Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.

22.4 Reactions of Carbohydrates 1149
water is eliminated, and so it is this OH that is lost most easily (Fig. 22.39). The
ring oxygen helps “kick out” the water molecule. Addition of alcohol at the elec-
trophilic C(1), followed by deprotonation, gives the glycoside. Overall, the hemiac-
etal OH of the original glucopyranose has been substituted by OR to form an acetal.
Specific glycosides are named by giving the alkyl group name of the alcohol used
to make the glycoside followed by the sugar name with the -ose replaced with -oside.
For example, if methyl alcohol is used to form an acetal with
D-glucopyranose, both
methyl α-
D-glucopyranoside and methyl β-D-glucopyranoside would be produced
(Fig. 22.40a). It is important to note that in water the acetal is more stable than the
hemiacetal. Under biological conditions, glycosides do not undergo mutarotation.
Methyl -D-glucopyranoside
(a)
HO
HO
OH
O
OCH
3
OH
WEB 3D
Methyl ␣-D-glucopyranoside Adenosine
(an N--ribofuranoside)
(b)
HO
HO
OH
O
OH
OCH
3
OH
NH
2
O
OH
HO
N
N
N
N
FIGURE 22.40 (a) The glycosides methyl α-D-glucopyranoside and methyl β-D-glucopyranoside,
acetals made by the reaction of methyl alcohol with
D-glucopyranose, and (b) N-β-glycoside
adenosine, an amino acetal made by the reaction of
D-ribose with the nitrogen-containing
compound adenine, a compound we shall see again in Chapter 23.
Nucleophiles other than alcohols can be used to make glycosides. For example,
N-glycosides are used in Nature in construction of RNA and DNA. Figure 22.40b
shows adenosine, an N-β-glycoside that is a component of RNA.
O
O
O
O
O
Br
O
O
O
Ag
2
O
ROH
O
O
O
O
O
O
O
O
O
O
OR
Glycosides are not stable in strongly acidic conditions. The tetramethoxy methyl
acetal formed by complete methylation of methyl α-
D-glucopyranoside through the
PROBLEM 22.20 The Koenigs–Knorr reaction is useful for making glycosides. In
this reaction, the pentaacetate compound of Figure 22.37 is treated with HBr to
give the C(1) bromide.The bromide is then replaced by an alcohol in the presence
of Ag
2
O.The reaction is very selective for the β anomer, but a series of experiments
has established that it is not a simple S
N
2 reaction. Show a mechanism that
explains the selective formation of the β anomer. Hint: Remember Chapter 21.
1150 CHAPTER 22 Carbohydrates
WORKED PROBLEM 22.21 Explain why in the reaction shown in Figure 22.41 it is only
the methoxyl group at the anomeric carbon that is substituted by a hydroxyl group.
ANSWER This kind of reaction takes place by protonation of an ether oxygen, loss
of alcohol to give a carbocation, and addition of water followed by deprotonation.
+
++
H
3
O
+
+
CH OCH
3
R
R
CH OCH
3
O
H
H
H
+
CH OH
H
CH OH
CH
HOH
2
R
R
R
R
R
R
R
R
O
H
H
..
..
..
..
..
..
..
..
..
..
..
..
O
+
+
CH
3
O
CH
3
O
CH
3
O
OCH
3
O
CH
3
OCH
2
CH
3
OCH
3
OH
CH
3
O
CH
3
O
+
+
O
CH
3
OCH
2
CH
3
O
CH
3
O
CH
3
O
O
CH
3
OCH
2
CH
3
O
CH
3
O
CH
3
O
HOCH
3
Resonance-stabilized carbocation
H
2
O
H
2
O
H
2
O
H
3
O
+
O
CH
3
OCH
2
CH
3
O
CH
3
O
CH
3
O
OH
O
CH
3
OCH
2
CH
3
O
CH
3
O
CH
3
O
O
HH
+
H
3
O
+
+
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
CH
3
OCH
2
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
All of the ether oxygens can be protonated, but only in one case can alcohol be
lost with assistance from the neighboring group to give a resonance-stabilized
carbocation. Addition of water to the cation followed by deprotonation leads
to the tetraether hemiacetal shown in Figure 22.41 (both anomers are formed).
cat.
