Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.


22.7 Summary 1169
Key Terms
aldaric acid (p. 1144)
aldohexose (p. 1129)
aldonic acid (p. 1144)
aldopentose (p. 1129)
aldose (p. 1126)
aldotetrose (p. 1129)
aldotriose (p. 1126)
anomeric carbon (p. 1134)
anomers (p. 1134)
carbohydrate (p. 1125)
cellulose (p. 1168)
configurational carbon (p. 1128)
disaccharide (p. 1161)
epimers (p. 1133)
Fischer projection (p. 1127)
furanose (p. 1132)
furanoside (p. 1148)
glycolysis (p. 1140)
glycoside (p. 1148)
Haworth form (p. 1136)
hexose (p. 1130)
ketose (p. 1128)
Kiliani–Fischer synthesis (p. 1139)
Lobry de Bruijn–Alberda van Ekenstein
reaction (p. 1141)
monosaccharide (p. 1161)
mutarotation (p. 1140)
nonreducing sugar (p. 1166)
osazone (p. 1145)
pentose (p. 1130)
polysaccharide (p. 1161)
pyranose (p. 1132)
pyranoside (p. 1148)
reducing sugar (p. 1133)
Ruff degradation (p. 1139)
saccharide (p. 1125)
starch (p. 1168)
sugar (p. 1125)
tetrose (p. 1129)
Reactions, Mechanisms, and Tools
Most of the reactions in this chapter can be found in the chem-
istry of carbonyl groups (Chapters 16 and 19) and alcohols
(Chapters 6 and 7); only a few new reactions appear.
One new twist to a known reaction is intramolecular hemiacetal
formation. Carbonyl groups react with nucleophiles in the addition
reaction (Chapter 16). When the nucleophile is an alcohol, hemi-
acetals are formed, but generally they are not favored at equilibri-
um. However, when a relatively strain-free ring can be formed in
an intramolecular hemiacetal formation, the cyclic form can be
favored. Such is the case for aldohexoses (and many other sugars),
which exist mainly in the six-membered pyranose forms (Fig. 22.12).
The equilibration of the cyclic hemiacetal with a small
amount of the open aldohexose form allows for either the α or
β anomer to produce an equilibrium mixture of the two
anomers in a phenomenon called mutarotation (Fig. 22.23).
The reactivity of the hemiacetal OH allows for a differen-
tiation of this OH from the others in the molecule. For exam-
ple, protonation of the C(1) hemiacetal OH leads to a
molecule that can form a resonance-stabilized carbocation on
loss of water. Nucleophiles such as alcohols can add to this
carbocation to give the acetals. Acetals of carbohydrates are
called glycosides. Only the OH at C(1) can react in this way
(Fig. 22.38).
Methods have been developed both to lengthen the carbon
chain in a carbohydrate (Kiliani–Fischer synthesis) and to
shorten it (Ruff degradation).
Reactions at selective positions in a carbohydrate molecule
can be accomplished using protecting groups. The unique
nature of the anomeric carbon allows for easy conversion of the
hemiacetal into an acetal.
Syntheses
Chain lengthening. The Kiliani–Fischer synthesis adds a
new C(1) aldehyde and generates a pair of C(2) stereoisomers
(Fig. 22.20).
Chain shortening. In the Ruff degradation, the original
aldehyde group is lost and a new aldehyde created at the old
C(2) (Fig. 22.21).
Osazone formation (Fig. 22.31).
Acetal and tetraether acetal derivatives of carbohydrates
(Figs. 22.36 and 22.38).
Aldaric and aldonic acids can be made through oxidation of
aldoses (Figs. 22.28 and 22.29).
Common Errors
At the heart of sugar chemistry is the difference between C(1),
the anomeric center, and the other carbons. If you lose track of
this difference, you lose the ability to follow the labeling studies
establishing the ring size of simple sugars and the points of
attachment of disaccharides.
It is also sometimes difficult to keep track of open and
closed (hemiacetal) forms. We typically write what is convenient,
not always good representations of structure. For example, open
forms of sugars might be written even though most of the ma-
terial is really tied up in the cyclic hemiacetal form. Often it is
the small amount of the open form that is reactive.This concept
can be confusing if you don’t have the equilibrium between open
and cyclic forms solidly in mind.

1170 CHAPTER 22 Carbohydrates
22.8 Additional Problems
PROBLEM 22.33 Glucosamine (2-amino-2-deoxy-D-glucopy-
ranose) is a very important substituted sugar. It is often used
for the treatment of osteoarthritis. Draw the β form of
glucosamine.
PROBLEM 22.34 Your task is to distinguish between D-talose
and
D-galactose. The following sequence is suggested. First,
oxidize to aldaric acids. Second, make the methyl esters.
Then ...? Fill in the last step. Oh, we forgot to tell you that at
the end of the second step your town’s elected officials passed
an ordinance forbidding any laboratory work because it would
be harmful to their chances for reelection. You are restricted to
spectroscopy.
PROBLEM 22.35 Use Table 22.2 (p. 1135) to calculate the
energy difference between the α- and β-pyranose forms of
D-allose at 25 °C.
