Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.

22.3 Formation of Carbohydrates 1139
This reaction is a modern variation of the Kiliani–Fischer synthesis (Emil Fischer,
again, and Heinrich Kiliani, 1855–1945). In the first step, sodium cyanide
(NaCN) adds to the ribose carbonyl group to make a cyanohydrin (p. 781). The
key point is that a new stereogenic carbon is generated in this reaction and both
(R) and (S) epimers will be formed. In other words, two products will be
formed, one with the new OH group on the right in the Fischer projection and
one with it on the left.
The second step of the Kiliani–Fischer synthesis, catalytic reduction with poi-
soned palladium (p. 452), gives a pair of imines that are hydrolyzed under the reac-
tion conditions to aldoses, each of them one carbon longer than the starting sugar.
The new sugars differ from each other only in their stereochemistry at C(2).In other
words, they are C(2) epimers.
PROBLEM 22.12 Use the Kiliani–Fischer synthesis starting with D-glyceraldehyde.
What new sugars are formed? It is not necessary to write mechanisms for the
reactions.
PROBLEM 22.13 The following two sugars are produced by Kiliani–Fischer syn-
thesis from an unknown sugar. What is the structure of that unknown sugar?
D-Idose
CH
2
OH
H
OHH
HO
OHH
HHO
CHO
D-Gulose
CH
2
OH
H
OHH
HO
OHH
OHH
CHO
22.3b Shortening Chains in Carbohydrates The Ruff degradation (Otto
Ruff, 1871–1939) is a method of shortening the backbone of a sugar by removal of
the aldehyde at C(1) and creating a new aldehyde at what was C(2) in the starting
sugar (Fig. 22.21). In this process, the aldehyde carbon of an aldose is first oxidized.
Then, the calcium salt of the resulting carboxylic acid is treated with ferric ion and
hydrogen peroxide to cause decarboxylation and oxidation of what was the C(2) carbon.
Ca Salt of
D-galactonic acid
CH
2
OH
H
OHH
HO
HHO
OHH
COO
–
Ca
2
D-Galactose
CH
2
OH
H
OHH
HO
HHO
OHH
CHO
D-Lyxose
(41%)
CH
2
OH
H
OH
HO
H
HHO
CHO
1. Br
2
/H
2
O
1
2. Ca(OH)
2
2
1. Fe
2
(SO
4
)
3
H
2
O/100 ⬚C
1
2. 30% H
2
O
2
2
+
FIGURE 22.21 The Ruff
degradation shortens the starting
sugar by one carbon. It is the
original aldehyde carbon that is
lost. In this example, the aldehyde
carbon of
D-galactose is oxidized
to give
D-galactonic acid, which is
converted into
D-lyxose.
1140 CHAPTER 22 Carbohydrates
The mechanism of this reaction remains obscure (chemistry is a living science—not
everything is fully understood) but one imagines that radicals are involved. Metal
ions can act as efficient electron-transfer agents, and a guess at the mechanism
might involve formation of the carboxyl radical, decarboxylation, capture of the
hydroxyl radical formed, and a final transformation of the hydrate into the alde-
hyde. Whatever the details of the mechanism, the stereochemistry at the original
C(2) is lost, but there can be no changes at the old C(3), C(4), or C(5).
Just as Nature can synthesize sugars from smaller carbon chains, there is a nat-
ural process called glycolysis (Fig. 22.22) that breaks down glucose into two mol-
ecules of a three-carbon compound called pyruvate.This chain-shortening pathway
is a critical part of the metabolism that occurs in almost all organisms. Glycolysis
provides cells with adenosine triphosphate (ATP) and NADH (p. 814).
HO
OH
+ 2 NAD
+
+ 2 ADP + 2 NADH2 + 2 ATP
O
OH
␣-D-Glucopyranose Pyruvate
+ phosphate
– H
2
O
– H
+
HO
OH
O
–
O
O
FIGURE 22.22 Glycolysis is a significant metabolic pathway. It involves ten intermediates and ultimately leads to
pyruvate. Energy storage is a side reaction in glycolysis that stores the glucose until energy demands bring it back into
the metabolic pathway.
Summary
Nature forms,stores,and uses chemical energy.Carbohydrates are the central chem-
ical in this dance of life. Chemists manipulate saccharides many ways, as well.
The Kiliani–Fischer synthesis lengthens a carbohydrate chain by one carbon atom.
The Ruff degradation shortens the chain by one carbon. Both reactions occur with-
out disturbing the remaining stereogenic carbons.
22.4 Reactions of Carbohydrates
22.4a Mutarotation Pure, crystallized α-D-glucopyranose has a specific
rotation (p. 159) of 112°. The pure β anomer has a specific rotation of 18.7°.
However, an aqueous solution of either anomer steadily changes specific rotation
until the value of 52.7° is reached.This phenomenon is called mutarotation and
is common for sugars. Our task is to explain it. The answer comes from the real-
ization that both α- and β-
D-glucopyranose are hemiacetals and exist in solution
in equilibrium with a small amount of the open-chain aldohexose (Fig. 22.23).
CH
2
OH
CHO
OH
H
H
HO
OH
OH
H
H
Open chain
(trace)
β Anomer
(64%)
HO
HO
α Anomer
(36%)
OH
O
OH
OH
HO
HO
OH
O
OH
OH
FIGURE 22.23 The α and β anomers
of a sugar can equilibrate through the
very small amount of the open form
present at equilibrium.
22.4 Reactions of Carbohydrates 1141
Once the acyclic form is produced, it can re-form the hemiacetal to make both the
α- and β-pyranose. Over time, an equilibrium mixture is formed and it is that
equilibrated pair of anomers that gives the specific rotation of 52.7°.