HCl
H
2
O
Acetal and
a tetraether
CH
3
O
CH
3
O
OCH
3
O
OCH
3
OH
OCH
3
Hemiacetal and
a tetraether
CH
3
O
CH
3
O
OCH
3
O
OCH
3
FIGURE 22.41 Acid hydrolysis of the
fully methylated acetal leads to a
hemiacetal in which only the
methoxyl group at the anomeric
carbon has been substituted by OH.
Williamson ether synthesis can be hydrolyzed in acid to a compound in which only the
acetal methoxyl group at C(1) has been transformed into a hydroxyl group (Fig. 22.41).
22.4 Reactions of Carbohydrates 1151
cat.
HCl
cat.
HCl
CH
3
OH
CH
3
OH
OH
HO
D-Glucopyranose
HO
O
OH
OH
OH
OH
HO
Methyl
D-glucopyranoside
HO
O
OCH
3
Methyl D-glucofuranoside
HIO
4
H
2
O
(cleaves 1,2-
diols at red lines)
O
O
HO
OCH
3
O
O
H
H
H
H
O
O
HIO
4
H
2
O
(cleaves 1,2-
diols at red lines)
Not observed
Observed
OH
OH
O
OH
OH
OCH
3
O
O
O
OCH
3
O
FIGURE 22.42 Formation of methyl D-glucopyranoside and further oxidation with
periodic acid gives the observed dialdehyde product, which is evidence that the
cyclic form of glucose is (mainly) a pyranose. A furanose would give a different
oxidation product.
22.4g Modern Carbohydrate Chemistry Many natural products and
pharmacologically active compounds are covalently attached to carbohydrates.
Carbohydrates attached to proteins, for instance, play a major role in cell sig-
naling, gene transcription, and immune response. Attaching a sugar to a sub-
stance can be a simple matter of one of the alcohols acting as a nucleophile.
Unfortunately there are so many alcohols on any given sugar that simply mix-
ing the reagents will yield multiple products. This procedure is not an accept-
able synthetic approach. Nature uses enzymes to control carbohydrate
attachments. Chemists control the reactions by employing selective protecting
groups (p. 788). For example, if the C(2) OH of
D-glucose needs to be attached
to a substrate, it stands to reason that we must protect the C(3), C(4), and C(6)
We can take advantage of these reactions of sugars, combined with an oxi-
dation, to determine whether the ring in cyclic glucose and other aldohexoses
is a furanose or a pyranose.
D-Glucose is first converted into its methyl glyco-
side by treatment with methyl alcohol in acid (Fig. 22.42). Recall that period-
ic acid cleaves 1,2-diols to dicarbonyl compounds. Therefore, treatment of
the methyl
D-glucopyranoside with HIO
4
leads to the dialdehyde shown in
Figure 22.42. If
D-glucose had reacted with methanol to give the furanoside,
a quite different product would have been obtained. This procedure is one
way in which the predominant ring structure, the six-membered pyranose, was
determined.
1152 CHAPTER 22 Carbohydrates
OH groups of the D-glucoside. Figure 22.43 shows the reactions one can use
to obtain each of the alcohol groups of α-
D-glucopyranoside independently
available for further modification. The reactions used for protecting are typi-
cally a combination of acetal formation, alkylation, acylation, silylation, and/or
reduction. Keep in mind that every carbohydrate is different. So, for example,
the method that works for
D-glucose may not work for D-mannose.
cat.
H
+
cat.
