Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.

23.2 Amino Acids 1179
As you can see from Table 23.1, amino acids are high-melting solids. Does this
not seem curious? Common amino acids have molecular weights between 75 and
204, and this molecular weight does not seem sufficient to produce molecules that
are solids. This conundrum leads directly to Section 23.2c.
23.2c Acid–Base Properties of Amino Acids Why are amino acids solids?
To answer this question, let’s ask another. What reaction do you expect between the
generic amine and the generic carboxylic acid of Figure 23.4?
A simple organic acid, such as acetic acid, has a pK
a
of about 4.5, and an ammo-
nium ion has a pK
a
of about 8–10. An amine and a carboxylic acid must react to
give the ammonium salt of Figure 23.5,because the carboxylic acid is a much stronger
acid than an ammonium ion. Exactly the same reaction must take place within a
single amino acid to form the “inner salt”or zwitterion, a molecule containing both
positively and negatively charged atoms.
O
..
..
C
..
..
OH
NH
2
R
R
+
..
?
FIGURE 23.4 What reaction must
occur between these two molecules?
O
..
..
..
..
OH
pK
a
~ 4.5 pK
a
~ 9
NH
2
R
HNH
2
RR
O
..
..
..
..
..
O
R
..
–
..
..
..
O
–
+
+
..
O
..
..
..
..
OH
R
O
..
..
R
A zwitterion
+
NH
3
NH
2
FIGURE 23.5 An amine and a carboxylic acid must undergo acid–base chemistry to give an ammonium
salt of the acid. In amino acids, an intramolecular version of the same reaction gives a zwitterion.
Amino acids are ionic compounds under normal con-
ditions,and therefore have high melting points.Other prop-
erties are in accord with this notion.They are miscible with
water, but quite insoluble in nonpolar organic solvents.They
have high dipole moments. Of course, the charge state of
an amino acid depends on the pH of the medium in which
it finds itself. Under neutral or nearly neutral conditions,
amino acids exist as the zwitterions of Figure 23.5. If we
lower the pH, eventually the carboxylate anion will proto-
nate, and the amino acid will exist primarily in the ammo-
nium ion form. Conversely, if we raise the pH, eventually
the ammonium ion site will be deprotonated, and the mol-
ecule will exist as the carboxylate anion (Fig. 23.6).
..
..
..
O
–
+
+
O
..
..
..
..
OH
R
O
..
..
R
Zwitterion
..
..
..
O
–
O
..
..
R
Carboxylate formAmmonium ion form
Neutral At high pHAt low pH
pH = 15pH = 1.0
H
3
N
H
2
N
H
3
N
..
FIGURE 23.6 At low pH (strongly acidic solution), the
zwitterion is protonated to give a net positively charged
molecule. At high pH (strongly basic solution), the zwitterion is
deprotonated to give a net negatively charged molecule.
PROBLEM 23.3 The pK
a
for a simple organic acid is 4–5. Note the pK
a
values of
the amino acids (column 5 in Table 23.1). Why are amino acids much more
acidic?
Amino acids can be described by a series of pK
a
values. The first refers to the
deprotonation of the carboxylic acid group, and it is in the range 1.7–2.4. The sec-
ond pK
a
value is for deprotonation of the ammonium ion, and is in the range
8.8–10.8 (Table 23.1).
The pH at which the concentration of the ammonium ion equals that of the car-
boxylate ion is called the isoelectric point (pI).At this pH the molecule will have a net
1180 CHAPTER 23 Amino Acids and Polyamino Acids (Peptides and Proteins)
zero charge.The zwitterion is the predominant form at this pH.This pH is defined
as the pI, which is a symbol for isoelectric point. For the amino acids with nonacidic
or basic side chains, the pI can be calculated by taking the midpoint between the pK
a
of the carboxylic acid and the pK
a
of the ammonium group.Thus,pI (pK
a
pK
a
)/2.
PROBLEM 23.4 Use the pK
a
data in Table 23.1 to calculate the pI (the isoelectric
point) for alanine.
As you can see from Table 23.1, many amino acids bear acidic or basic side chains,
which complicates the picture somewhat and can make the determination of the iso-
electric point a bit more difficult. These side groups are also involved in acid–base
equilibria and there are pK
a
values associated with them. For example, arginine will
be doubly protonated at very low pH (Fig. 23.7).