PROBLEM 22.36 Calculate the equilibrium ratio of α- and
β-
D-glucopyranose from the specific rotations of the pure
anomers (α 112°, β 18.7°; equilibrium value 52.7°).
You might compare your calculation with the values given in
Table 22.2.
PROBLEM 22.37 The disaccharide maltose (C
12
H
22
O
11
) can
be hydrolyzed in acid to two molecules of
D-glucose. It is also
hydrolyzed by the enzyme maltase, a molecule known to
cleave only α-glycosidic linkages. Maltose can be oxidized by
bromine in water to maltobionic acid (MBA, C
12
H
22
O
12
).
When MBA is treated with methyl iodide and base, followed
by acid hydrolysis, the products are 2,3,4,6-tetramethyl-
D-glu-
copyranose and 2,3,5,6-tetramethyl-
D-gluconic acid. Provide a
three-dimensional structure for maltose and explain your rea-
soning.
PROBLEM 22.38 Draw chair structures for the nonreducing
disaccharides composed of one molecule of
D-allose and one
molecule of
D-altrose. First determine how many are possible,
in principle, and then adopt some scheme for indicating what
they are.
PROBLEM 22.26 Draw the following molecules:
(a) A Fischer projection of any
D-aldoheptose.
(b) A Fischer projection of any
L-ketopentose.
(c) A Fischer projection of a
D-aldotetrose that gives a meso
diacid on oxidation with dilute nitric acid.
(d) Methyl α-
D-galactopyranoside in chair form.
(e) The osazone of
D-allose in Fischer projection.
(f) Phenyl β-
D-ribofuranoside in Haworth form.
PROBLEM 22.27 Make clear three-dimensional chair drawings
of all the pyranose forms of
D-altrose. Before you start, decide
how many will there be.
PROBLEM 22.28
(a) What sugar or sugars would result from the Ruff degrada-
tion applied to
D-gulose?
(b) What sugar or sugars would result from the Kiliani–Fischer
synthesis applied to
D-lyxose?
PROBLEM 22.29
(a) What other sugar, if any, would give the same aldaric acid as
D-talose when oxidized with nitric acid?
(b) What other sugar, if any, would give the same aldaric acid as
D-xylose when oxidized with nitric acid?
(c) What other sugar, if any, would give the same aldaric acid as
D-idose when oxidized with nitric acid?
PROBLEM 22.30 What other sugar would give the same
osazone as
L-talose?
PROBLEM 22.31 What are the principal organic products
when
D-lyxose is treated under the following conditions?
Mechanisms are not necessary.
D-Lyxose is drawn in the
open form, but it is mostly in the cyclic form in aqueous
solution. Keep this fact in mind as you determine the
products.
CHO
OH
H
H
H
HO
HO
CH
2
OH
D-Lyxose
?
(a) NaBH
4
, H
2
O
(b) Br
2
, H
2
O
(c) HNO
3
(d) excess CH
3
I, Ag
2
O
(e) cat. HCl, CH
3
OH
CHO
OH
OH
OH
H
H
H
CH
2
OH
D-Ribose
?
(a) Ac
2
O, H
3
O
+
(b) CH
3
OH, H
3
O
+
(c) PhNHNH
2
(3 equiv.)
(d) 1. NaCN
2. H
2
/Pd, poisoned
3. H
2
O
(e ) 1. Br
2
/H
2
O
2. Ca(OH)
2
3. Fe
2
(SO
4
)
3
4. H
2
O
2
PROBLEM 22.32 What are the principal organic products
when
D-ribose is treated under the following conditions?
Mechanisms are not necessary.
D-Ribose is drawn in the open
form, but it is mostly cyclic.
22.8 Additional Problems 1171
PROBLEM 22.39
Write a mechanism for the acid-induced
transformation of a
D-aldopentose into furfural.
PROBLEM 22.40 Figures 22.25 and 22.26 give one mecha-
nism for the Lobry de Bruijn–Alberda van Ekenstein reaction.
Use the example given, in which
D-glucose equilibrates with
D-mannose and D-fructose, and provide another mechanism in
which a hydride shift occurs.
PROBLEM 22.41 When α-D-xylopyranose is treated with
hydrochloric acid in methyl alcohol, the major products are two
isomers of methyl
D-xylopyranoside.
(a) Draw the starting material and the two major products and
explain how the two major products are formed from the
single starting material.
(b) There are two minor products formed as well. Both are
methyl furanosides. What are these two products and how
are they formed?
PROBLEM 22.42 Outline a mechanism for the following
reaction:
CHO
CHO
OH
OH
OH
H
H
H
CH
2
OH
Furfural
H
3
O
+
H
2
O
O
-D-Glucopyranose
(40%)
Amino
-D-gluco-N-pyranoside
NH
3
CH
3
OH
25 °C, 2 days
HO
HO
OH
O
OH
OH
HO
HO
OH
O
NH
2
OH
-D-Glucopyranose
cat.
HCl
25 °C
HHO
OHH
CH
2
OH
OHH
OHH
HO
HO
OH
O
OH
OH
SH
SS
PROBLEM 22.43 Outline a mechanism for the following
reaction:
PROBLEM 22.44 When D-glucose, or any polysaccharide
containing
D-glucose units such as cellulose, is pyrolyzed with
an acid catalyst, a new compound, levoglucosan (1), is formed.