H
+
Bu
2
SnO
Methyl ␣-D-glucopyranoside
OH
HO
HO
O
OH
79%
NaCNBH
3
Et
3
N
LiAlH
4
R
3
SiCl
pyridine
O
Ph
CH
3
OH
O
O
CH
3
H
3
C
O
Ph
O
O
HO
HO
OCH
3
OCH
3
2 equiv. NaH
80%
O
Ph
Ph
Ph
O
O
O
O
OCH
3
89%
O
Ph
Ph
Ph
O
HO
O
O
OCH
3
O
Ph
Ph
Ph
HO
O
O
O
OCH
3
82%
H
3
C
H
3
C
HO
O
OH
<50%
O
O
OCH
3
OCH
3
OCH
3
95%
H
3
C
H
3
C
HO
O
OSiR
3
O
O
OCH
3
OCH
3
OCH
3
Ph
2
Br
72%
C(6) OH free
C(4) OH free
C(2) OH free
C(3) OH free
Ph
O
Ph
O
O
O
HO
OCH
3
O
O
Ph Cl
98%
O
Ph
Sn
Bu
Bu
O
O
O
O
OCH
3
FIGURE 22.43 Manipulation of
the various alcohol groups in an
α-
D-glucopyranoside.
Summary
Reactions in carbohydrates can occur at the carbonyl group or at one of the sever-
al alcohols. Aldehydes can be oxidized or reduced.They also can react with amines
to form imines. The alcohols can be alkylated or acylated. Selective protection of
the glycoside alcohols is possible with careful manipulation of protecting groups.
22.5 The Fischer Determination of the Structure of D-Glucose 1153
22.5 The Fischer Determination of the Structure
of
D-Glucose (and the 15 Other Aldohexoses)
In the following pages, we will approximate the reasoning that led Emil Fischer to the
structure of
D-glucose.The structures of the other 15 aldohexoses can be determined as
we go along. As a bonus, we will also derive the structures of the eight aldopentoses. A
risk in this kind of discussion,which straightens out the actual twisted history of the Fischer
determination, is that the work will seem too easy, too straightforward. Fischer’s papers
appeared in 1891, less than two decades after van’t Hoff ’s (Jacobus Henricus van’t Hoff,
1852–1911, received the first Nobel Prize in Chemistry in 1901) and Le Bel’s ( Joseph
Achille Le Bel, 1847–1930) independent hypothesis of the tetrahedral carbon atom.
Determining stereochemistry was no simple matter, and Fischer’s work depended criti-
cally on a close analysis of the stereochemical relationships of complex molecules.
Moreover, the experimental work was extraordinarily difficult and slow.
5
Fischer’s work
was monumental and remains an example of the best we humans are able to accomplish.
Fischer recognized that it was impossible for him to solve one of the central problems
in this endeavor,the separation of the
D series from the enantiomeric L series. In the 1880s
there was no way to determine absolute configuration,which is hardly surprising given that
the structural hypothesis of the tetrahedral carbon atom was barely 15 years old at the time.
However, Fischer recognized that the 16 aldohexoses must be composed of eight pairs of
mirror images and that if he knew the structures of one set, he automatically could draw
the structures of the mirror-image set.The problem was to tell which was which, and this
he knew he could not do.So, he did the next best thing, he was arbitrary; he just guessed.
The odds aren’t bad, 50:50, and there are times when it is best to accept what is possible
and not give up because a perfect solution to the problem at hand isn’t available.So Fischer
simply defined one set of eight isomers as the
D series, knowing that it might be necessary
for history to correct him. In the event, fortune smiled and he made the right guess. Be
sure that you understand that the correctness of Fischer’s guess wasn’t due to cleverness—
his success was not the result of a shrewd guess or the product of a well-developed intu-
ition; there really was no way to know at the time. He was just lucky.