“H
+
”
Arginine (Arg)
at pH = 7
lower pH
pK
a
= 9.1
pK
a
= 12.5
pK
a
= 2.2
+
+
O
H
3
N
H
2
N
NH
2
OH
+
+
O
H
3
N
H
2
N
N
H
NH
2
–
O
N
H
..
..
..
..
..
.. ..
..
..
..
..
..
..
FIGURE 23.7 Arginine is twice
protonated at low pH. Because the
side chain has a basic group, this
amino acid has three pK
a
values.
PROBLEM 23.5 Notice which nitrogen on the arginine side chain is protonated
under acidic conditions. Explain why it is the nitrogen shown that is protonated.
The most acidic group is the carboxylic acid, pK
a
2.2. Second is the ammo-
nium ion, pK
a
9.1. The side chain group has a pK
a
of 12.5 and will be the last
position deprotonated as the solution becomes less acidic.
PROBLEM 23.6 Which ring nitrogen in histidine (Table 23.1) will be protonated
first as the solution becomes more acidic?
These acid–base properties give rise to an especially powerful separation method
widely used in analysis of amino acid mixtures, called electrophoresis. It works this
way. A mixture of amino acids is placed on moistened paper. At a given pH, each
amino acid exists as either a positive ion, a neutral
zwitterion, or a negative ion. When a current is
applied to the paper, the positively charged ions will
migrate to the cathode (negative pole), the neutral
zwitterions remain stationary, and the negatively
charged ions move to the anode (positive pole) at
rates depending on the pH and the strength of the
applied current. The net result is a separation, as the
amino acids in the mixture migrate at different rates.
For example, at pH 5.5, the amino acids Lys, Phe,
and Glu exist in the forms shown in Figure 23.8.
Lys
(cation = 1+)
COO
–
H
3
N
+
Phe
(neutral)
Glu
(anion = 1–)
+
COO
–
H
3
N
+
COO
–
COO
–
H
3
N
+
NH
3
FIGURE 23.8 The charge states of Lys, Phe, and Glu at pH 5.5.
23.2 Amino Acids 1181
When a current is applied, Phe will not move, Lys will migrate to the cathode, and
Glu to the anode (Fig. 23.9).
Start (current off) Turn current on
Cathode
–
Anode
+
Mix of Lys, Phe, Glu
Cathode
–
Anode
+
Lys (moves
to cathode)
Phe (no
movement)
Glu (moves
to anode)
FIGURE 23.9 Separation of three amino acids by electrophoresis.
23.2d Syntheses of Amino Acids One very simple method for making
an amino acid is to alkylate ammonia using the S
N
2 reaction of ammonia with an
α-halo acid (Fig. 23.10).
THE GENERAL CASE
SPECIFIC EXAMPLES
..
NH
3
(S
N
2)
..
NH
3
/H
2
O1.
1.
2. neutralization
2. neutralization
48 h, 25 °C
excess
Glycine
(65%)
2-Aminohexanoic acid
(norleucine)
(65%)
..
..
R
COOH
COOH
..
NH
4
Br
..
..
..
..
..
..
..
..
..
..
Cl
COO
–
R
COO
–+
..
NH
3
/H
2
O
25 h, 55 °C
COO
–
COOH
..
NH
2
NH
3
+
Br
NH
3
+
FIGURE 23.10 Simple alkylation of
an α-halo acid will give an α-amino
acid.
In Chapter 7 (p. 312), a closely related alkylation method appeared as a means
of producing alkylamines. A problem for this synthetic procedure is the ease of
overalkylation.The simple alkylation reaction is not generally a practical method for
1182 CHAPTER 23 Amino Acids and Polyamino Acids (Peptides and Proteins)
PROBLEM 23.7 Devise simple syntheses of the following α-bromo acids starting
from alcohols containing no more than four carbon atoms and inorganic reagents
of your choice (p. 950).
(a)
O
..
..
..
..
..
..
OH
..
Br
(b)
O
..
..
..
..
..
..
OH
..
Br
PROBLEM 23.8 Why are α-bromo acids (or their carboxylate salts) more easily
alkylated than simple alkyl bromides in the S
N
2 reaction (p. 542)?