D-Fructose
(30.9%)
CH
2
OH
CH
2
OH
H
OH
OHH
H
HO
O
D-Glucopyranose
(63.4%)
HO
HO
OH
O
OH
OH
OH
D-Mannopyranose
(2.4%)
HO
HO
O
OH
OH
Ca(OH)
2
H
2
O
10 days
Ca(OH)
2
H
2
O
10 days
FIGURE 22.24 In base, D-glucopyranose equilibrates with D-mannopyranose and D-fructose.
PROBLEM 22.14 Write a mechanism for the acid-catalyzed mutarotation of
D-glucopyranose.
4
This “name reaction” is even better than it sounds. One might be forgiven for assuming that someone meet-
ing these two chemists on a Dutch street in 1880 might say, “Goedemorgen, Lobry; hoe gaat het, Alberda?,”
should he or she be so presumptuously familiar as to address them by their first names. But that assumption
would be dead wrong! The reaction title, alas, incorporates only parts of their names. The reaction is named
for Cornelius Adriaan van Troostenbery Lobry de Bruijn (1857–1904) and the slightly less spectacularly named
Willem Alberda van Ekenstein (1858–1937).
PROBLEM 22.15 Although D-fructose is shown in Figure 22.24 in the open-chain
form, it exists mostly ( 67%) in the pyranose form and partly ( 31%) in the
furanose form in which the hydroxyl group at C(5) is tied up in hemiacetal for-
mation with the ketone. Draw Fischer projections for the pyranose and furanose
forms of
D-fructose.
''
The mechanism of the Lobry de Bruijn–Alberda van Ekenstein reaction is sim-
pler than its title. As we have pointed out several times now, the predominant cyclic
forms of these sugars are in equilibrium with small amounts of the open-chain iso-
mers. In base, the α hydrogen in the open aldo form can be removed to produce a
22.4b Epimerization in Base In base, sugars rapidly equilibrate with other
sugars, which is a more profound change than the equilibration of anomers
we saw in the preceding section. Again, let’s use D-glucose as an example. As
shown in Figure 22.24,
D-glucopyranose equilibrates with another D-aldohexose,
D-mannopyranose, and a ketose, D-fructose, when treated with aqueous base. This
process goes by the absolutely delightful name of the Lobry de Bruijn–Alberda van
Ekenstein reaction.
4
1142 CHAPTER 22 Carbohydrates
D-Glucose
(open form)
OH
OH
H
OH
OHH
H
H
HO
CH
2
OH
base
H
2
O
protonate
base
protonate
H
2
O
OH
H
OH
OHH
H
HO
HHO
CH
2
OH
O
Resonance-stabilized
enolate
H
OH
OHH
H
HO
CH
2
OH
D-Mannose
(open form)
–
OH
H
D-Glucopyranose
HO
HO
OH
O
OH
D-Mannopyranose
HO
HO
O
OH
OH
OH
OH
FIGURE 22.25 A mechanism for
the equilibration of
D-glucose and
D-mannose involves formation of an
enolate followed by reprotonation.
resonance-stabilized enolate (Fig. 22.25). Reprotonation regenerates D-glucose, if
reattachment occurs from the same side as proton loss, but
D-mannose if reattach-
ment is from the opposite side.
CH
2
OH
OH
H
OH
OHH
H
HO
OH
H
OH
OHH
H
HO
CH
2
OH
O
H
O
H
Enolate
(remember that protonation
on carbon gives
D-glucose
and
D-mannose)
–
CH
2
OH
OH
H
OH
OHH
H
HO
protonate on
oxygen
O
H
OH
OHH
H
HO
CH
2
OH
D-FructoseDouble enol
–
HO
H
HO
FIGURE 22.26 Protonation on oxygen of the enolate generates a double enol, which leads to D-fructose, D-glucose, or
D-mannose.
PROBLEM 22.16 Make a three-dimensional drawing of the enolate in Figure 22.25
to show how both D-glucose and D-mannose can be formed on reprotonation.
