Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.

23.4 Peptide Chemistry 1189
..
O
..
..
O
..
..
N
H
H
H
R
R
R
R
H
3
N
..
N
..
N
..
O
..
O
..
..
..
..
O
..
..
R
H
3
N
O
..
..
–
–
..
O
..
..
R
H
3
N
O
..
..
–
..
O
..
..
R
H
3
N
O
..
..
–
..
O
..
..
R
H
3
N
O
..
..
–
Amide
bonds
Amide
bond
++
+
++
O
..
..
FIGURE 23.19 Peptides are
polyamino acids linked through
amide bonds.
By far, the most important reaction of amino acids is their polymerization to
peptides and proteins through the formation of new amide bonds (Fig. 23.19).The
following sections are devoted to this reaction and some of its consequences.
O
..
..
..
O
..
..
N
H
H
H
3
N
..
N
..
O
..
O
..
..
..
..
O
..
..
Alanine
A tripeptide: Alanylserylvaline
Amino
terminus
Carboxy
terminus
.
Ala Ser
Val
.
.
.
ASV
Serine Valine
H
3
N
O
..
..
–
–
..
O
..
..
..
..
H
3
N
O
..
..
–
..
O
..
..
H
3
N
O
..
..
–
Amide links to be made
OH
..
..
OH
++
+
+
WEB 3D
FIGURE 23.20 The tripeptide alanylserylvaline, .The amino acid at the amino terminus starts
the name, and the amino acid at the carboxy terminus ends it.
Ala
.
Ser
.
Val
Summary
Many of the reactions of carboxylic acids and amines appear in the chemistry of
amino acids.Acylation and Fischer esterification are two examples.There are some
new reactions, however, that involve both the amino and the acid groups. Reaction
with ninhydrin is an example.
23.4 Peptide Chemistry
23.4a Nomenclature and Structure Figure 23.20 shows the schematic
construction of a three amino acid peptide (a tripeptide) made from alanine, serine,
and valine. Don’t confuse this picture with real synthetic procedures—we will get to
them soon enough. Peptides are usually written with the amino terminus on the
left, proceeding toward the carboxy terminus on the right. They are always named
from the amino terminus to the carboxy terminus. So the tripeptide in Figure 23.20
is alanylserylvaline, or .Ala
.
Ser
.
Val, or A
.
S
.
V
N
H
H
H
H
3
N
..
N
..
COO
–
O
..
..
..
O
..
..
NH
2
Ph
..
..
N
H
H
H
3
N
HO
..
N
..
COO
–
O
..
..
O
..
..
O
..
..
SCH
3
N
..
..
..
+
+
PROBLEM 23.15 Draw the structures for
.
PROBLEM 23.16 Name the peptides drawn below.
Thr
.
PheGlu
.
andAsp
.
His
.
Cys,Pro
.
Gly
.
Tyr,
1190 CHAPTER 23 Amino Acids and Polyamino Acids (Peptides and Proteins)
A cross-linked pair of chains—
the link is a disulfide (S
S) bridge
O
Cys
..
..
O
..
..
..
..
N
H
..
N
H
..
H
N
..
R
oxidation
Disulfide
O
..
..
O
..
..
N
H
..
R
SH
..
..
SH
Cys
H
N
..
H
N
..
O
..
..
O
..
..
..
..
N
H
..
N
H
..
H
N
..
R
O
..
..
O
..
..
N
H
..
R
H
N
..
H
N
..
S
..
..
S
FIGURE 23.21 Formation of disulfide
bonds can link one polyamino acid
chain to another.
The sequence of amino acids in a peptide or protein constitutes its primary struc-
ture.These chains of amino acids are linked in another way.Connections between chains
or within the same chain are often formed through disulfide bridges (Fig. 23.21).
Cysteine is usually the amino acid involved in these bridges, which are made through
oxidation of the thiol groups (p. 809).
There are other important structural features of polyamino acids. The amide
groups prefer planarity and the hydrogen bonds between the NH hydrogens and
amide carbonyl groups, along with the structural constraints imposed by the disul-
fide links, lead to regions of order in most large peptides.
23.4 Peptide Chemistry 1191
WORKED PROBLEM 23.17 Explain why amides prefer planarity.
ANSWER Amides are stabilized by resonance.
NH
2
R
R
R
C
CC
O
O
=
–
..
..
..
..
..
..
NH
2
NH
2
O
+
This resonance stabilization is maximized when the 2p orbitals on N, C, and O
overlap as much as possible, which requires sp
2
hybridization and planarity.