A successful variation of the simple alkylation synthesis involves a protect-
ed, disguised amine, and is called the Gabriel synthesis (Siegmund Gabriel,
1851–1924). In this procedure, bromomalonic ester is first treated with
potassium phthalimide in an S
N
2 displacement of bromide ion (Fig. 23.11).
CO
2
COOH
COOH
25–110 °C, 1 h
K
+
+
++
O
..
..
O
..
..
O
..
..
O
..
..
O
O
..
..
..
..
EtOOC
EtOOC
H
EtOOC
EtOOC
H
(97%)
Methionine
(85%)
(59%)
1. NaOH/H
2
O
2. HCl, Δ
..
..
–
N
CH
3
S
N
2
..
..
S
..
..
..
..
..
SCH
3
Cl
base
(alkylation step)
hydrolysis and
decarboxylation
..
..
..
..
..
SCH
3
H
3
N
EtOOC
EtOOC
Br
N
N
COOH
..
..
Bromomalonic
ester
Potassium
phthalimide
Phthalic acid
+
WEB 3D
FIGURE 23.11 The Gabriel synthesis uses bromomalonic ester in the first step.
producing alkylamines,but it is useful in making α-amino acids.The α-bromo acids
are easily available,and a vast excess of ammonia can be used to minimize overalkyl-
ation.Moreover, the first formed α-amino acids are not especially effective competi-
tors in the S
N
2 reaction, because they are sterically congested and less basic than
simple amines. This technique is an effective way of making both “natural” and
“unnatural” amino acids.
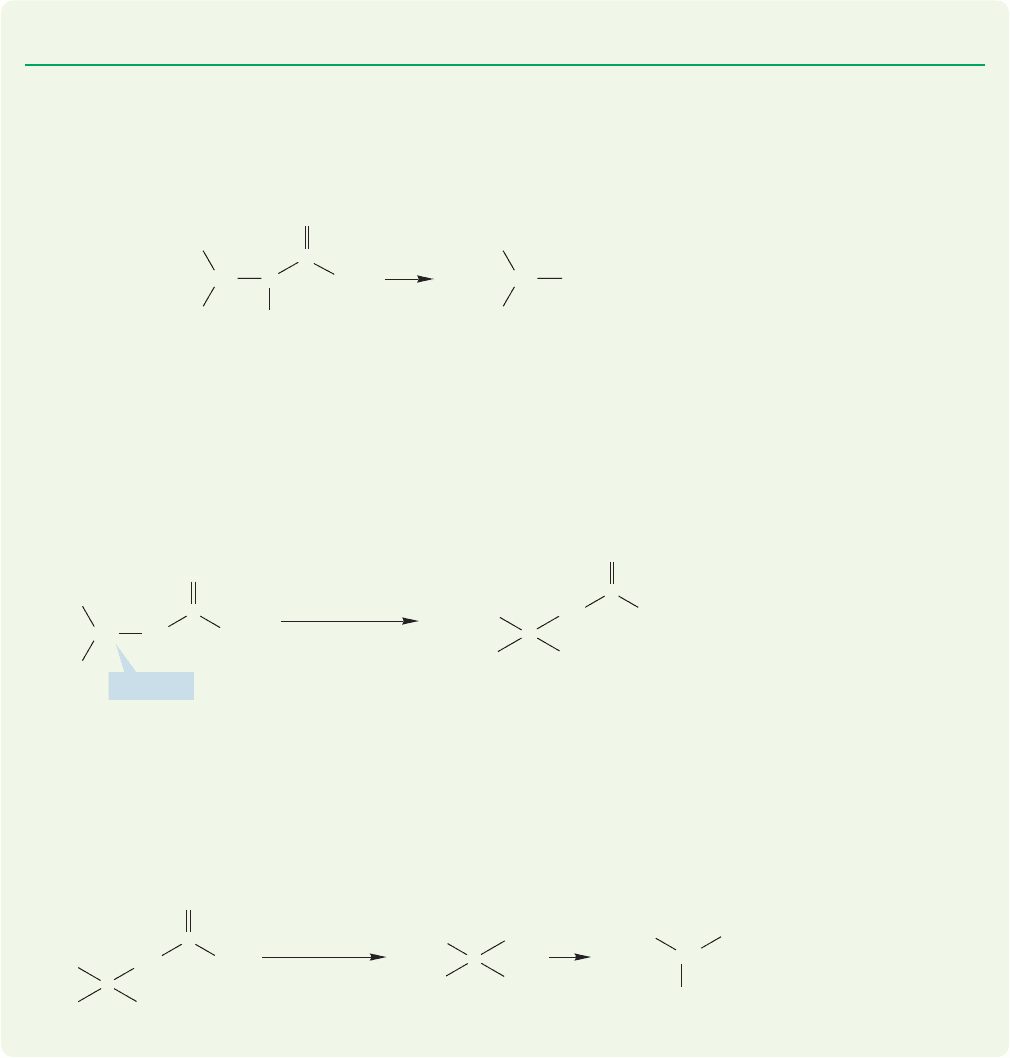
PROBLEM 23.9 Provide detailed mechanisms for the reactions of Figure 23.11.