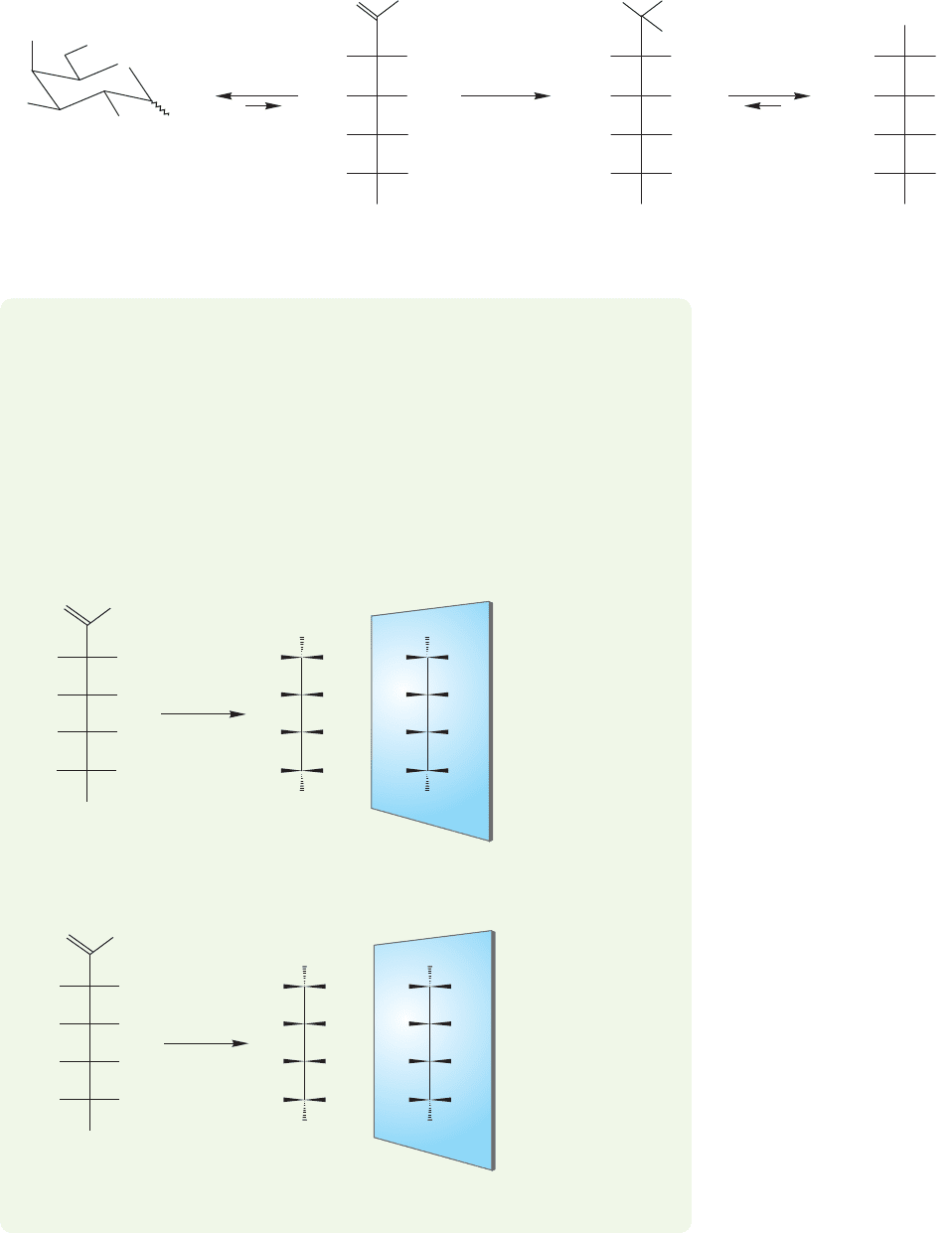
If protonation occurs at the enolate oxygen,a “double enol”shown in Figure 22.26
is formed.This compound can re-form a carbon–oxygen double bond in two ways.
The double enol can form either an aldehyde or a ketone. If an aldehyde is gener-
ated,the epimeric aldohexoses
D-glucose and D-mannose shown in Figure 22.25 are
produced. If a ketone is formed, it is
D-fructose that is the product.
22.4 Reactions of Carbohydrates 1143
22.4c Reduction We have already seen one aldose reduction, the reaction of
D-glucose with sodium borohydride to give 1,2,3,4,5,6-hexanehexaol (Fig. 22.10).
Any sugar that has a hemiacetal can be reduced by these conditions (Fig. 22.27).
NaBH
4
OH
H
H
OHH
HO
H
HO
CH
2
OH
H
2
O
OH
H
H
OHH
HO
H
HO
CH
2
OH
CH
2
OH
H
H
OH
OHH
HO
H
HO
CH
2
OH
D-Galactopyranose
HO
HO
OH
O
OH
OH
D-Galactose
(open form)
Alkoxide 1,2,3,4,5,6-
Hexanehexaol
O
H
H
H
O
–
FIGURE 22.27 Reduction of
D-galactopyranose to convert the
aldehyde into an alcohol proceeds
through the small amount of the
open, aldo form present at
equilibrium. As the open form is
used up, it is regenerated through
equilibration with the pyranose form.
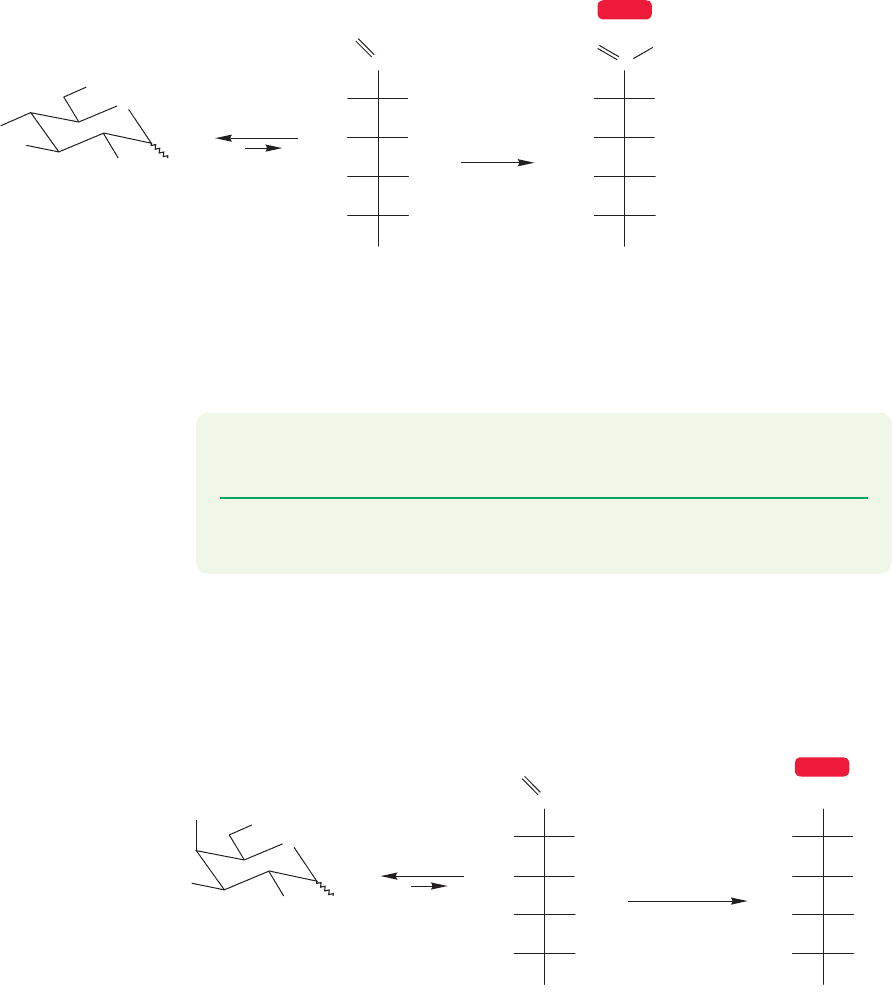
WORKED PROBLEM 22.17 Reduction of D-altrose with sodium borohydride in
water gives an optically active molecule, D-altritol. However, the same procedure
applied to D-allose gives an optically inactive product. Explain.