Write a mechanism for the formation of 1 from
D-glucose
under these conditions.
OH
OH
OH
O
O
1
pyridine
60 °C
TsCl
C
12
H
20
O
6
acetone
125 °C
NaI
A
(C
12
H
19
O
6
Ts)
Raney Ni
B
(C
12
H
19
IO
5
)
1% H
2
SO
4
C
(C
12
H
20
O
5
)
D
(C
6
H
12
O
5
)
PROBLEM 22.45 Treatment of α-D-galactopyranose with ace-
tone and sulfuric acid catalyst leads to a compound that has the
formula C
12
H
20
O
6
and still contains a primary alcohol. Draw a
structure for this compound and explain its formation.
PROBLEM 22.46 Draw structures for molecules A–D, and
explain the reactions of the starting material, which is the prod-
uct formed in Problem 22.45.
1172 CHAPTER 22 Carbohydrates
PROBLEM 22.47 The primary alcohol of a sugar will partici-
pate in product formation with many carbonyl compounds. For
example, many saccharides react with benzaldehyde (PhCHO)
in acid to give a six-membered ring in which the primary alco-
hol is incorporated. However, as shown in Problem 22.45, ace-
tone leads to formation of a five-membered ring rather than a
six-membered ring. Draw a detailed three-dimensional struc-
ture (a) for the six-membered ring formed when benzaldehyde
reacts with the partial sugar shown and (b) for the six-mem-
bered ring formed when acetone reacts with the partial sugar.
(c) Justify the product you have shown in each case.
PROBLEM 22.50 The antioxidant vitamin C (shown below) is
an essential compound for our diet. The common dietary
sources of this vitamin are fruits and vegetables. Interestingly,
most other animals are able to biosynthesize the molecule and
thus don’t rely on a plant-based diet to obtain this necessary
substance. The biological pathway for vitamin C formation
begins with
D-glucose. Based on the structure of vitamin C,
do you think glucofuranose is a precursor? Draw glucofuranose
and suggest the overall steps (oxidation, reduction, substitution,
elimination) that might be used to make this conversion to
vitamin C.
PROBLEM 22.48 The anomeric effect is the observed increase
in stabilization of electronegative groups in the α position
compared to the β position for
D sugars in the pyranose form.
One possible explanation for this increase in stabilization is
hyperconjugation. Explain how hyperconjugation can be used
to justify the anomeric effect.
PROBLEM 22.49 Write a mechanism for the reaction shown
in Figure 22.30.
H
3
O
+
PhCHO
A schematic view
of a sugar
HO
OCH
2
O
HO
HO
HOCH
2
Ph
PROBLEM 22.51 Maltose is a disaccharide that differs very
little from cellobiose. It has the same properties as cellobiose
described in Problem 22.24, but is not cleaved by lactase
(it is cleaved by another enzyme, maltase). Provide a three-
dimensional structure for maltose. Hint: How else can the two
sugars be linked?
Vitamin C
H
H
CH
2
OH
HO
HO OH
O
O

Special Topic
Amino Acids and
Polyamino Acids
(Peptides and Proteins)
1173
23.1 Preview
23.2 Amino Acids
23.3 Reactions of Amino Acids
23.4 Peptide Chemistry
23.5 Nucleosides, Nucleotides,
and Nucleic Acids
23.6 Summary
23.7 Additional Problems
23
AMINO ACIDS ARE US The enzymes that catalyze the bioorganic chemistry in all
humans are composed of the same 20 amino acids.

1174 CHAPTER 23 Amino Acids and Polyamino Acids (Peptides and Proteins)
When I returned from the physical shock of Nagasaki ..., I tried to persuade
my colleagues in governments and the United Nations that Nagasaki should
be preserved exactly as it was then. I wanted all future conferences on
disarmament...to be held in that ashy, clinical sea of rubble. I still think as I
did then, that only in this forbidding context could statesmen make realistic
judgements of the problems which they handle on our behalf.
Alas, my official colleagues thought nothing of my scheme. On the
contrary, they pointed out to me that delegates would be uncomfortable
in Nagasaki.
—JACOB BRONOWSKI
1
23.1 Preview
For many people, fascination with science depends on the relationship between the ma-
terial they study and biological processes.This connection seems natural and no one can
deny the excitement of today’s incredible pace of discovery in biological chemistry and
molecular biology. It’s quite impossible to be a decent molecular biologist without being
first a chemist.Organic chemistry is particularly,if not uniquely,important.Molecular biol-
ogy is organic chemistry (and inorganic and physical chemistry) applied to molecules of
a scale not generally encountered in traditional, “small molecule” organic chemistry.
2
Molecular size and the attendant complexity of structure generate genuinely new
chemistry. We have already seen how important molecular shape is in small mol-
ecules,and now, in molecules of biological size, stereochemistry becomes more com-
plex and even more vital to understanding mechanism.To a great extent, biological
action depends on shape. Much of the immense architecture of amino acid poly-
mers serves “only”to bring a reactive group to an appropriate location on a substrate
molecule, which is itself held by the enzyme in just the right position for reaction.