WORKED PROBLEM 23.10 In a simpler version of the Gabriel synthesis,
acetamidomalonic ester is used rather than bromomalonic ester. Sketch out the
steps in this related amino acid synthesis.
O
..
..
C
..
N
H
Acetamidomalonic ester Phenylalanine
CH
3
CH
2
OOC
CH
3
CH
2
OOC
CH CH
3
..
NH
2
HOOC
PhCH
2
CH
?
CH
3
O
NH
CH
C
1. NaOEt/EtOH
S
N
2
(87%)
2. PhCH
2
Cl
EtOOC
EtOOC
CH
3
CH
2
Ph
NH
C
EtOOC
EtOOC
O
C
Doubly α
ANSWER It is essentially the same process as that of Figure 23.11 and Problem
23.9. Advantage is first taken of the acidity of the doubly α hydrogen to introduce
an R group.
Now acid (or base) is used to convert the esters into carboxylic acids and to
hydrolyze the amide. Heating decarboxylates the malonic acid and liberates the
amino acid.
EtOOC
EtOOC
CH
2
Ph
C
HOOC
HOOC
CH
2
Ph
CH
2
Ph
CO
2
(80%)
+
NH
2
C
Δ
CH
3
O
NH
C
1. NaOH/H
2
O
2. H
2
O/H
3
O
(neutralize)
HOOC
NH
2
CH
+
23.2 Amino Acids 1183
The nitrogen atom destined to become the amine nitrogen of the amino acid is
introduced at this point, and rendered nonnucleophilic by the flanking carbonyl
groups.In the next step,the malonic ester product is treated with base and an active
alkyl halide to introduce the R group of the amino acid.Finally, treatment with acid
or base, followed by neutralization, uncovers the amine. The phthalimide group is
hydrolyzed to phthalic acid, and the malonic ester is hydrolyzed to a malonic acid.
Gentle heating induces decarboxylation to give the amino acid.There are many vari-
ations on this general theme.