ANSWER This problem is easy, but it is critical for understanding the upcoming
material describing the Fischer proof of the stereochemistry of glucose. The
important point is to see that reduction of the aldehyde to the alcohol makes the
molecule more symmetrical by making both ends of the chain CH
2
OH groups.
D-Allose becomes a meso compound (and therefore optically inactive) because
the reduced product now has a plane of symmetry. Reduction of D-altrose gives
an alcohol that lacks symmetry and is still optically active.
O
H
OHH
OHH
Open form of
D-allose
(optically active)
Product
(meso)
Mirror image
of product
The product and
its mirror image
are superimposable,
therefore the
product is
optically inactive
The product and
its mirror image
are not superimposable,
therefore the
product is
optically active
CH
2
OH
CH
2
OH
CH
2
OH
H
2
O
NaBH
4
OHH
OH
OH
OH
OH
OHH
H
H
H
H
CH
2
OH
CH
2
OH
H
H
H
H
HO
HO
HO
HO
Mirror
Mirror
OHH
HHO
Open form of
D-altrose
(optically active)
Product
(optically active)
CH
2
OH
CH
2
OH
CH
2
OH
H
2
O
NaBH
4
OHH
OH
OH
H
OH
OHH
H
H
H
CH
2
OH
CH
2
OH
H
H
H
HO
H
HO
HO
HO OH
Mirror image
of product
O
H
1144 CHAPTER 22 Carbohydrates
22.4d Oxidation Sugars contain numerous oxidizable groups, and methods
have been developed in which one or more of them may be oxidized in the pres-
ence of the others. Mild oxidation converts only the aldehyde into a carboxylic acid;
the primary and secondary hydroxyls are not touched. The reagent of choice is
bromine in water, and the product is called an aldonic acid (Fig. 22.28).
CH
O
OH
H
OH
OHH
H
H
HO
CH
2
OH
Br
2
O
H
2
O
0 ⬚C
H
2
O
D-Glucose
(open form)
C
OH
H
OH
OH
OH
H
H
H
HO
CH
2
OH
D-Gluconic acid
(~ 96%)
In an aldonic acid,
the end groups are
still different
HO
D-Glucopyranose
HO
OH
O
OH
OH
WEB 3D
FIGURE 22.28 Oxidation of an aldose with bromine in water gives an aldonic acid. Note that the functional groups at
the two ends of the molecule are still different from each other.
PROBLEM 22.18 Write a mechanism for the oxidation shown in Figure 22.28.
Hint for the first step: What reaction is likely between an aldehyde and water?
PROBLEM 22.19 Examination of the NMR and IR spectra of typical aldonic acids
often shows little evidence of the carboxylic acid group. Explain this observation.
CH
O
OH
H
OHH
H
HO
HHO
CH
2
OH
NaNO
2
H
2
O
HNO
3
0 ⬚C, 4 h
D-Galactose
(open form)
COOH
OH
H
OHH
H
HO
HHO
COOH
D-Galactaric acid
(89%)
D-Galactopyranose
HO
HO
OH
O
OH
OH
WEB 3D
FIGURE 22.29 Oxidation of a
sugar with nitric acid generates an
aldaric acid, which has a carboxylic
acid group at each end of the
molecule.