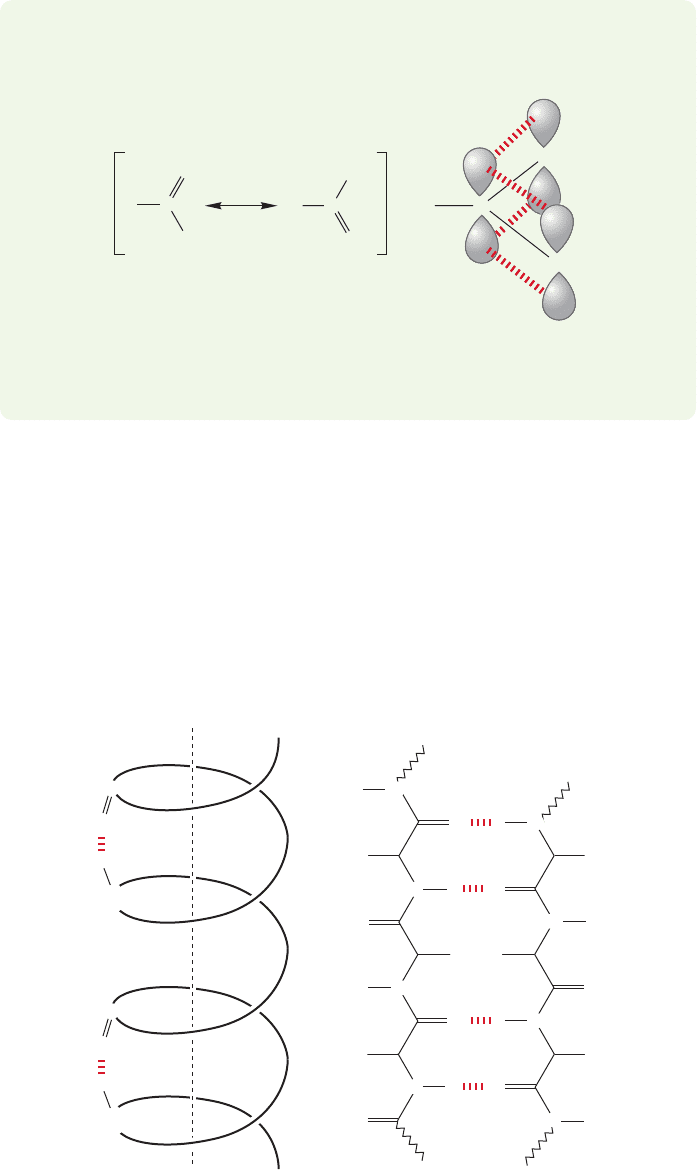
α-Helix
Axis of helix
β-Pleated sheet
N
..
..
R
R
R
R
R
R
H
N
N
N
N
N
N
H
H
H
..
..
H
H
N
H
..
..
..
..
..
O
..
..
O
..
..
O
..
..
O
..
O
..
..
H
O
..
..
O
..
..
H
O
..
..
C
N
..
H
O
..
..
C
N
..
FIGURE 23.22 Two examples
of ordered secondary structure
in a polypeptide, the α-helix and
β-pleated sheet.
There are even higher structural orders for these molecules. It might have been
that polypeptides were best described as ordered regions of secondary structure
(α-helix or β-pleated sheets) connected by sections of random coil. In such a case,
Especially common are regions arranged in an ␣-helix and repeating folded sec-
tions known as -pleated sheets (Fig. 23.22). Both of these regions allow regular
patterns of hydrogen bonding to develop, thereby imposing order on the sequence
of amino acids. In the regions that appear as an α-helix the hydrogen bonds are
between the coils of the helix, whereas in the pleated sheets they hold lengths of the
chains in roughly parallel lines. Other regions consist of a disordered, random coil,
series of amino acids. The combination of these structural features determines the
folding pattern, or secondary structure of the polypeptide.
a protein would have no fixed overall structure beyond the sections defined by
secondary structure. But this idea has been shown not to be correct. X-Ray
structure determinations of crystallized proteins have revealed these species to
have specific, three-dimensional structures.
3
This tertiary structure of the pro-
tein is determined partly by hydrogen bonding, but also by van der Waals and
electrostatic forces.These giant molecules are composed of a carbon backbone
to which the amino acid side chains,or R groups,are attached.These R groups
are either hydrocarbon-like, nonpolar groups such as methyl,isopropyl,or ben-
zyl, or highly polar amino, hydroxyl, or sulfhydryl groups. In the natural envi-
ronment of a protein, water, it should be no surprise to find that the protein
adjusts its shape so that the polar, hydrophilic groups are aimed outward toward
the polar solvent, whereas the nonpolar,“greasy”hydrophobic hydrocarbon por-
tions cluster inside the molecule, maximally protected from the hostile aque-
ous environment (Fig. 23.23).