1184 CHAPTER 23 Amino Acids and Polyamino Acids (Peptides and Proteins)
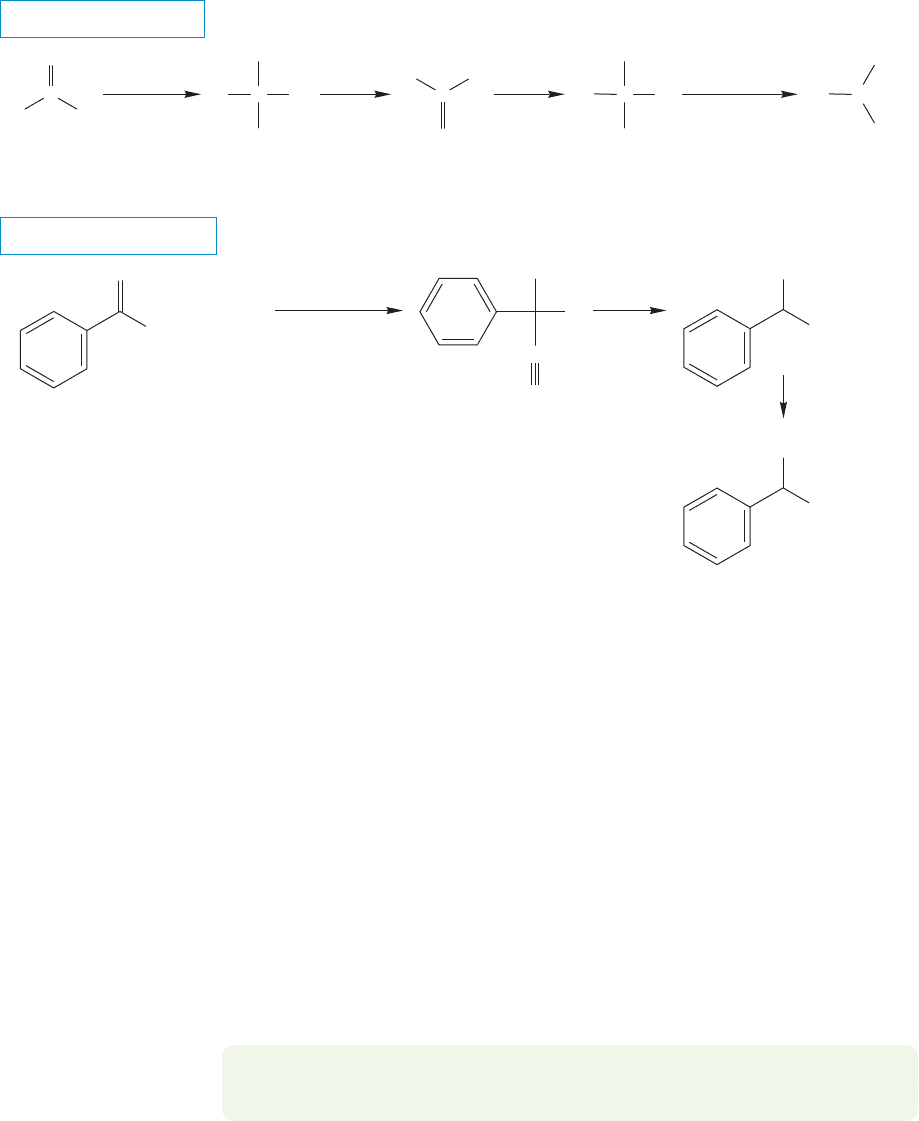
In the Strecker synthesis (Adolph Strecker, 1822–1871), a cyanide is used as
the source of the carboxylic acid portion of an amino acid (Fig. 23.12). An aldehyde
is treated with ammonia, the ultimate source of the amino group, and cyanide ion.
In the first step of the reaction, a carbinolamine is formed (p. 790) and water is lost
to give an imine.The imine is then attacked by cyanide to give an α-amino cyanide.
Acid hydrolysis of the nitrile gives the acid (p. 904).
THE GENERAL CASE
A SPECIFIC EXAMPLE
+
..
KCN
Carbinolamine
..
..
NH
2
C
OH
Overall yield
(36%)
O
..
..
C
H
O
..
..
H
COO
–
NaCN
R
HR
R
H
NH
3
..
..
..
C
NH
2
CO
2
H
NH
3
NH
3
N
H
NH
2
C
CN
R
CH
R
H
..
NH
..
..
..
H
2
O
..
KCN
C
..
..
..
H
3
O
..
+
+
/H
2
O
1. H
2
O/CH
3
OH
NH
4
+
45 °C, 2 h
HCl
2 h
Cl
–
Cl
–
NH
3
/H
2
O
–
Imine
+
COOH
+
NH
3
FIGURE 23.12 The Strecker amino
acid synthesis.
The first step in this reaction, imine formation, is analogous to the first step in
the Mannich condensation in which the role of the amine is also to produce a reac-
tive imine (p. 1003).This imine can next be attacked by the nucleophilic cyanide to
give the tetrahedral α-amino nitrile.
As we have seen, amino acids in Nature are almost all optically active (S) enan-
tiomers, or
L forms (save glycine, which is achiral). None of the syntheses outlined
in this section automatically produces the natural (S) enantiomer specifically. In order
to obtain the single enantiomer, we either need a method for resolution (separation)
of the racemic mixture of enantiomers formed in these syntheses or a method of
selectively producing the (S) enantiomers.