Because the hemiacetal present in most sugars is a latent aldehyde, and because
an aldehyde is susceptible to oxidation, several oxidants have been used to detect
sugars. These metal reagents (usually copper based) undergo a color change as the
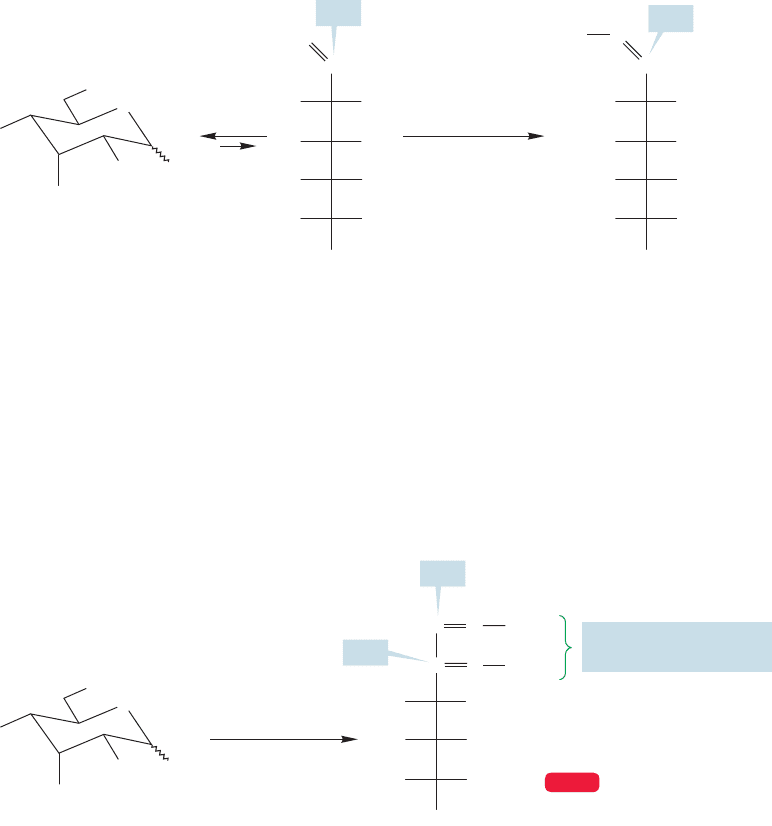
More vigorous oxidation of a sugar with nitrous or nitric acid creates an aldaric
acid in which both the aldehyde and the primary alcohol have been oxidized to car-
boxylic acids (Fig. 22.29). Notice that this reaction destroys the difference between
the two ends of the molecule.There is an acid group at both the top and bottom of
the Fischer projection.
22.4 Reactions of Carbohydrates 1145
aldehyde is oxidized and the metal reduced. It is this kind of redox reaction that has
given us the term reducing sugar. If a sugar has a hemiacetal, it is in equilibrium with
an aldehyde and so can reduce the metal reagent.
In even more vigorous oxidations, the secondary hydroxyl groups are attacked and
the six-carbon backbone can be ruptured.You already know that vicinal diols (1,2-diols)
are cleaved on treatment with periodate (p. 807). When sugars are treated with perio-
date, mixtures of products are obtained, as the various 1,2-diols are cleaved. Treatment
with periodate can chop the carbon backbone into small fragments, and the structures
of the fragments can often be used in structure determination.
22.4e Osazone Formation Treatment of aldoses with phenylhydrazine under
acidic conditions initiates an odd and very useful reaction. What would we expect? We
know that the cyclic forms of aldoses are in equilibrium with the open-chain isomers,
which contain a free aldehyde group. Aldehydes react with substituted hydrazines to
generate hydrazones (p. 793), and so it is no surprise to see the reaction of Figure 22.30
in which the carbonyl group at C(1) has formed a phenylhydrazone derivative.
CH
O
CH
2
OH
H
3
O
+
OH
PhNHNH
2
OH
OH
OHH
H
H
H
D-Allose
(open form)
CH
N
PhNH
CH
2
OH
OH
OH
OH
OHH
H
H
H
A phenylhydrazone
C(1)
C(1)
D-Allopyranose
HO
OH
O
OH
OH
OH
FIGURE 22.30 The small amount of free aldehyde present at equilibrium accounts for
phenylhydrazone formation at C(1).
What is a surprise is to find that in the presence of excess hydrazine not only C(1)
but also C(2) is transformed into a phenylhydrazone.The product is a 1,2-diphenyl-
hydrazone, called an osazone (Fig. 22.31). Here are two clues to the mechanism of
the reaction.First, it takes three equivalents of phenylhydrazine to complete the reac-
tion and, second, aniline and ammonia are by-products.
HO
OH
O
OH
OH
OH
CH
3
COOH
3 PhNHNH
2
Both C(1) and C(2) are
now phenylhydrazones
NH
2
NH
3
Ph
NHPh
NHPh
HC
C
CH
2
OH
N
N
OH
OHH
H
OHH
An osazone
(76%)
++
2 H
2
O+
C(1)
C(2)
D-Allopyranose
WEB 3D
FIGURE 22.31 Osazone formation
from an aldose involves conversion
of both C(1) and C(2) into
phenylhydrazones.
