Leu
H
3
N
+
FIGURE 23.44 In any successful
strategy, we must activate the carboxy
end and block the amino end of
one amino acid while blocking
reaction at the carboxy end of the
other amino acid.
Amino groups can be blocked by converting them into carbamates through sim-
ple addition–elimination reactions (p.901).Two popular methods involve the trans-
formation of the free amine into a carbamate by reaction with either di-tert-butyl
dicarbonate or benzyl chloroformate. Biochemists are even more addicted to
acronyms than are organic chemists,and these protecting groups are called tBoc and
Cbz, respectively (Fig. 23.45).
FIGURE 23.46 A carboxy end can be protected by
converting it into an ester.
COO
–
H
3
N
+
H
2
N
H
2
OEt
O
OEt
..
..
HOEt
..
..
..
..
..
..
+
Leucine Leucine ester
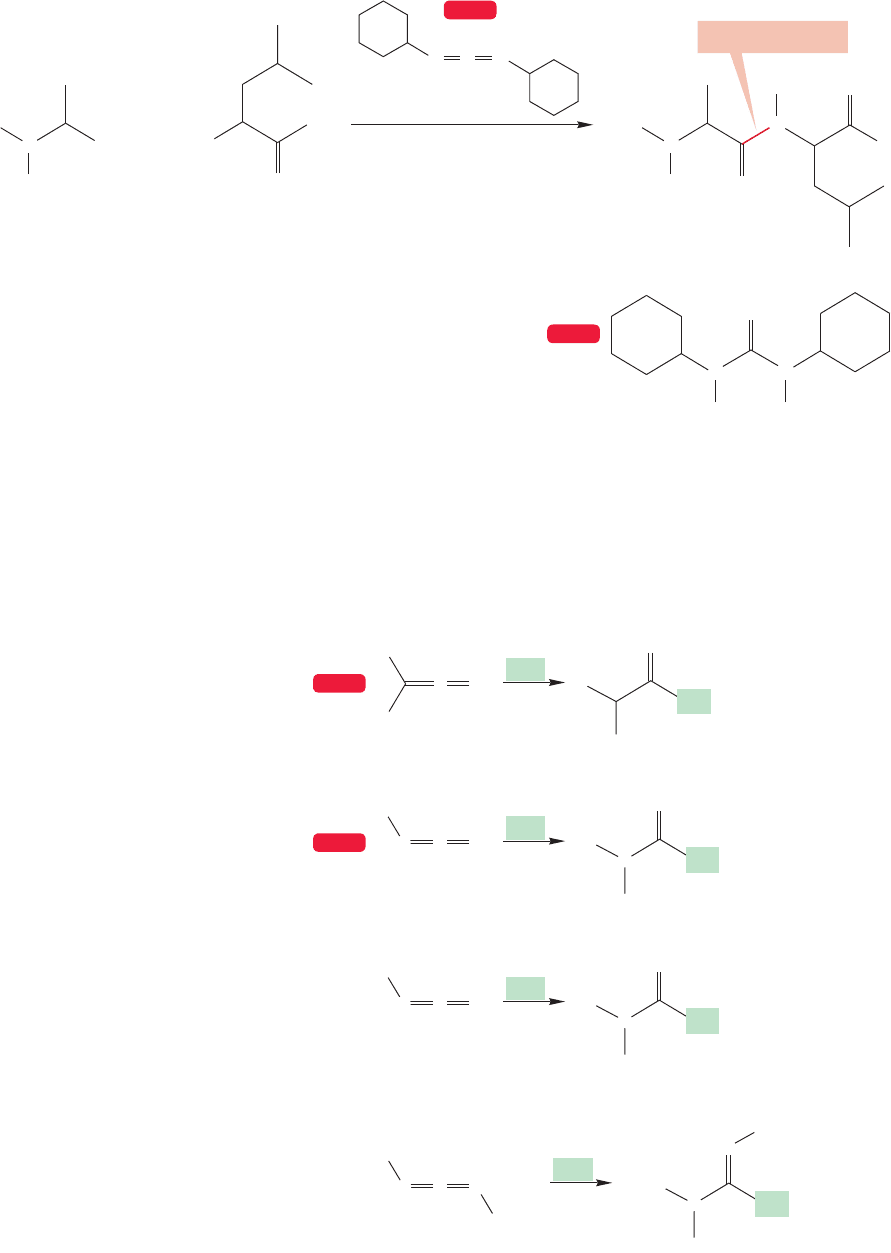
1206 CHAPTER 23 Amino Acids and Polyamino Acids (Peptides and Proteins)
COOH
N
H
2
N
H
+
C
O
OR
..
..
..
..
..
..
..
..
..
..
N
N
NN
H
H
+
HH
O
OR
..
..