23.2e Resolution of Amino Acids The standard method is the one we first
discussed in our chapter on chirality (p.169) and involves transformation of the pair
of enantiomers, which have identical physical properties, into a pair of diastere-
omers, which do not, and can be separated by physical means (most often crystal-
lization or chromatography).
PROBLEM 23.11 There actually is one physical property not shared by enantiomers.
What is it?
Optically active amines are used to transform the enantiomeric amino acids in
a racemic mixture into diastereomeric ammonium salts that can be separated by
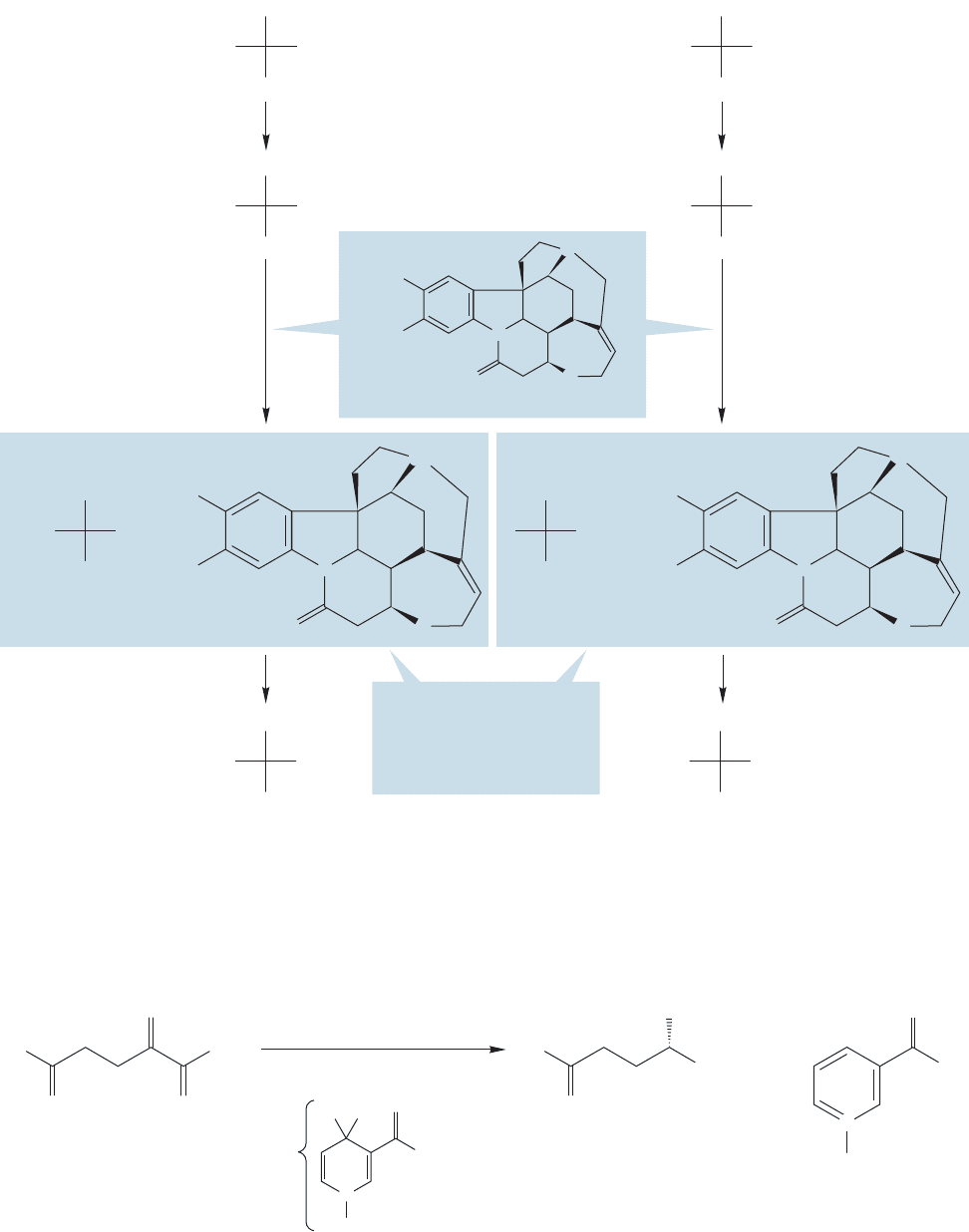
23.2 Amino Acids 1185
COO
–
CH
3
Ac
2
O
(S) =
L
HH
3
N
COOH
CH
3
HAcNH
Brucine
COO
–
CH
3
Ac
2
O
(R) =
D
NH
3
H
COOH
CH
3
CH
3
O
CH
3
O
NHAcH
NaOH/H
2
O NaOH/H
2
O
+
+
COO
–
CH
3
HH
2
N
COO
–
CH
3
NH
2
H
N
O
N
O
COO
–
CH
3
H
COO
–
CH
3
H
+
CH
3
O
CH
3
O
N
O
O
CH
3
O
CH
3
O
N
O
O
AcNH
NHAc
+
NH
NH
These ammonium salts of
the (R) and (S) carboxylate
anions are diastereomeric
and can be separated by
fractional crystallization
FIGURE 23.13 Resolution
of a pair of enantiomeric
amino acids.