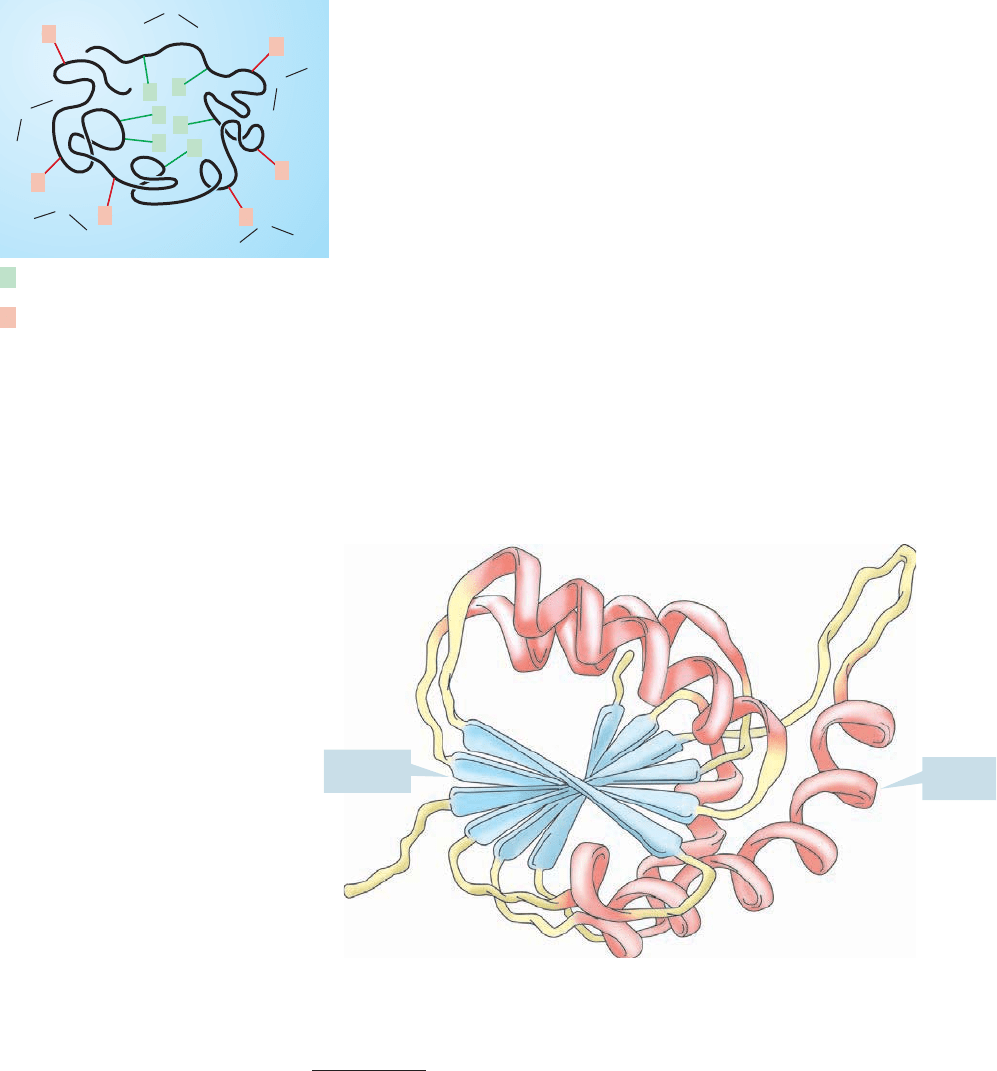
Many proteins adopt globular shapes such as that shown in Figure 23.23, which
maximizes interior hydrophobic and exterior hydrophilic interactions. Other proteins
are fibrous, taking the form of a superhelix composed of ropelike coils of α-helices.
Figure 23.24 shows a typical protein, the enzyme lactate dehydrogenase,which incor-
porates five α-helices and six β-sheets.
1192 CHAPTER 23 Amino Acids and Polyamino Acids (Peptides and Proteins)
3
It is a general problem that until recently the structures of proteins could only be determined by X-ray
diffraction studies on crystallized materials. Work was limited by the availability of crystals, and crystal-
lization of these molecules is usually difficult. Successful formation of X-ray quality crystals of a protein is
cause for celebration (and publication) even before the work of structure determination begins. But how do
we know that the structure of the molecule in solution is the same as that in the solid state crystal?