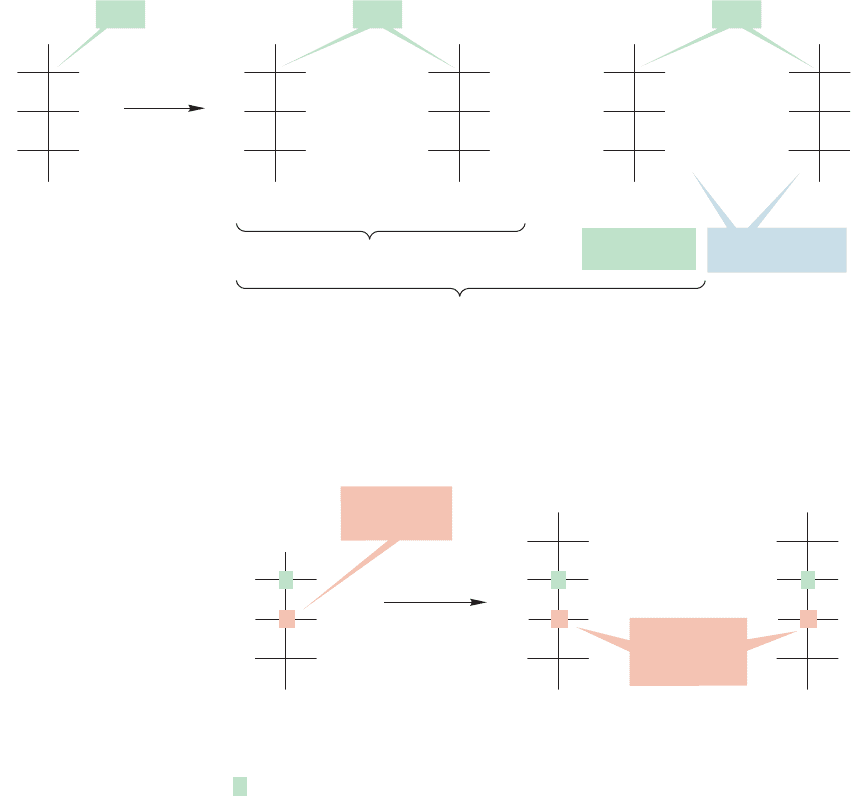
The Fischer proof starts with arabinose, arbitrarily assumed to be the
D enan-
tiomer. The first critical observation was that the Kiliani–Fischer synthesis applied
to D-arabinose led to a pair of D-aldohexoses (as it must).These were D-glucose and
D-mannose (Fig. 22.44). Because this synthesis must produce a pair of sugars that
5
Here is what Fischer had to say to his mentor, Adolf von Baeyer, about this matter in 1889. “The investiga-
tions on sugars are proceeding very gradually....Unfortunately, the experimental difficulties in this group are
so great, that a single experiment takes more time in weeks than other classes of compounds take in hours, so
only very rarely a student is found who can be used for this work.”
Kiliani–Fischer
synthesis
CH
2
OH
?
OHH
?
?
?
D-Arabinose D-Glucose and D-mannose
share these partial structures
CHO
C
C
CH
2
OH
?
OHH
OHH
??
CHO
C
C
CH
2
OH
??
OHH
HHO
??
CHO
C
C
C(3)
C(2)
C(4)
C(1)
?
+
FIGURE 22.44 The first step in
Fischer’s determination of the
structure of glucose.The
Kiliani–Fischer synthesis applied to
D-arabinose led to D-glucose and
D-mannose, which must share the
partial structures shown.
1154 CHAPTER 22 Carbohydrates
Kiliani–
Fischer
synthesis
CH
2
OH
?
OHH
H
D-Arabinose
CHO
C
C Known from Figure 22.45
CH
2
OH
?
OHH
OHH
H
CHO
C
HO
CH
2
OH
??
OHH
HHO
H
CHO
C
HO
These two structures are shared by
D-glucose and D-mannose
C(3) in
arabinose
C C
C
HO
C(4) in
glucose
and mannose
+
?
?