O
..
..
..
..
Dicyclohexylurea
(DCU)
New peptide bond
O
..
..
(DCC)
tBoc
tBoc
N
..
N
..
WEB 3D
WEB 3D
FIGURE 23.47 Peptide bond
formation can be achieved using
dicyclohexylcarbodiimide (DCC).
drating agent. The hydrated form of DCC is dicyclohexylurea (DCU), which is a
product of the reaction (Fig. 23.47).
C
A ketene
O
..
..
Amides
O
..
..
C
Isocyanates
O
..
..
Ureas
O
..
..
..
R
H
N
C
Isothiocyanates
S
S
..
..
Thioureas
..
..
..
R
H
N
C
NH
3
..
NH
3
..
NH
3
..
NH
3
..
..
..
..
R
R
R
R
H
N
N
N
Carbodiimides
Guanidines
NH
2
..
NH
2
..
NH
2
..
NH
2
..
R
R
R
R
N
..
R
N
..
R
N
..
WEB 3D
WEB 3D
FIGURE 23.48 Cumulated double
bonds (DCC in this case) react with
all manner of nucleophiles, including
amines and carboxylate anions.
What is the mechanism of the coupling reaction of Figure 23.47? Remember
that other cumulated double bonds (p. 512) such as ketenes (p. 515), isocyanates
(p. 918), and isothiocyanates (p.1198) all react rapidly with nucleophiles.The relat-
ed DCC is no exception,and it is attacked by amines to give molecules called guani-
dines. Carboxylates are nucleophiles too, and will also add to DCC (Fig. 23.48).
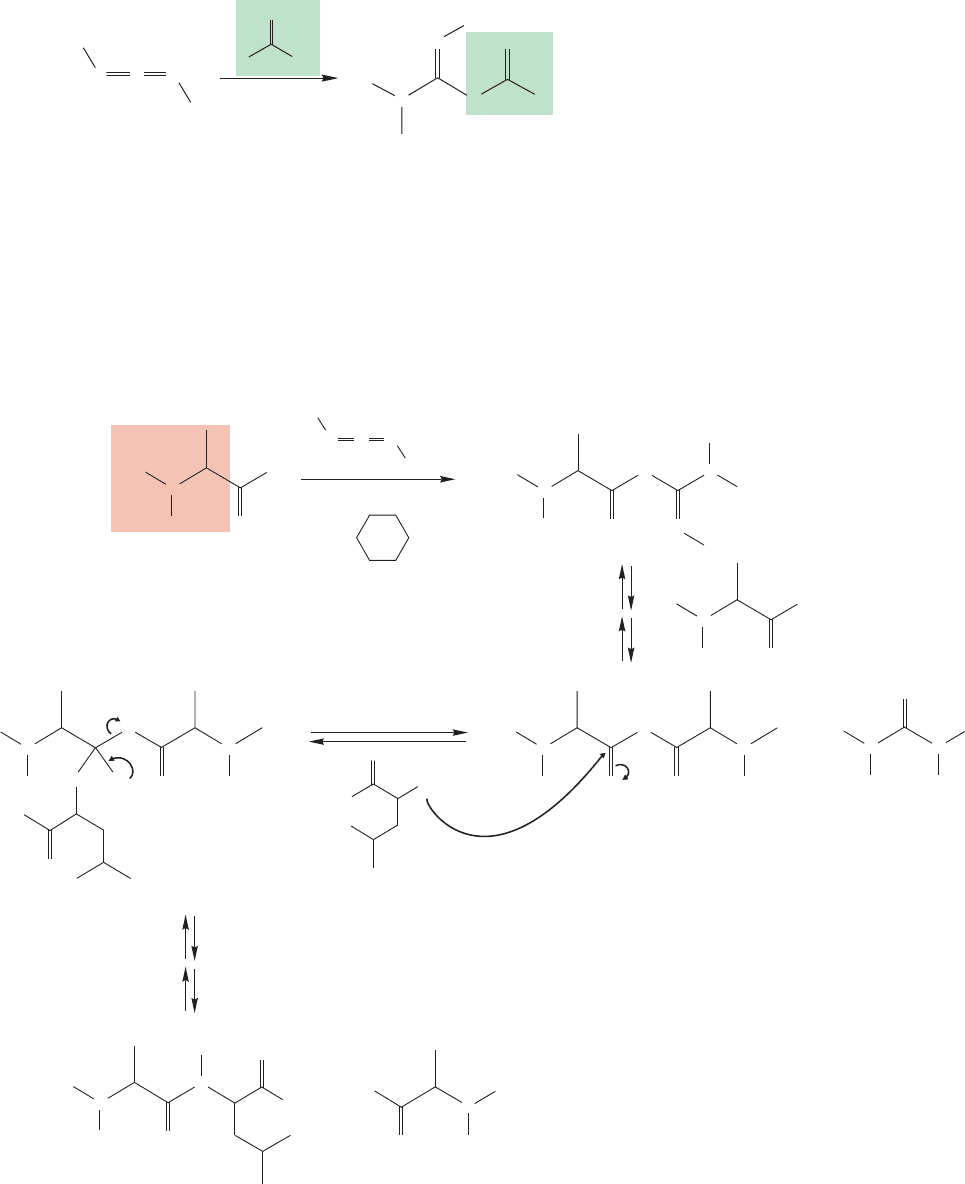
23.4 Peptide Chemistry 1207
So in the coupling reaction of Figure 23.47 the unprotected carboxylic acid group
of alanine adds to DCC to give an anhydride-like intermediate that can react with
a second alanine carboxylic acid to give the anhydride.The amino acid leucine then
adds,via an addition–elimination mechanism,to give the dipeptide (Fig. 23.49). Note
that the dipeptide is still protected in two places.