Remember, biological activity is tied intimately to the detailed structure of these molecules—might we not
be led astray by a structure that owed its shape to crystal packing forces in the solid state,and that was quite
different in solution? Indeed we might. These days, it is becoming possible to use very high-field NMR
spectrometers,along with increasingly sophisticated NMR pulse techniques,to determine structures in solu-
tion. Remember that high-field spectrometers are anything but cheap; the cost is about $10
3
per megahertz,
often of your tax dollars.
α-Helix
β-Sheets
FIGURE 23.24 Lactate dehydrogenase is a large protein that has well-defined tertiary
structure.
R
R
R
R
R
R
R = Hydrocarbon-like,
nonpolar side chains
R = Polar side chains
R
R
R
R
R
R
H
O
H
H
O
H
H
O
H
H
O
H
H
O
H
FIGURE 23.23 A globular protein,
ordered so as to put the nonpolar side
chains in the inside of the “glob” and
the polar side chains outside
interacting with the polar solvent
medium.
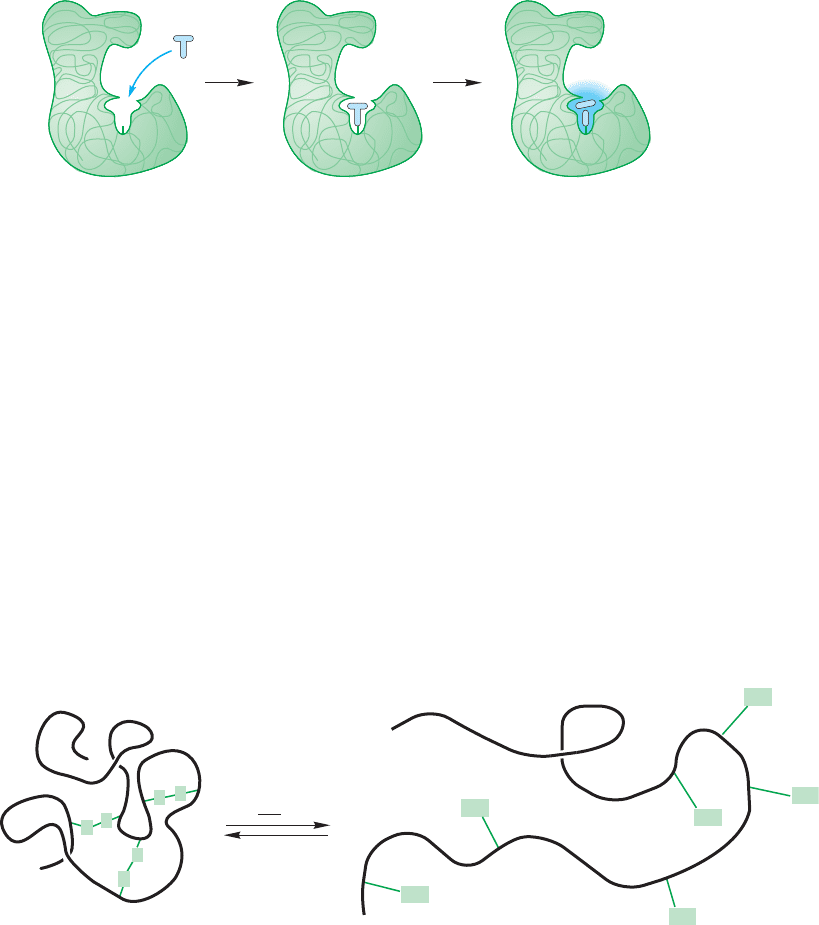
23.4 Peptide Chemistry 1193
This tertiary structure of a protein is extraordinarily important because it deter-
mines the shape of these huge molecules and biological activity depends intimately
on these shapes. Evolution has produced proteins that have pockets into which spe-
cific molecules called substrates fit. This enzyme–substrate binding allows for the
proper orientation for reaction of the substrate.The reaction could be complicated,
or as simple as the hydrolysis of an ester. In other molecules, substrates are bound
only for transportation purposes.These binding sites are a direct consequence of the
primary structure that determines the secondary and tertiary structures. Figure 23.25
gives a schematic representation of this process.
FIGURE 23.25 A binding site in a
protein captures (binds) a small
molecule for transport, or reaction
with appropriately located reactive
groups.