FIGURE 22.46 What we now know
about the structures of
D-arabinose,
D-glucose, and D-mannose. For
D-arabinose, only the configuration
at C(3), which becomes C(4) in
D-glucose and D-mannose, is left to
be determined.
have different configurations only at the newly generated stereogenic carbon [C(2)
in the two hexoses],
D-glucose and D-mannose must have the partial structures
shown on the right in Figure 22.44.
Let’s take a moment to look carefully at Figure 22.44. What do we know, and how
do we know it? Fischer has a flask containing one of the enantiomers of the pentose called
arabinose, but doesn’t know whether this enantiomer is
D or L, that is, whether the OH
at C(4) is on the right or left.He “solves”this problem by guessing,by assuming the sugar
he has in the flask is a member of the
D series defined as having the OH at C(4) on the
right. He has no way of knowing that almost all natural sugars are of the
D series, but he
guesses correctly. Fischer also has no idea of the relative arrangement of the OH groups
at C(3) and C(2) in the arabinose.Kiliani–Fischer synthesis applied to
D-arabinose gives
D-glucose and D-mannose, one of which has the newly created OH on the carbon adja-
cent to the aldehyde on the right, the other of which has it on the left.
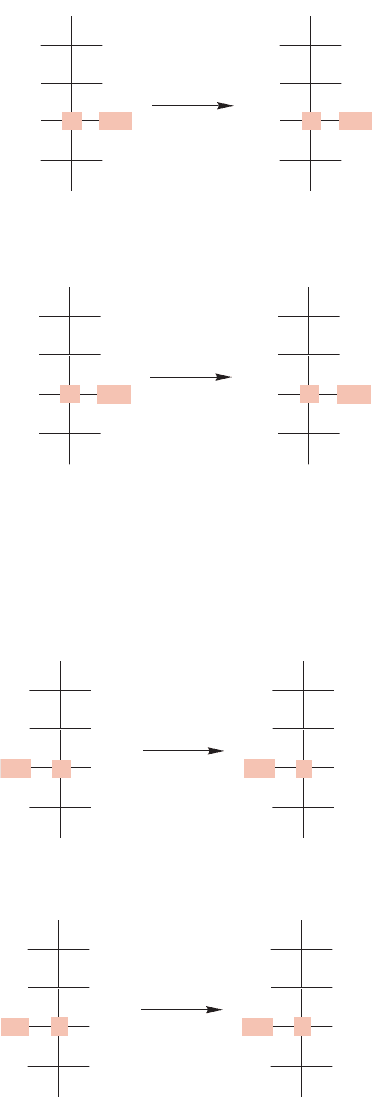
The second step in the Fischer proof determines the configuration of C(2) in
D-ara-
binose. Fischer observed that the oxidation of
D-arabinose with nitric acid gives an opti-
cally active diacid.The possibilities are shown in Figure 22.45.There are only three diacids
that can be produced from a D-aldopentose,and two of them are meso.In the third,which
is the optically active diacid, the OH at C(2) is on the left.Thus, the C(2) in
D-arabinose
must have OH on the left. Because C(2) in
D-arabinose becomes C(3) in the D-glucose
and
D-mannose of Figure 22.44, we know that C(3) in both hexoses has the OH on
the left.
HNO
3
CH
2
OH
?
OHH
?
?
?
meso
(not formed)
meso Optically active
OH at C(2) is
on the left
Optically active
CHO
C(2)
OH
OHH
H
H
COOH
OH
OHH
H
OHH
COOH
C(2) C(2)
H
OHH
HO
OHH
COOH
COOHCOOH COOH
HO
H
OHH
HO
H
COOH
COOH
HO
These are the three possible diacids
These structures
are the same
FIGURE 22.45 Oxidation of
D-arabinose leads to an optically active
diacid, which shows that the OH at
C(2) in D-arabinose is on the left.