This chapter can only begin to introduce this subject and attempt to show you how
important organic chemistry is to it.The choice of subjects is certainly arbitrary—there
is a vast number of possible topics.This chapter is held together, somewhat tenuous-
ly it must be admitted, by nitrogen atoms. Nitrogen atoms are always components of
the alkaloids we saw in Chapter 6, and of amino acids, the subjects of this chapter.
ESSENTIAL SKILLS AND DETAILS
1. In this chapter, we pass from small molecules to big ones. It is necessary to be able to
apply old “small molecule” chemistry in these new larger and more complex molecules.
2. Manipulation of blocking and activating groups is necessary for successful peptide synthesis,
so you have to know which to use,and when and how to attach and detach them.
3. It is only introduced here, but surely the general mechanism for polynucleotide
replication is important. If you go on in biology or biochemistry, you’ll learn much
more about it.
1
Jacob Bronowski (1908–1974) was a mathematician,poet, and playwright.He was the author and compelling
narrator of the brilliant television series, The Ascent of Man. Don’t miss it if it is replayed.
2
Scales change depending on your point of view.To a theoretician, a molecule with 10–20 nonhydrogen “heavy
atoms” is often too large to be easily approached. Daunting problems are presented, if not of intellectual
content, certainly of cost. Even with the decrease in price of computer time, calculations on molecules of
“biological”size often simply cost too much.To a molecular biologist, a molecule of only 10 heavy atoms would
be laughably tiny—hardly visible on the scale of proteins and enzymes.
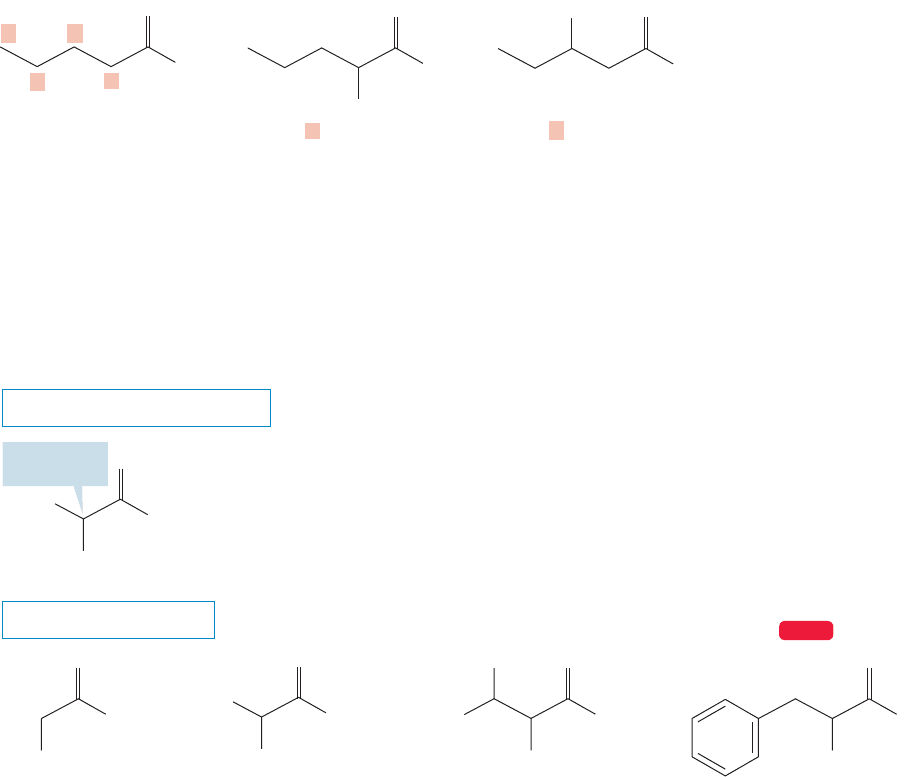
23.2 Amino Acids 1175
An α-amino acid
O
..
..
..
β
γα
..
..
OH
NH
2
A β-amino acid
O
..
..
..
..
..
OH
NH
2
O
..
..
..
..
OH
δ
FIGURE 23.1 The amino group can
be located on the hydrocarbon chain
with a Greek letter, α, β, γ, or δ.
O
OH
R
NH
2
THE GENERAL STRUCTURE
SPECIFIC EXAMPLES
2-Aminopropanoic acid
(alanine)
2-Amino-3-methylbutanoic acid
(valine)
2-Amino-3-phenylpropanoic acid
(phenylalanine)
2-Aminoacetic acid
(glycine)
O
..
..
..
..
OH
..
O
..
..
..
..
OH
O
..
..
..
..
OH
H
3
C
O
..
..
..
..
..
OH
NH
2
CH
3
..
NH
2
H
3
C
NH
2
..
NH
2
1
2
3
1
2
2
3
1
2
3
1
WEB 3D
Stereogenic
carbon
FIGURE 23.2 An α-amino acid is just
an “R-substituted”2-aminoacetic
acid.The IUPAC and the common
names are given for several naturally
occurring amino acids.