How do we know that the same tertiary structure is adopted by every pro-
tein molecule of a given amino acid sequence? One clue is that synthetic pro-
teins have the same biological activity as the natural proteins. If we reproduce
the primary sequence of a protein correctly, the proper biological activity
appears. Because this biological activity depends directly on the details of shape,
we can conclude that the molecule must be folding properly into the appropri-
ate tertiary structure.
Another clue comes from experiments in which the tertiary structure of a pro-
tein is deliberately disrupted, which is called denaturing the protein. Many ways
of denaturing a protein are irreversible.When an egg is cooked, the thermal ener-
gy denatures the proteins in the egg white in an irreversible way. Once the egg is
fried, there is no unfrying it. Similar chemistry can be induced by pH change.The
curdling of milk products is an example. But some denaturing reactions are
reversible. For example, disulfide bonds in a protein can be broken through treat-
ment with thiols (Fig. 23.26). This disruption of the secondary structure trans-
forms the ordered sections of the protein into the random coil arrangements.
S
S
S
S
SH
SH
SH
SH
SH
HS
Active globular protein
R
SH
oxidize
Denatured (random coil)
S
S
FIGURE 23.26 A reversible method of denaturing proteins, of destroying the secondary and
tertiary structure, is to break the disulfide bonds. Air oxidization re-forms the disulfides;
the higher order structures are regenerated, and biological activity is reestablished.
1194 CHAPTER 23 Amino Acids and Polyamino Acids (Peptides and Proteins)
Similar reversible disruptions of order can be achieved through treatment with urea
(in a reaction whose precise mechanism remains unknown), or, sometimes, through
heating.If these denatured proteins are allowed to stand,the thiol groups are reox-
idized by air to the appropriate disulfide linkages and the original tertiary struc-
ture with its biological activity returns!
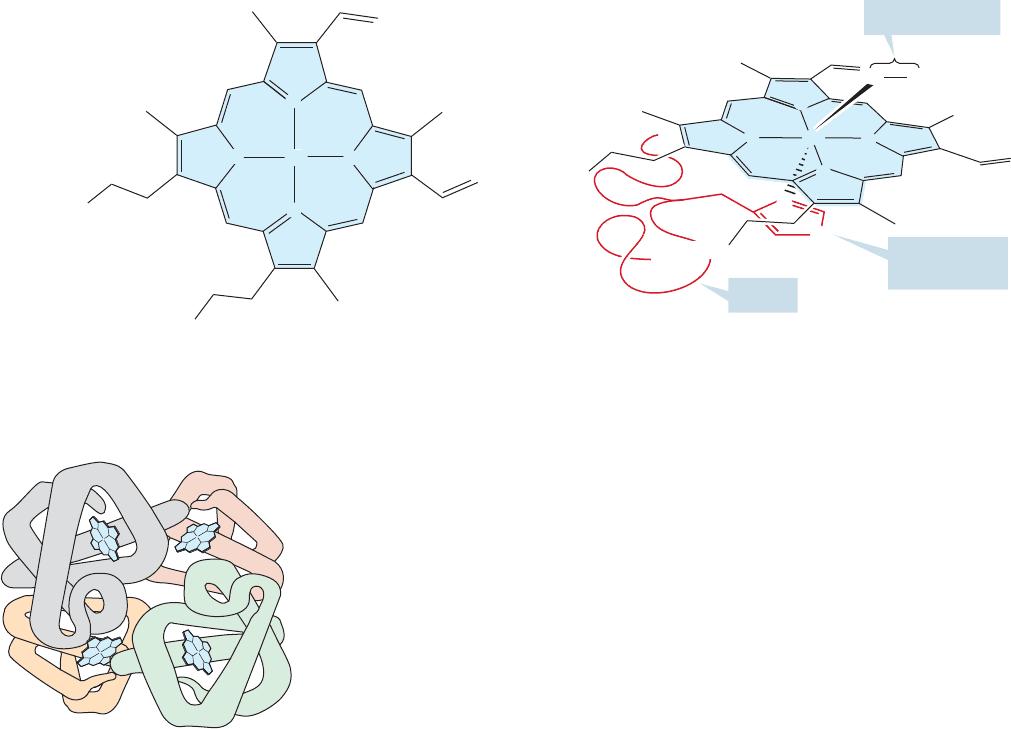
There is even higher order to some proteins. Particularly large proteins are
collections of smaller polypeptides held together by intramolecular attractions—
van der Waals and electrostatic forces—into superstructures with quaternary
structure. The classic example of quaternary structure is hemoglobin, the pro-
tein responsible for oxygen absorption and transport in humans. To accomplish
these biological functions the protein incorporates four heme units,each consist-
ing of an iron atom surrounded by heterocyclic rings which coordinate with the
metal (Fig. 23.27).