Now we know a bit more about the partial structures of D-arabinose, D-glucose,and
D-mannose. We can specify all but one OH position in D-arabinose and in the two
D-aldohexoses. We do not yet know the position of the OH at C(3) of D-arabinose,
which becomes C(4) in the
D-aldohexoses, and we also do not know which partial
structure belongs to
D-glucose and which to D-mannose (Fig. 22.46).
22.5 The Fischer Determination of the Structure of D-Glucose 1155
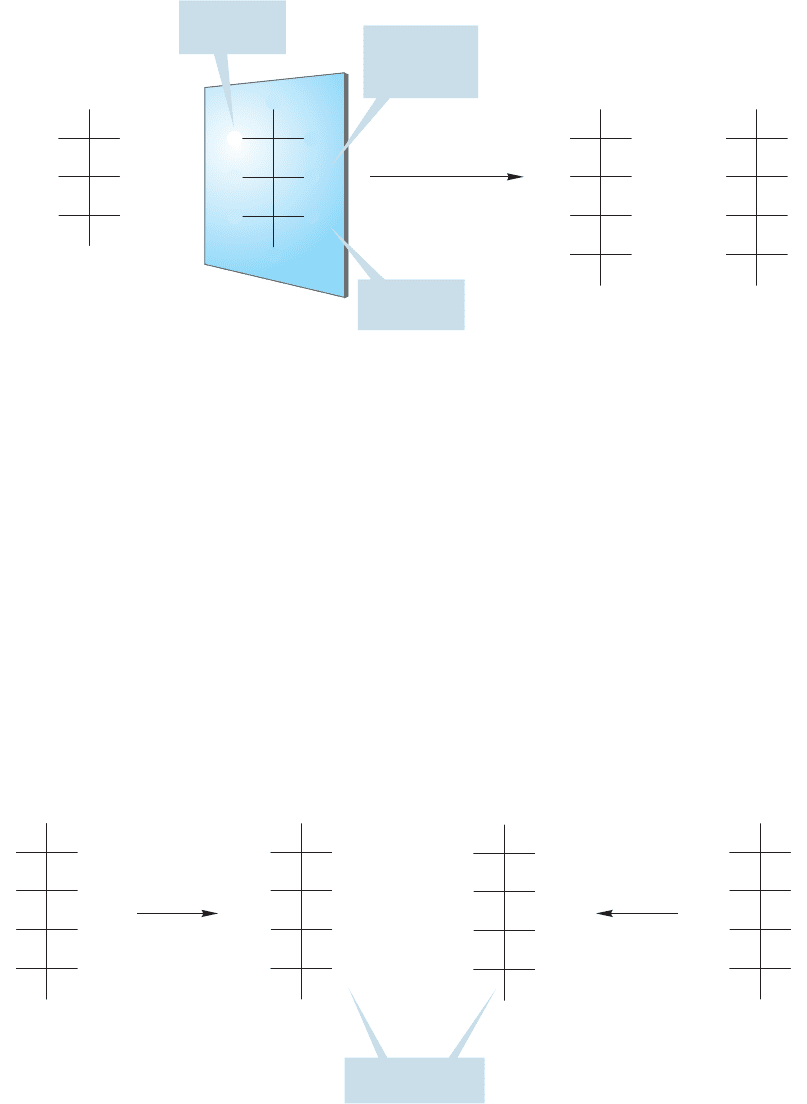
In the third step, the position of the last OH in D-arabinose is fixed by the obser-
vation that both aldohexoses,
D-glucose and D-mannose, give optically active diacids
when oxidized with nitric acid.There are two possibilities for the OH at C(4) in the
aldohexoses: It is either on the left or on the right. If it is on the right, both sugars
give an optically active diacid, in agreement with the experimental data (Fig. 22.47).