23.2 Amino Acids
23.2a Nomenclature There is a systematic nomenclature for amino acids, but
as we have so often seen, the real world eschews the system and goes right on using
familiar old common names. The generic term amino acid itself is shorthand for
␣-amino acid. It is the α that locates the amino group on the carbon adjacent to
the carboxylate carbon and distinguishes the α-amino acids from the isomeric but
less important β and γ compounds (Fig. 23.1).
The IUPAC naming system uses a 2 instead of the α and names the α-amino
acids as members of a series of 2-amino carboxylic acids (Fig. 23.2). In practice, the
systematic name is rarely used, and common names are retained for most of these
molecules.Biochemists tend to view amino acids as substituted α-aminoacetic acids.
In this frame of mind, there is an R group on C(2).This R group is called the amino
acid side chain. Note that as long as R doesn’t equal H, the amino acids contain a
stereogenic carbon atom.
Three-letter codes have been developed for the amino acids and are very com-
monly used, especially when describing the polymeric amino acids known as pep-
tides or in especially large examples, as proteins. A peptide is a molecule that is
composed of two or more amino acids that are connected by an amide bond. The
connecting amide bond is referred to as a peptide bond. A protein is just a long
polypeptide chain.The boundary between the smaller peptides, or polypeptides,and
the larger proteins is neither fixed nor always clear, and the terms are sometimes
used interchangeably. More recently, an even shorter set of one-letter abbreviations
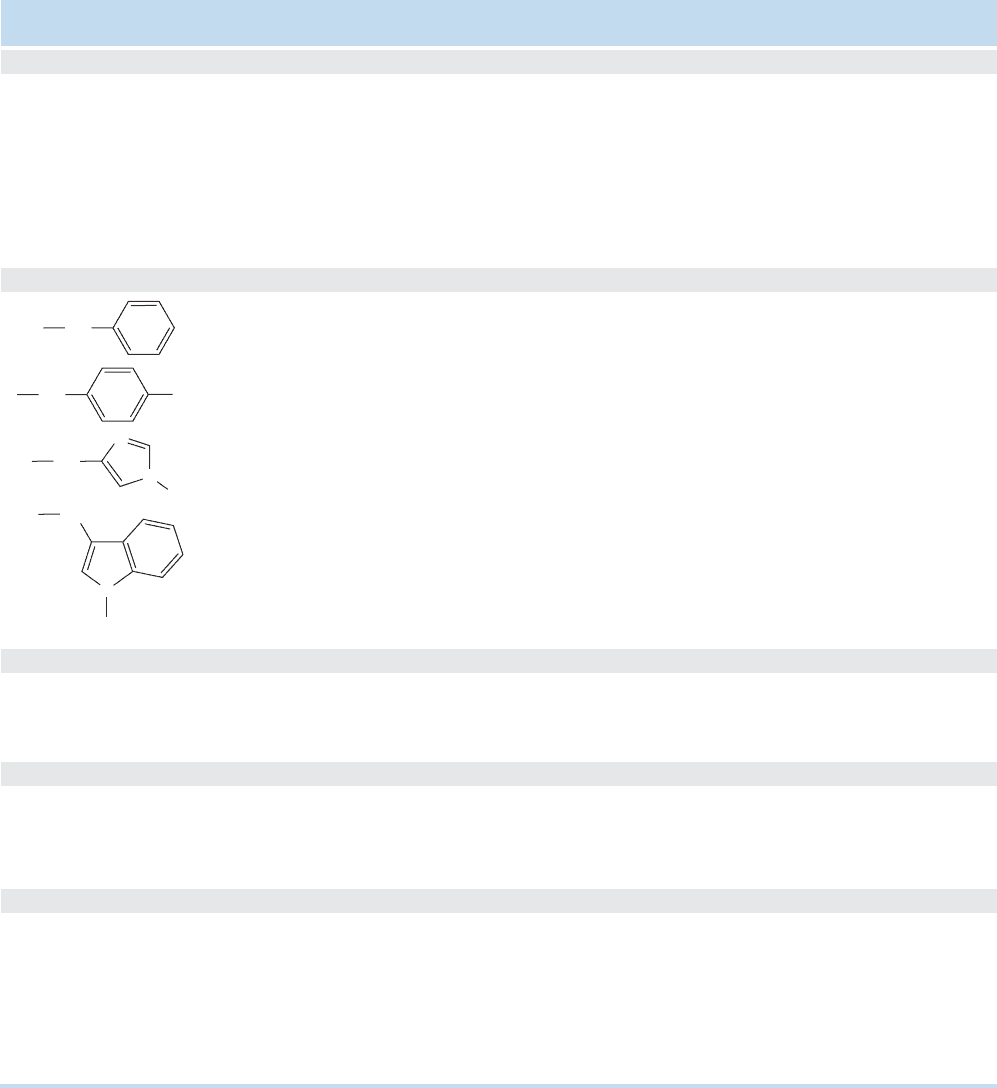
1176 CHAPTER 23 Amino Acids and Polyamino Acids (Peptides and Proteins)
has come into use. Table 23.1 gives the names and abbreviations for the 20 com-
mon amino acids and some of their physical properties. Notice that R groups of
many kinds appear, from simple hydrocarbon chains to more complicated acidic
and basic groups.