N
Imidazole
from histidine
HOOC
HOOC
Heme Bound heme unit
N
N
N
N
Protein
Fe
Fe
Coordinated O
2
N
N
N
N
OO
N
H
HOOC
HOOC
FIGURE 23.27 The heme unit and an oxygen-coordinated heme structure of hemoglobin.
The protein surrounding the heme is called globin, and in each globin subunit
a histidine side chain (an imidazole) is poised to hold the heme in position. There
are four subunits in hemoglobin, two pairs of slightly different peptide chains
(Fig.23.28).These are held together by electrostatic and van der Waals forces as well
as hydrogen bonding, and take the shape of a giant tetrahedron.
Given all this general information about structure,we still face two difficult prob-
lems. First, how do we determine the amino acid sequence of an unknown protein,
and second, once we know that structure, how can we synthesize it?
23.4b Determination of Protein Structure If suitable crystals can be
obtained, and if the X-ray diffraction pattern for the crystal can be solved in a rea-
sonable amount of time, the structure of the protein is apparent. However, this
process is not yet a general procedure. Crystallization of these molecules remains
difficult, and although the determination of the structure of small molecules by
X-ray is now routine and rapid, the determination of the structures of proteins
remains difficult and time consuming.
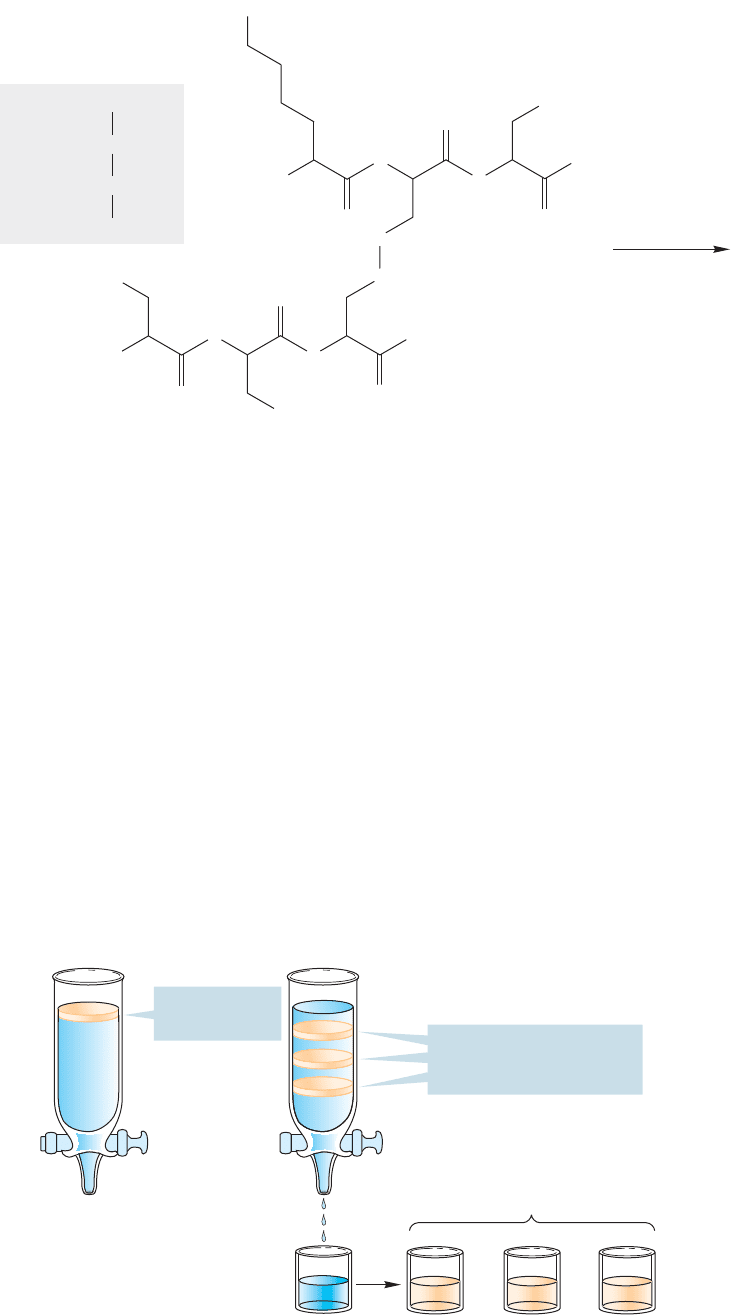
In principle, we can get an overall picture of the composition of a protein by first
destroying all disulfide links in the molecule, and then hydrolyzing all the peptide
FIGURE 23.28 The quaternary
structure of hemoglobin. Each heme
unit is attached to a globin subunit.