Optically active
diacid
CH
2
OH
HNO
3
OHH
OHH
HHO
H
CHO
HO
COOH
OHH
HHO
H
COOH
HO
OHH
CC
Optically active
diacid
CH
2
OH
OHH
OHH
OHH
C
H
CHO
HO
COOH
OHH
OHH
H
COOH
HO
OHH
HNO
3
C
If the OH at C(4) is on the right
FIGURE 22.47 If the last unknown
OH of
D-arabinose is on the right,
then oxidation of the derived
D-glucose and D-mannose with nitric
acid produces two optically active
diacids.This result matches the
experimental results.
CH
2
OH
HNO
3
OHH
HHO
HHO
H
CHO
HO
COOH
OHH
HHO
H
COOH
HO
Optically active
diacid
HHO
CC
CH
2
OH
OHH
HHO
OHH
H
CHO
HO
COOH
OHH
OHH
H
COOH
HO
meso Diacid!
HHO
HNO
3
C C
If the OH at C(4) had been on the left
FIGURE 22.48 However, if the
unknown OH had been on the left,
one possible diacid is meso, not
optically active, which does not
match the experimental results.
On the other hand, if the OH at C(4) is on the left, one of the diacids is meso,
not optically active, which does not match the experimental data (Fig. 22.48).
1156 CHAPTER 22 Carbohydrates
Known from
Figure 22.45
Known from
Figures 22.47
and 22.48
By definition,
a
D sugar
CH
2
OH
HHO
OHH
H
CHO
HO
CH
2
OH
OHH
HHO
OH
CHO
H
L-Arabinose D-Arabinose
AB
CH
2
OH
OHH
OHH
OHH
H
CHO
HO
CH
2
OH
OHH
OHH
HHO
H
CHO
HO
Kiliani–Fischer
synthesis
These two structures are shared by
D-glucose and D-mannose
Mirror
FIGURE 22.49 Now we know the structure of D-arabinose, L-arabinose, and the two structures A and
B, one of which is
D-glucose and the other D-mannose.
D-Glucose
X or Y = OH
CH
2
OH
Gulose
OHH
OHH
YX
H
CHO
HO
COOH
OHH
OHH
YX
H
COOH
HO
COOH
XY
OHH
HHO
H
COOH
HO
CH
2
OH
XY
OHH
HHO
H
CHO
HO
HNO
3
HNO
3
These structures
are the same
FIGURE 22.50 Oxidation with nitric acid forms an aldaric acid. Both the aldehyde end and the primary
alcohol end are converted into the same group, a carboxylic acid. Because
D-glucose and gulose give the same
aldaric acid, they must differ by turning the Fischer projection end to end.
So, the correct position of the OH at C(3) in D-arabinose and at C(4) in the two
D-aldohexoses, D-glucose and D-mannose, must be on the right. D-Glucose and
D-mannose share the two structures in Figure 22.49. The next problem is to tell
which is which.
We now know the full structure of
D-arabinose because the positions of all three
OH groups have been determined. Of course, because we know the structure of
D-arabinose,we also know the structure of its mirror image, L-arabinose (Fig. 22.49).
Now Fischer had to decide which of the two structures A and B in Figure 22.49
was D-glucose and which was D-mannose. The final observation he needed was
that
D-glucose and another hexose, gulose, gave the same optically active diacid
when oxidized with nitric acid (Fig. 22.50). We can find what the structures of
D-glucose and the other sugar (gulose) are in the following way.Treatment with nitric
acid oxidizes the aldehyde and primary alcohol groups at the two ends of the car-
bon chain to carboxylic acids. No stereogenic atoms are altered in the process.
22.5 The Fischer Determination of the Structure of D-Glucose 1157
CH
2
OH
HNO
3
OHH
OHH
OHH
H
CHO
HO
COOH
OHH
OHH
H
COOH
HO
A
OHH
COOH
HNO
3
HHO
OHH
H
COOH
HO
H
HO
CH
2
OH
HHO
HHO
H
CHO
HO
Gulose?
OHH
These structures
are the same
FIGURE 22.51 If D-glucose has structure A, then gulose has the structure shown. Oxidation
of gulose gives the same aldaric acid as oxidation of
D-glucose.Turning the Fischer
projection of the oxidized product of gulose 180° shows you the two oxidized products are
the same.