TABLE 23.1 Properties of 20 Common Amino Acids
R
(Side Chain)
Simple Alkane Groups
Common
Name
a
mp (°C)
L-Form
b
pK
a
(COOH)
pK
a
(
+
NH
3
)
pK
a
(Side Chain)
Isoelectric
Point (pI
)
Abbreviations
¬H Glycine 229 (dec) 2.3 9.6 6.0Gly, G
¬CH
3
Alanine 297 (dec) 2.3 9.7 6.0Ala, A
¬CH(CH
3
)
2
Valine* 315 (dec) 2.3 9.7 6.0Val, V
¬CH
2
CH(CH
3
)
2
Leucine* 337 (dec) 2.3 9.6 6.0Leu, L
¬CHCH
3
(CH
2
CH
3
) Isoleucine* 284 (dec) 2.3 9.6 6.0Ile, I
Aromatic Groups
Phenylalanine* 283 (dec) 1.8 9.2 5.9Phe, F
Tyrosine 316 (dec) 2.2 9.1 10.1 5.7Tyr, Y
Histidine* 288 (dec) 1.8 9.0 6.0 7.6His, H
Tryptophan* 290 (dec) 2.4 9.4 5.9Trp, W
Groups containing an OH (see also Tyr)
¬CH
2
OH Serine 228 (dec) 2.2 9.2 5.7Ser, S
¬CH(CH
3
)OH reonine* 226 (dec) 2.2 9.2 5.6r, T
Groups Containing S
¬CH
2
SH Cysteine 1.7 10.8 8.3 5.0Cys, C
¬CH
2
CH
2
SCH
3
Methionine* 283 (dec) 2.3 9.2 5.8Met, M
Groups Containing a Carbonyl Group
¬CH
2
COOH Aspartic Acid 270 (dec) 1.9 9.6 3.7 2.9Asp, D
¬CH
2
CH
2
COOH Glutamic Acid 211 (dec) 2.2 9.7 4.3 3.2Glu, E
¬CH
2
CONH
2
Asparagine 214 (dec) 2.0 8.8 5.4Asn, N
¬CH
2
CH
2
CONH
2
Glutamine 185 (dec) 2.2 9.1 5.7Gln, Q
CH
2
CH
2
CH
2
CH
2
OH
N
N
H
N
H
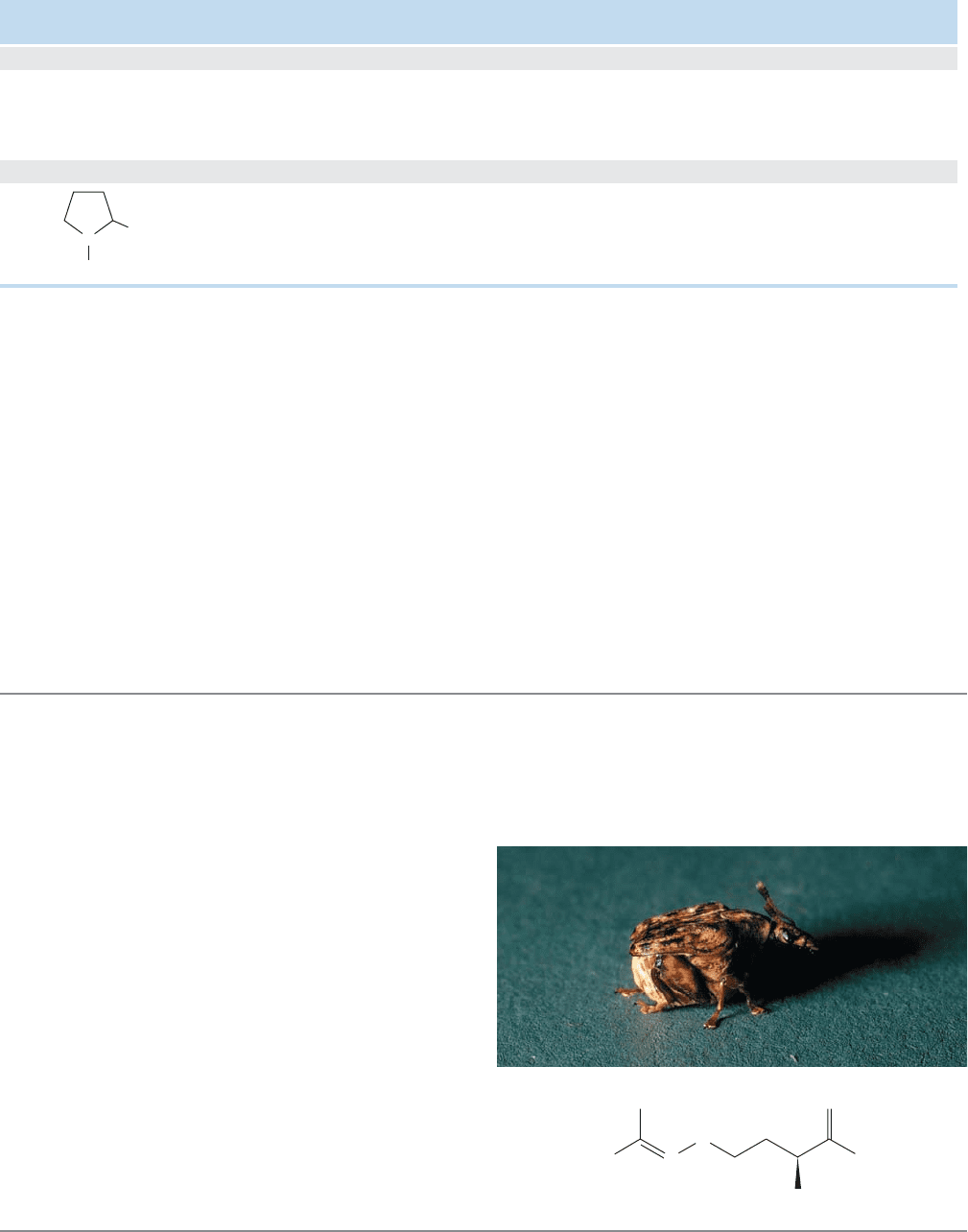
TABLE 23.1 Properties of 20 Common Amino Acids
R
(Side Chain)
Common
Name
a
mp (°C)
L-Form
b
pK
a
(COOH)
pK
a
(
+
NH
3
)
pK
a
(Side Chain)
Isoelectric
Point (pI
)
Abbreviations
Groups Containing an Amino Group (see also Trp and His)
¬(CH
2
)
4
NH
2
Lysine*
¬(CH
2
)
3
NH—C
=
NH(NH
2
) Arginine*
224 (dec) 2.2 8.9 10.3 9.7Lys, K
A Secondary Amine
207 (dec) 2.2 9.1 12.5 10.8Arg, R
Proline 221 (dec) 2.0 10.6 6.1Pro, P
a
Essential amino acids are marked by an asterisk (*).
b
Decomposition is abbreviated by dec.
COOH
N
H
CANAVANINE: AN UNUSUAL AMINO ACID
We tend to think of plants as passive, defenseless creatures,
growing quietly at the mercy of herbivorous predators. But
this notion seems odd; shouldn’t evolutionary pressure lead to
defense mechanisms in which the poor plant finds a way to
prevent animals from eating it? Indeed it has, and plants are
full of all sorts of toxins evolved to discourage predators. Of
course, these defenses lead to evolutionary pressure on the
predator population, and sometimes one species will work its
way around the plant’s defenses. The signal that this has hap-
pened is the presence of a single predator. All others have
been discouraged, but one has found a way to defeat the
plant’s defense systems.Take, for example, Dioclea megacarpa,a
vine whose only predator is the beetle Caryedes brasiliensis