NH
3
enzyme
“glutamate dehydrogenase”
..
␣-Ketoglutaric acid
O
..
..
O
..
..
O
HH
O
..
..
..
..
..
..
HO
..
..
OH
..
..
COO
–
L-Glutamic acid
O
..
..
HO
..
..
N
Sugar
N
Sugar
NH
2
O
..
..
NH
2
..
+
NH
3
+
NAD
+
NADH
+
FIGURE 23.14 Synthesis of an optically active L-amino acid through reductive amination.
However, it seems that there must be a way to avoid the tedium of fractional
crystallization and make the optically active compounds directly. After all, Nature
does this somehow, and we should be clever enough to find out how this happens
and imitate the process. One method Nature uses to make optically active amino
acids involves a reductive amination (Fig. 23.14). Ammonia and an α-keto carboxylic
crystallization.The chiral amines of choice are optically pure alkaloids (p.255), which
are readily available in Nature. The process of separation is outlined in Figure 23.13.
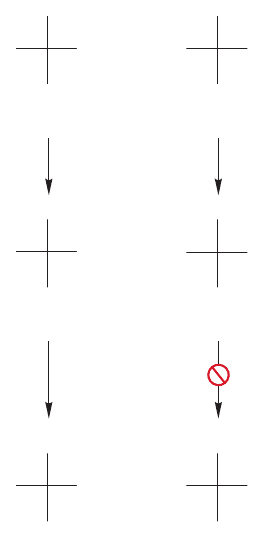
1186 CHAPTER 23 Amino Acids and Polyamino Acids (Peptides and Proteins)
acid react in the presence of an enzyme and a source of hydride, usually NADH
(p. 814), to produce an optically active chiral amino acid.
There are two procedures used by chemists that imitate the natural pathway.
In one, actual microorganisms are used in fermentation reactions that can direct-
ly produce some
L-amino acids. In the other, enzymes are used to react with one
enantiomer of a racemic pair. As you might guess, it is usually the
L isomer that
is “eaten” by the enzyme, because enzymes have evolved in an environment of
L
isomers. So it is usually the D isomers that are ignored by enzymes. In an extraor-
dinarily clever procedure, this preference for natural,
L, enantiomers can be used
to achieve a kinetic resolution (Fig. 23.15). The mixture of enantiomers is first
acetylated to create amide linkages. An enzyme, hog-kidney acylase, then
hydrolyzes the amide linkages of only
L-amino acids. So, treatment of the pair of
acetylated amino acids leads to formation of the free
L-amino acid. The acylat-
ed
D enantiomer is unaffected by the enzyme, which has evolved to react only
with natural
L-amino acids. The free L-amino acid can then easily be separated
from the residual
D acetylated material.