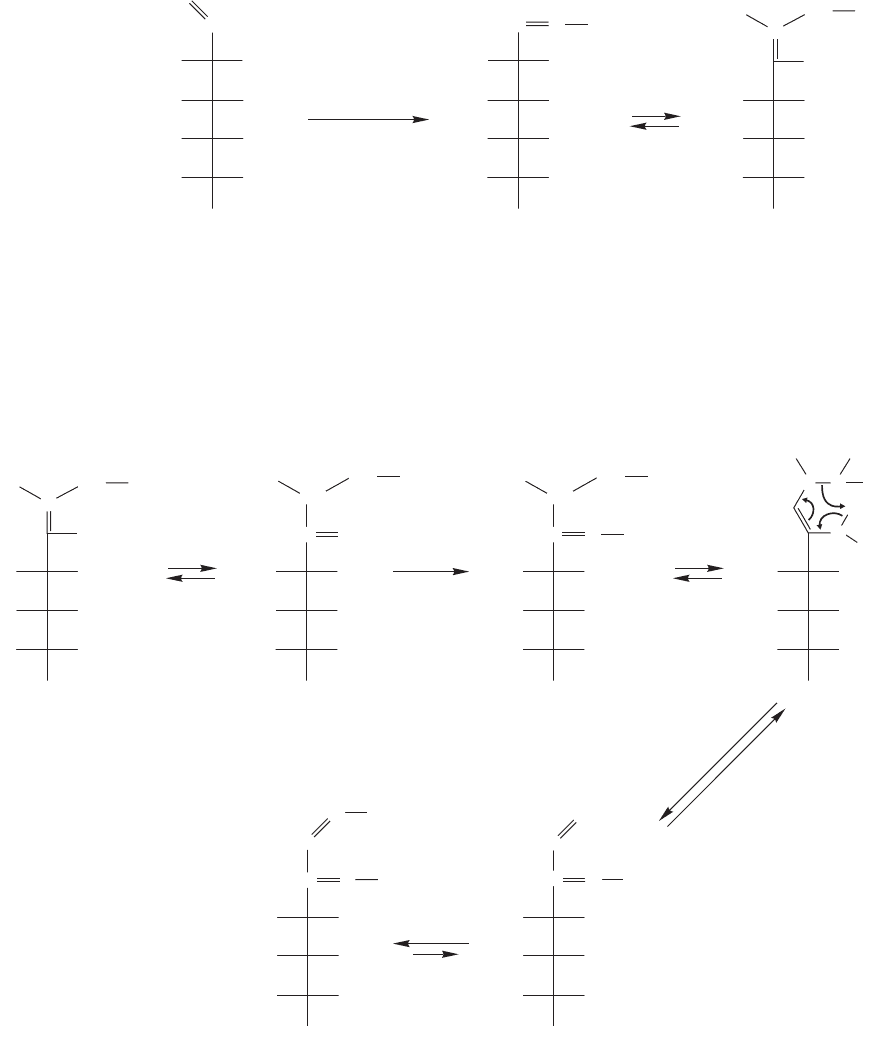
The mechanism begins with the reaction at C(1) to form a phenylhydrazone
(p. 793), which consumes the first equivalent of phenylhydrazine.This hydrazone is
an imine and imines are in equilibrium with enamines (p. 796), just as ketones are
in equilibrium with enols.The imine–enamine equilibrium is shown in Figure 22.32.
1146 CHAPTER 22 Carbohydrates
CH
O
CH
2
OH
OH
OH
OHH
H
OHH
H
OHH
PhNHNH
2
NHPhHC
CH
2
OH
N
OH
OHH
H
OHH
Phenylhydrazone,
an imine
OH
NHPh
CH
2
OH
OH
OHH
H
OHH
An enamine and an enol
C
HNH
FIGURE 22.32 The
phenylhydrazone formed when
an aldose reacts with
phenylhydrazine is an imine
and therefore in equilibrium
with an enamine.This enamine
is also an enol.
HH
PhNHNH
2
PhNHNH
2
CO
CH
2
OH
OH
OHH
H
OHH
Ketone
HC
CH
2
OH
NH
OH
OHH
H
OHH
Diimine
Aniline
OH
NHPh
CH
2
OH
OH
OHH
H
OHH
A
n enol and an enamine
C
HNH
NHPh
H
NH
NHPhN
CH
C
CH
2
OH
OH
OHH
H
OHH
An osazone
NHPh
N
HC
C
CH
2
OH
OH
OHH
H
OHH
New phenylhydrazone
NHPh
H
NH
CH
NHPh
NH
2
Ph
NH
3
N
NHPh
N
C
CH
2
OH
OH
OHH
H
OHH
NHPh
+
+
N N
N
H
Ph
FIGURE 22.33 Reaction between
phenylhydrazine and the ketone
form of the substituted enol leads
to a phenylhydrazone different
from the one formed in the
reaction of Figure 22.32. Aniline
is now eliminated to give a
diimine. Reaction with a third
equivalent of phenylhydrazine
leads to the osazone.
Notice that osazone formation destroys the stereogenicity of C(2). Thus,
the same osazone can be formed from two sugars that are epimeric at C(2).
Figure 22.34 makes this point using D-glucose and D-mannose.When we work
through the Fischer proof of the structure of glucose and the other aldohex-
oses, we will make use of this point a number of times, so remember it.
In this case, the enamine is also an enol and is therefore in equilibrium with a ketone
that can react with a second equivalent of phenylhydrazine (Fig. 22.33). Now ani-
line (PhNH
2
) is eliminated through a series of steps, perhaps as shown in Figure
22.33, to give a diimine, which reacts with the third equivalent of phenylhydrazine
in an exchange of one imine for another to give the final osazone product.
22.4 Reactions of Carbohydrates 1147
PhNHNH
2
PhNHNH
2
HC
CH
2
OH
H
OH
OHH
H
HO
The same osazone!
CH
2
OH
H
OH
OHH
H
HO
HHO
D-Mannose
O
CH
NHPhN
NHPh
N
C
C(1)
C(2)
CH
2
OH
H
OH
OHH
H
HO
OHH
D-Glucose
O
CH
C(1)
C(2)
FIGURE 22.34
An osazone can
be formed from
two different
carbohydrates that
are epimeric at C(2).
The stereochemistry
at C(2) is destroyed
in the reaction.
FIGURE 22.35 Treatment of a sugar
with excess methyl iodide and silver
oxide leads to formation of a
polyether, in this case a hexaether.