23.4 Peptide Chemistry 1195
bonds. Remember: These peptide bonds are amides which means acid hydrolysis
should convert them into acids (p. 902). This procedure regenerates all the amino
acids of which the molecule is built. Figure 23.29 shows a hypothetical example. Of
course, this technique does nothing to reveal either primary structure or any higher
structure.
In practice, the fragments formed when the disulfide bonds are broken are first
separated from one another and then hydrolyzed. This separation is no trivial task,
but several methods are now available,including electrophoresis (p.1180) which works
for peptides as well as amino acids. Various kinds of chromatography are also effec-
tive.In gel-filtration chromatography the mixture is passed over a column of poly-
mer beads which,on the molecular level, contain holes into which the smaller peptide
fragments fit more easily than the larger pieces. Accordingly, the larger fragments
pass more rapidly down the column, and a separation is achieved. Ion-exchange
chromatography uses electrostatic attractions to hold more highly charged frag-
ments on the column longer than more nearly neutral molecules. As in any chro-
matographic technique, the more tightly held molecules move more slowly than
those less tightly held by the medium (Fig. 23.30).
1. RSH
2 Cys Ser2 Phe
++
Lys
+
..
..
2.H
3
O
..
+
/H
2
O
O
..
..
O
..
..
O
..
..
..
..
O
..
..
O
..
..
O
..
..
OH
Lys
.
Cys
.
Phe
Phe
.
Ser
.
Cys
..
..
OH
Ph
Ph
..
..
OH
..
..
N
H
..
N
H
..
H
N
..
H
N
..
H
2
N
..
H
2
N
..
S
..
..
S
S
S
NH
2
..
FIGURE 23.29 Cleavage of a
hexapeptide to its constituent amino
acids.The disulfide bonds are first
broken to produce two fragments,
which are hydrolyzed to break the
amide bonds.
Mix of peptide
fragments
Solutions of separated peptide
fragments ready for sequencing
Individual peptide
fragments move down the
column at different rates
FIGURE 23.30 A schematic
description of chromatographic
separation of peptide fragments.
1196 CHAPTER 23 Amino Acids and Polyamino Acids (Peptides and Proteins)
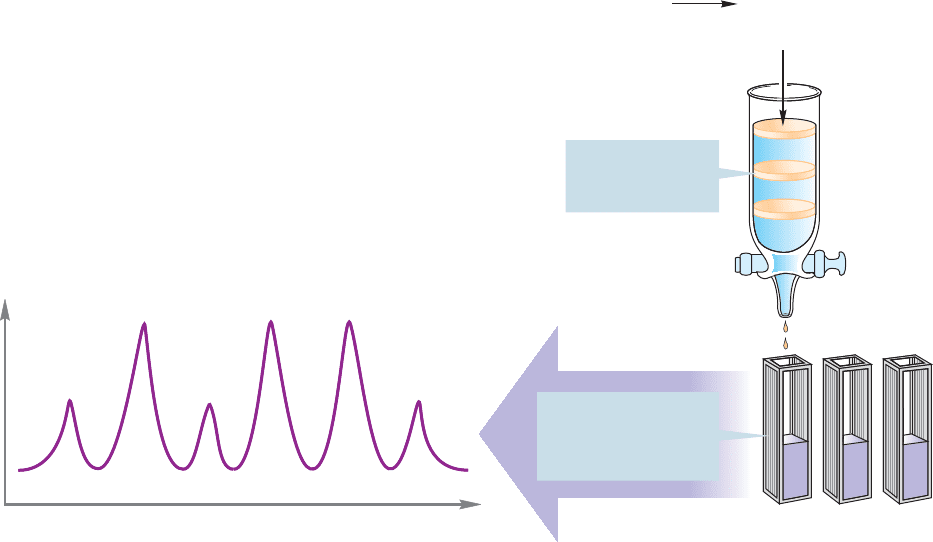
Once the peptide fragments are separated, each can be hydrolyzed to its con-
stituent amino acids. Now we need methods for determining how much of each
amino acid is present. Again chromatography is used. The amino acids are separated
on an ion-exchange column.As each amino acid is eluted from the column,it emerges
into a chamber in which it reacts with ninhydrin (p. 1187) to form a purple solution.