Free L enantiomer Acetylated
D enantiomer
Ac
2
O
HOAc
Ac
2
O
HOAc
+
H
3
N
COO
–
R
H
(S) =
L
+
NH
3
COO
–
R
H
(R) =
D
+
H
3
N
COO
–
R
H
(S) =
L
COOH
R
H
(R) =
D
hog-kidney
acylase
hog-kidney acylase
(no reaction with
D enantiomer)
COOH
R
H
(S) =
L
NHAc
NHAc
AcNH
COOH
R
H
(R) =
D
A racemic mixture
of amino acids
A racemic mixture of
acetylated amino acids
Easily separable mixture
+
+
+
FIGURE 23.15 A kinetic resolution of a pair of enantiomeric amino acids.
23.3 Reactions of Amino Acids
The simple expectation that the chemical reactions of the amino acids are a
combination of carboxylic acid (Chapter 17) and amine (Chapter 6) chemistry
is right. Indeed, we have already seen some of these reactions in the acid–base
23.3 Reactions of Amino Acids 1187
1. Ac
2
O
HOOC
HOOCHOOC
CH
3
O
..
..
O
..
..
..
..
..
HN
..
N
..
HOOC
NH
2
..
(91%)
(>72%)
NH
2
..
O
..
..
2. H
2
O
20 min
Cl
H
FIGURE 23.16 The amino group of an amino acid can be acylated.
78 °C
CH
3
CH
2
OH
HCl
H
3
N
OCH
2
CH
3
(~ 100%)
COO
–
+
H
3
N
+
O
FIGURE 23.17 Fischer esterification of the acid group of an
amino acid.
chemistry described in Section 23.2c. A few more important and useful exam-
ples follow.
23.3a Acylation Reactions The amino group of amino acids can be
acylated using all manner of acylating agents. Typical examples of effective
acylating agents are acetic anhydride, benzoyl chloride, and acetyl chloride
(Fig. 23.16).
23.3b Esterification Reactions The carboxylic acid group of amino acids
can be esterified using typical esterification reactions. Fischer esterification is the
simplest process (Fig. 23.17).
PROBLEM 23.12 Write a mechanism for the acylation reactions of Figure 23.16.
PROBLEM 23.13 If amino acids are typically in their zwitterionic form, how are
acylation and esterification reactions,which depend on the presence of free amine
or acid groups, possible?
23.3c Reaction with Ninhydrin The hydrate of indan-1,2,3-trione is
called ninhydrin. Amino acids react with ninhydrin to form a deep purple mol-
ecule. It is this purple color that is used to detect amino acids. Although it may
1188 CHAPTER 23 Amino Acids and Polyamino Acids (Peptides and Proteins)
seem curious at first, all amino acids except proline, regardless of the identity of
the R group, form the same purple molecule on reaction with ninhydrin. How
can this be?
Like all hydrates, ninhydrin is in equilibrium with the related carbonyl com-
pound (Fig. 23.18).The amino acid first reacts with the trione to form an imine
through the small amount of the free amine form of the amino acid present
at equilibrium. We have seen this kind of reaction many times (p. 793). Next,
decarboxylation of the imine produces a second imine that is hydrolyzed under
the aqueous conditions to give an amine. It is in this hydrolysis step that the
R group of the original amino acid is lost. Still another imine, the third in this
sequence, is then formed through reaction of the amine with another molecule
of the ninhydrin trione. The extensive conjugation in the product leads to a
very low energy absorption in the visible region (p. 529), and the observed
purple color.
O
O
O
Ninhydrin Ninhydrin trione
Second imine
R Group of original
amino acid lost here
..
..
O
..
O
..
..
O
..
..
O
..
..
OH
..
..
OH
..
..
..
..
..
..
O
O
..
..
..
..
O
O
..
..
..
..
..
..
..
..
..
COO
–
–
–
–
(–)
(–) (–)
(–)
(–)
–
R
NH
2
CO
2
hydrolysis
H
2
O
R
N
O
O
..
..
..
..
..
O
..
..
..
..
O
R
N
O
..
..
..
..
O
..
..
N
decarboxylation
First imine
+
+
H
R
..
O
Third imine—purple!
..
..
H
3
N
+
O
..
..
O
..
..
O
..
..
WEB 3D
FIGURE 23.18 Formation of a purple molecule through reaction of an amino acid with the ninhydrin trione.
Each amino acid in Table 23.1 except proline gives the same purple product.
PROBLEM 23.14 Why is the trione of Figure 23.18 hydrated at the middle car-
bonyl rather than either of the side carbonyl groups?