shown in the photo. This plant uses canavanine in place of the
closely related arginine (replace the O in canavanine with
CH
2
and you get arginine). In most herbivores, replacement
of arginine with canavanine leads to incorrect protein folding
and arrested development. The single successful beetle has
developed an enzyme that can tell the difference between
arginine and canavanine, as well as an enzyme specially
designed to destroy canavanine. So, C. brasiliensis happily
munches away on Dioclea while all other beetles stay away.
Why pick out this particular set of 20 from the vast number conceivable? Because
these are the 20 amino acids encountered in peptides and proteins in Nature.Your body
can synthesize 10 of these 20 from the food you eat. One might think that a reason-
able way for your insides to proceed would be to break down ingested proteins to their
constituent amino acids, and then use these directly. But this is not how it works.
Instead, amino acids are metabolized (broken down chemically) into much simpler
molecules from which the 10 amino acids are resynthesized. But even your body is
not a perfect synthetic chemist—unless you are a superior mutant,there are 10 of these
20 amino acids it is unable to make. These you do use directly. These 10 you must
ingest, and if your diet fails to include one or more of these essential amino acids you
will not last long.The essential amino acids are marked with an asterisk in Table 23.1.
(continued)
23.2 Amino Acids 1177
H
2
N
OH
ONH
2
NH
2
N
O
1178 CHAPTER 23 Amino Acids and Polyamino Acids (Peptides and Proteins)
23.2b Structure of Amino Acids All but one of the 20 common amino
acids is a primary amine.The only exception is proline, which is an amino acid con-
taining a secondary amine (see Table 23.1). All 20 of the biologically important
amino acids but the achiral glycine and cysteine are found in the (S) configuration.
Many (R) amino acids are known in Nature, but most play no important role in
human biochemistry. Why? That’s a good question and no certain answer exists.
Did the first resolution of enantiomers (p. 169) produce an (S) amino acid, which
then determined the sense of chirality of all the amino acids formed thereafter? Is
the preference for (S) no more than the result of some primeval accident? No one
knows. If it is, we may one day encounter (R) civilizations somewhere in the uni-
verse, provided we are sufficiently lucky and clever to survive long enough.
PROBLEM 23.1 Draw tetrahedral three-dimensional structures for (R) and (S)
valine and aspartic acid.