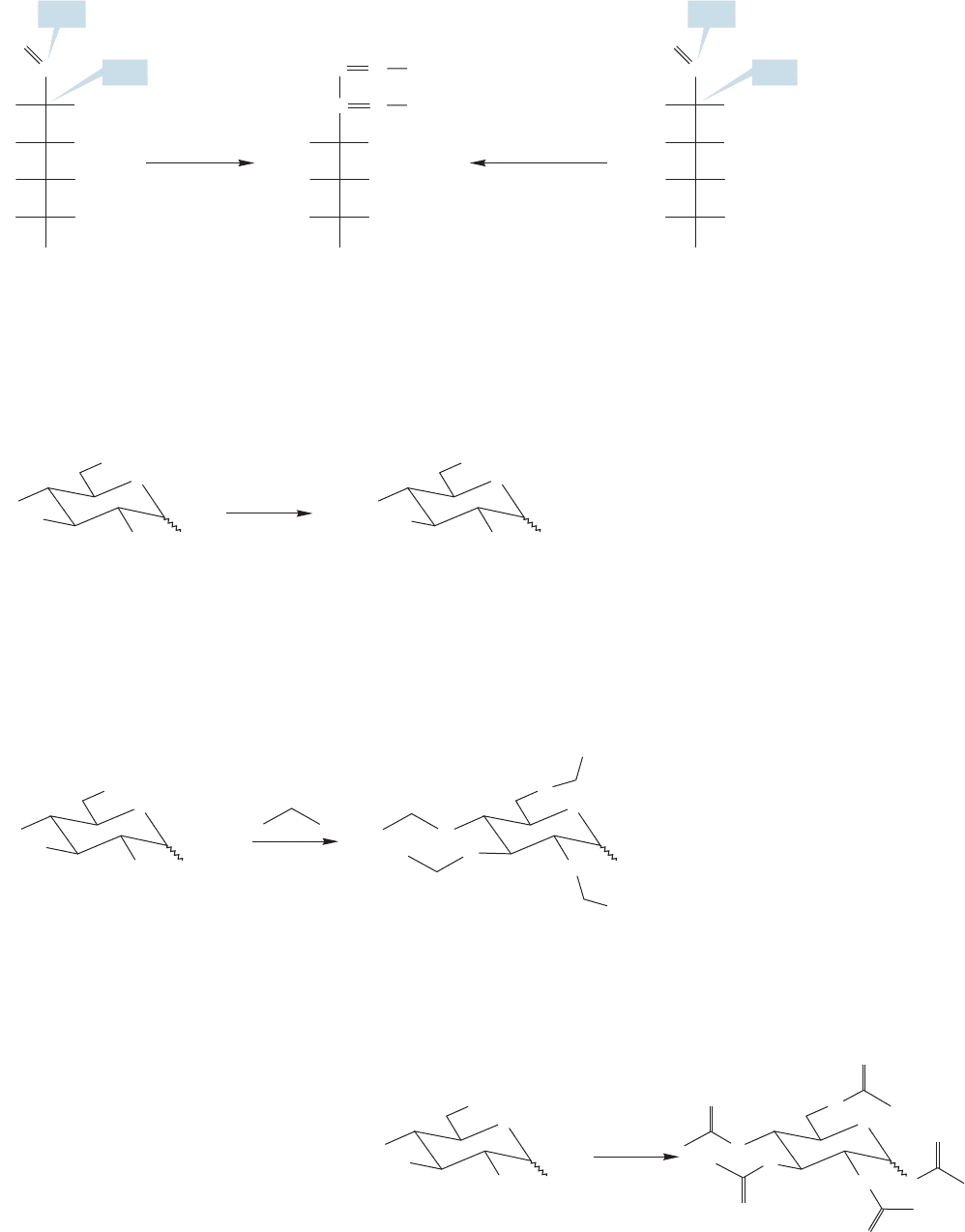
22.4f Ether and Ester Formation Alcohols can be made into ethers. One
classic method in carbohydrate chemistry involves the treatment of a sugar with
excess methyl iodide and silver oxide, which leads to methylation at every free
hydroxyl group in the molecule to form a polyether (Fig. 22.35).
HO
excess
KOH
dioxane
Methyl
D-Glucopyranoside
HO
OH
O
OCH
3
OH
O
Ph
(~ 95%)
O
O
O
OPh
Ph
Ph
ClPh
OCH
3
FIGURE 22.36 Benzylation through a
series of Williamson ether syntheses.
HO
excess
CH
3
I
Ag
2
O
D-Glucopyranose
HO
OH
O
OH
OH
CH
3
O
(~ 55%)
Methyl 2,3,4,6-tetramethoxy-
D-glucopyranoside
CH
3
O
OCH
3
OCH
3
O
OCH
3
difference between the alkoxy group at C(1) and the four benzyl ethers.The C(1) alkoxy
group is part of an acetal. The acetal is stable in basic conditions. If the ether-forming
reaction were run on the hemiacetal, then the basic conditions would drive the sugar to
the open-chain form and lead to multiple
products.But use of the acetal produces the
polyether in which neither the alkoxy group
at C(1) nor the pyranose ring connection is
disturbed in the benzylation reaction.
A carbohydrate reacting with acetic
anhydride or acetyl chloride will produce
a polyacetylated compound in which all of
the free hydroxyl groups are esterified
(Fig. 22.37).
98 ⬚C, 8.5 h,
acid catalyst
O
O
O
O
O
O
HO
D-Glucopyranose
HO
OH
O
OH
A pentaester
(58%)
O
O
O
O
excess
Ac
2
O
OH
O
FIGURE 22.37 The free hydroxyl groups of
glucopyranose are esterified with acetic anhydride.