tBoc
tBoc
tBoc
tBoc
tBoc
N
R
R
N
H
Protected alanine
O
OH
(DCC)
C
R=
..
..
..
..
..
..
Anhydride
(two steps)
addition–
elimination
N
N
H
H
O
O
..
..
..
..
..
N
R
R
..
tBoc
..
N
HO
OH
..
..
..
..
R
..
N
H
R
..
N
H
O
..
..
..
N
H
..
N
H
O
O
..
..
..
..
O
..
..
tBoc
..
N
H
tBoc
+
..
N
..
elimination
deprotonation
Dipeptide
(protecting groups still present)
..
N
H
O
O
..
..
..
..
..
O
..
..
–
O
..
..
Protected
leucine
Protected
alanine
addition
NH
2
H
2
N
O
EtO
..
..
..
..
EtO
..
..
..
N
..
N
H
H
+
O
..
..
O
..
..
..
..
..
OEt
+
O
H
tBoc
O
..
..
–
..
..
..
..
N
FIGURE 23.49 The mechanism of
amino acid coupling using DCC.
Now all we have to do is remove these protecting groups and,at last, we will have
made our dipeptide, .The tBoc blocking group is removed by treatment with
very mild acid, which does not cleave amide bonds. If the Cbz protecting group is
Ala
.
Leu
..
..
..
R
N
..
R
R
N
C
Carbodiimides
An anhydride-like
intermediate
..
..
..
O
O
R
H
N
1. R
2. H
3
O / H
2
O
+
O
–
O
..
..
..
..
..
..
..
..
R
N
..
FIGURE 23.48 (continued)

1208 CHAPTER 23 Amino Acids and Polyamino Acids (Peptides and Proteins)
Deprotect tBoc
H
H
O
+
1. mild acid
CF
3
COOH
at 25 ⬚C
2. NaOH/H
2
O
3. neutralize
Ala
.
Leu
Ala
.
Leu
Ala
.
Leu
Deprotect Cbz
H
H
O
H
2
Pd/C
N
N
O
O
PhCH
2
O
OEt
Deprotect ester
1. NaOH
H
2
O
2. neutralize
H
N
O
O
OEt
O
N
N
OEt
H
O
O
N
tBoc
H
3
N
H
2
N
O
–
FIGURE 23.50 Methods for removing the protecting groups used in peptide synthesis.
used, it can be easily removed by catalytic hydrogenation (Fig. 23.50).The carboxylic
acid on the carboxy terminus can be regenerated by treatment of the ester with base.
The ester will hydrolyze faster than the amide.
What about the yield for these reactions? Remember, this process, with all its
protection and deprotection steps, must be repeated many, many times in the syn-
thesis of a large peptide or a protein.These steps are efficient—they proceed in high
yield because there are essentially no side
reactions.The problem of tedious repetition
has been solved in an old-fashioned way—
by automation.Polypeptides can be synthe-
sized by a machine invented by R. Bruce
Merrifield (1921–2006). In Merrifield’s
procedure, the carboxy end of a tBoc-pro-
tected amino acid is first anchored to a
material constructed of polystyrene in
which some phenyl rings have been substi-
tuted with chloromethyl groups.The amino
acid displaces the chlorine through an S
N
2
reaction (Fig. 23.51).
tBoc
H
N
R
O
Modified polystyrene
Ph Ph Ph
Cl
Cl
Ph Ph Ph
O
H
N
R
O
tBoc
O
N
H
R
tBoc
O
O
–
FIGURE 23.51 Polystyrene can be
chloromethylated in a Friedel–Crafts
procedure, and the chlorines replaced
through S
N
2 reaction with the unprotected
carboxy end of an amino acid. This
technique anchors an amino acid to the
polymer chain.