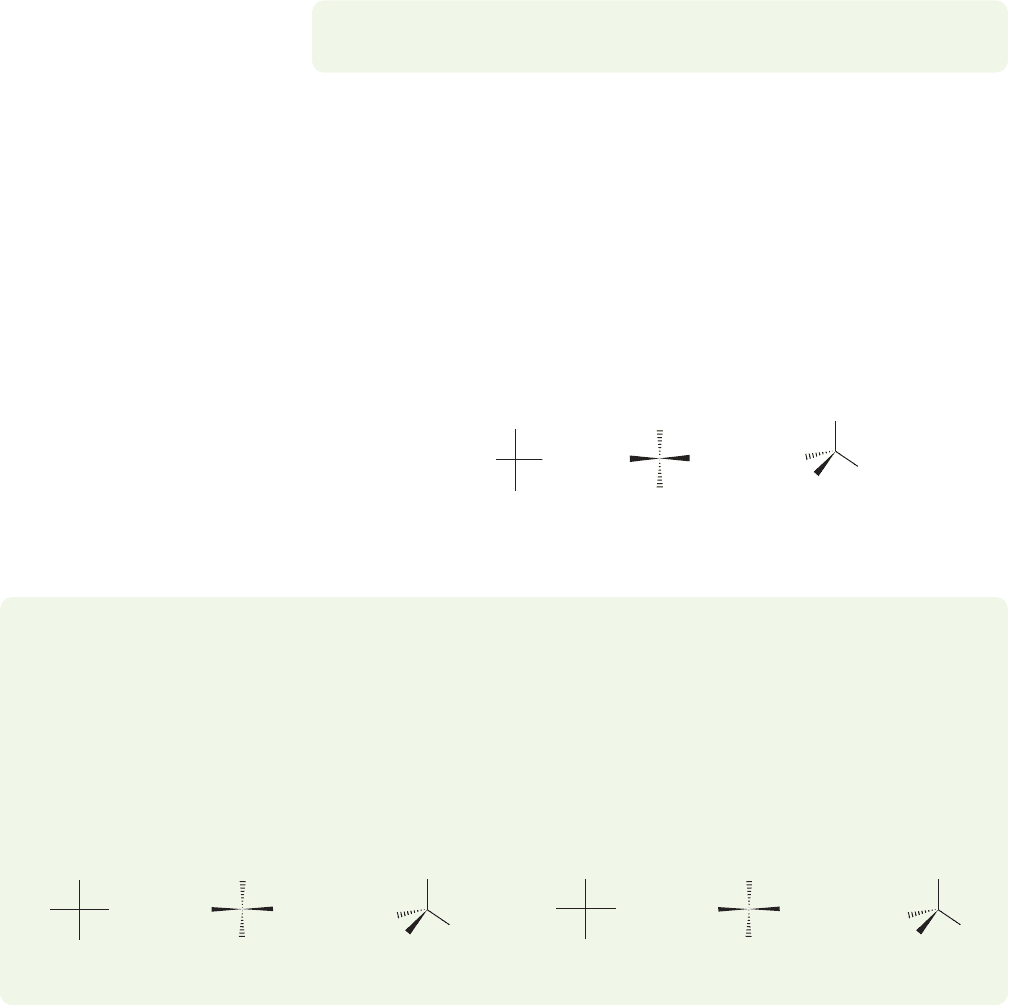
Amino acids are usually not described as (S) or (R). Instead, the Fischer projec-
tion system is used (p. 1127). The acid group is drawn at the top, and, as with the
sugar Fischer projections, vertical lines represent bonds retreating from you and hor-
izontal lines represent bonds coming toward you.The carbon chain is the backbone
of the Fischer projection, which means the side chain is placed at the bottom of the
drawing. The position of the amino group in the Fischer projection determines
whether the amino acid is
D or L. If the amino group is on the left, then it is an
L-amino acid.If the amino group is on the right, then it is a D-amino acid.As shown
in Figure 23.3, typically it is the
L-amino acid that is in the (S) configuration. It is
interesting that Nature depends most on the
D-carbohydrates and the L-amino acids.
==
H
3
C
H
HOOC
H
2
N
COOH
COOH
CH
3
H
NH
2
(S)-AlanineFischer projection
of
L-alanine
H
H
2
N
CH
3
FIGURE 23.3 An (S) amino acid is
usually
L in the Fischer convention.
L-Valine
1
3
4
2
H
2
N
CH(CH
3
)
2
H
2
N
CH
2
SH
H
COOH
COOH
H
=
H
2
N
CH(CH
3
)
2
H
COOH
=
(CH
3
)
2
CH
(S) Enantiomer
L-Cysteine
=
H
2
N
CH
2
SH
H
COOH
=
(R) Enantiomer
H
HOOC
NH
2
1
2
4
3
HSCH
2
H
HOOC
NH
2
WORKED PROBLEM 23.2 Draw the L isomers of valine and cysteine in Fischer
projection. Determine the (R) or (S) configuration of each.
ANSWER Remember the convention for Fischer projections. To convert the
Fischer projection into a 3-D structure, draw the horizontal bonds as solid wedges
coming toward you and vertical bonds as dashed wedges retreating from you.
Now the 3-D image can be rotated in space to put the lowest atomic number (the
hydrogen) pointing toward the right. Notice that
L-cysteine is the only L-amino
acid in Table 23.1 that is in the (R) configuration.