One of the classic S
N
2 reactions is the Williamson ether synthesis (p. 315), the for-
mation of an ether from an alkoxide and an alkyl halide. When an aldohexose is treat-
ed with benzyl chloride and potassium hydroxide,a series of Williamson ether syntheses
results in formation of a tetraether and an acetal (Fig. 22.36). Be careful to notice the
1148 CHAPTER 22 Carbohydrates
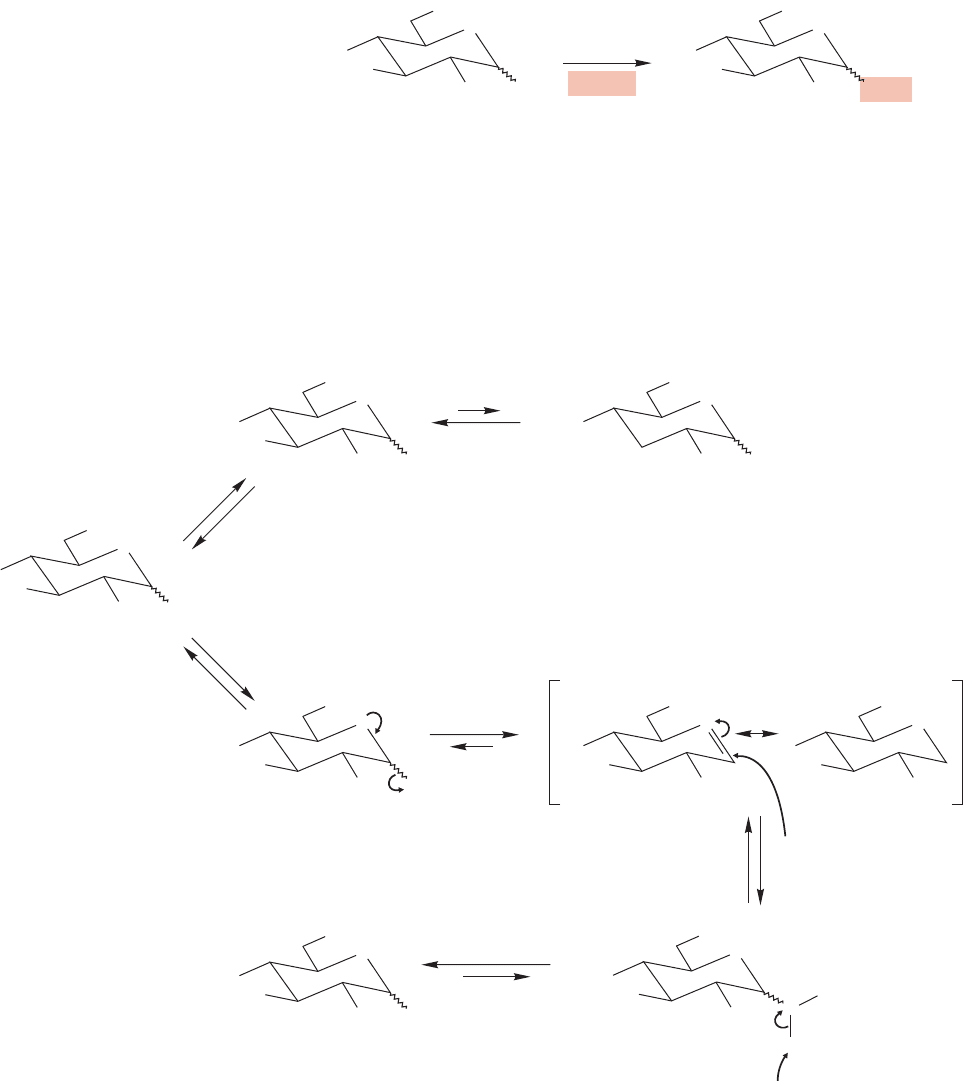
Notice that in both the alkylation and acylation reactions the oxygen tied up in
the ring remains unaffected by the reaction.It can form neither an ether nor an ester.
The presence of the hemiacetal form allows for selective ether formation when
a sugar is treated with dilute acidic alcohol. The product is an acetal (Fig. 22.38).
The generic name for an acetal of any sugar is glycoside. Pyranose glycosides are
called pyranosides, and furanose glycosides are furanosides.
Why is it that only the hydroxyl group at the anomeric carbon is substituted
by a methoxyl group in this reaction? The first step is protonation of one of the
many OH groups (Fig. 22.39). All the OH groups are reversibly protonated, but
only one protonation leads to a resonance-stabilized cation when a molecule of
cat.
HCl
CH
3
OH
64 ⬚C, 72 h
HO
D-Glucopyranose
HO
OH
O
OH
OH
HO
OCH
3
A glycoside (in this
case a pyranoside)
(~ 50%)
HO
OH
O
OH
FIGURE 22.38 Treatment of a sugar
with dilute HCl and alcohol converts
only the anomeric OH into an acetal.
Such a molecule is called a glycoside.
H
2
OR
HO
D-Glucopyranose
(a hemiacetal)
HO
OH
O
OH
A pyranoside
(an acetal)
HO HO
H
2
O
OH
O
OH
OH
O
OH
H
2
OR
protonation
H
2
O
elimination
+
+
+
+
+
+
+
+
HO HO
HO
OH
O
OH
OH
O
OH
HO
HO
OH
O
OH
HO
deprotonation
HO
HO
OH
O
OH
HO
HO
OH
O
R
H
OH
ROH
addition
ROH
ROH
2
OH OH
OH
OH
2
OR
O
+
+
FIGURE 22.39 All OH groups in a sugar can be reversibly protonated. In addition to the protonation at C(3) shown, the top
reaction represents protonation at C(2), C(4), and C(6).The bottom reaction shows protonation of the OH at the anomeric
C(1). Only in this intermediate can an oxygen “kick out” water and provide resonance stabilization for the resulting cation.
Addition of alcohol at C(1) followed by a deprotonation gives the glycoside.