Therefore the only way two different aldoses can be oxidized to give the same
aldaric acid is if those two sugars differ by turning the Fischer projection end to
end (Fig. 22.50).
There are two possibilities for the structure of
D-glucose, A and B.IfD-glucose
has structure A, then gulose, the sugar that gives the same aldaric acid on oxidation,
must have the structure shown at the right in Figure 22.51.
Let’s see what is required if
D-glucose has structure B. In this case,gulose must
have the structure shown at the right in Figure 22.52 in order to give the same aldar-
ic acid.This time, however, the logic fails, because this gulose would have to be iden-
tical with
D-glucose, an impossibility. Both would have the structure of B (Fig.
22.52)!
D-Glucose cannot be B, because there can be no second sugar that gives
the same diacid.Therefore,
D-glucose must have structure A, and structure B must
be
D-mannose. We also know the structure of gulose from this logic. Notice that
it is an
L series sugar!
Gulose? No! This is
B again
These structures
are the same
CH
2
OH
HNO
3
OHH
OHH
HHO
H
CHO
HO
COOH
OHH
HHO
H
COOH
HO
If
D-glucose
is B
OHH
COOH
OHH
HHO
H
COOH
HO
OHH
HNO
3
CH
2
OH
OHH
HHO
H
CHO
HO
OHH
FIGURE 22.52 If D-glucose is structure B, then gulose must have the structure on the right so that it
can give the same aldaric acid. But this putative structure of gulose is the same as
D-glucose. It does
not fit Fischer’s observation that
D-glucose and another hexose give the same diacid on oxidation with
nitric acid.
1158 CHAPTER 22 Carbohydrates
Now let’s take stock of what we know, which is rather more than we might think
at first.We have the structures of 6 of the 16 possible aldohexoses, because we know
D-glucose, D-mannose, and L-gulose and their enantiomers L-glucose, L-man-
nose, and
D-gulose. In addition, we know the structures of D- and L-arabinose
(Fig. 22.53).
CH
2
OH
OHH
OHH
OHH
H
D-Glucose
CHO
HO
CH
2
OH
HHO
HHO
HHO
OH
L-Glucose
CHO
H
CH
2
OH
OHH
OHH
H
D-Arabinose
CHO
HO
CH
2
OH
HHO
HHO
OH
L-Arabinose
CHO
H
CH
2
OH
OHH
OHH
HHO
H
D-Mannose
CHO
HO
CH
2
OH
HHO
HHO
OHH
OH
L-Mannose
CHO
H
CH
2
OH
OHH
HHO
OHH
OH
D-Gulose
CHO
H
CH
2
OH
HHO
OHH
HHO
H
L-Gulose
CHO
HO
FIGURE 22.53 We now know the structures of these aldohexoses and aldopentoses.
3 equiv.
PhNHNH
2
3 equiv.
PhNHNH
2
CH
2
OH
OHH
OHH
H
D-Arabinose
CHO
C
HO
CH
2
OH
OHH
OHH
OH
D-Ribose
CHO
C
H
CH
2
OH
OHH
OHH
N NHPh
The same osazone
HC
C
N NHPh
C(2) C(2)
FIGURE 22.54 D-Arabinose and D-ribose give the same osazone and therefore can differ only at
C(2).The structure of
D-ribose is therefore known. L-Ribose is simply the mirror image of the
D isomer.
There are many ways to determine the structures of the remaining aldopentoses
and aldohexoses. Here is just one possible sequence. Let’s first deal with the three
remaining D-aldopentoses, given that we know the structure of D-arabinose. The
aldopentose
D-ribose gives the same osazone as D-arabinose (Fig. 22.54), which
means that these two sugars must differ only in the configuration at C(2).Therefore
D-ribose must have the structure shown in Figure 22.54.