The intensity of the purple color is proportional to the amount of amino acid pres-
ent, and can be plotted against time or amount of solvent used for elution from the
column.This procedure produces a chromatogram consisting of a series of peaks of
various sizes. How do we tell which peak corresponds to which amino acid? This
identification is done by running samples of known amino acids through the col-
umn, determining their retention times, and matching these with those of the
unknown amino acids (Fig. 23.31).
But this technique only gives us the coarsest picture of the structure of the pro-
tein: We know the constituent amino acids and their relative amounts, but we have
no idea of their order, the primary structure. An important task is to find a way to
determine the sequence of amino acids in a protein. The first step in making this
determination is again to destroy the disulfide links and separate the constituent
polypeptides. A simple method of determining the amino acid at the amino termi-
nus is called the Sanger degradation, after Frederick Sanger (b. 1918). A peptide is
allowed to react with 2,4-dinitrofluorobenzene, which undergoes a nucleophilic aro-
matic substitution reaction (p.675).A product is formed that labels the terminal amino
Polypeptide
Mixture of
individual
amino acids
in here
Amino acid 2
Amino acid 1
..
..
H
3
O
..
+
H
2
O
Intensity of purple color =
amount of amino acid
~
Time
(volume of solvent)
Ion-exchange
column separates
amino acids
Ninhydrin chamber—
a spectrometer
detects the purple
color
Amino
acid 1
Amino
acid 2
Amino
acid 3
FIGURE 23.31 Peptide fragments can be hydrolyzed to constituent amino acids, which can then be separated
by chromatography. Reaction of each amino acid with ninhydrin gives a purple color that can be quantitatively
analyzed spectroscopically.The intensity of the purple color is proportional to the amount of amino acid
formed.
23.4 Peptide Chemistry 1197
+
+
+
Identified as the amino terminus
(hydrolysis
of the amide
links)
(adduct)
O
..
..
O
..
..
O
..
..
OH
..
..
N
H
..
H
N
..
H
2
N
NO
2
NO
2
NO
2
..
R R
O
..
..
O
..
..
O
..
..
OH
..
..
N
H
..
H
N
..
H
N
..
R
O
..
..
OH
..
..
..
..
..
F
O
2
N
NO
2
O
..
..
H
N
..
R
O
2
N
..
..
H
3
O
..
+
/H
2
O
OH
..
..
O
..
..
OH
..
..
H
3
N
H
3
N
R
R
R
R
R
FIGURE 23.32 The amino acid at the
amino terminus can be identified by
reaction with 2,4-dinitrofluorobenzene,
followed by hydrolysis. Only the amino
acid at the amino terminus of the chain
will be labeled.
It is also possible to determine the amino acid at the other end of a peptide chain,
the carboxy terminus. The method of choice uses an enzymatic reaction in which one
of a number of carboxypeptidases specifically cleaves the terminal amino acid at the
carboxy end of the chain. As each carboxy terminal amino acid reacts with the enzyme,
a new amino acid is revealed to be cleaved in turn by more carboxypeptidase. Careful
monitoring of the production of amino acids as a function of time can give a good idea
of the sequence (Fig. 23.33). Still, it is clear that things will get messy as long peptides
are turned into a soup containing an increasingly complex mixture of amino acids.
First to appear
Second to appear
O
..
..
N
H
H
R
R
R
R
H
3
N
..
N
H
..
N
..
O
N
H
H
R
R
R
..
N
..
O
O
enzyme
(carboxypeptidase)
enzyme
enzyme
O
..
..
..
..
..
..
..
..
..
..
R
Last to appear
R
+
+
H
3
N
+
N
H
R
R
..
O
R
..
O
–
..
..
..
O
–
..
..
..
O
–
..
..
..
O
–
..
..
..
O
–
..
..
R
+
+
H
3
N
+
O
..
..
O
..
..
O
..
..
O
..
..
O
..
..
O
..
..
O
..
..
H
3
N
+
H
3
N
+
H
3
N
+
H
3
N
+
..
O
–
..
..
..
O
–
..
..
FIGURE 23.33 Carboxypeptidase is
an enzyme that cleaves only at the
carboxy terminus of a polypeptide.
group with a 2,4-dinitrobenzene group (Fig. 23.32). Hydrolysis now generates a num-
ber of amino acids, but only the amino acid terminus is labeled! Unfortunately, we
have had to destroy the entire peptide to make this determination!
PROBLEM 23.18 Write a mechanism for adduct formation in Figure 23.32. What
is the function of the nitro groups?
A much better method would be one that included no overall hydrolysis step
and that could be more controlled than the cleavage reactions set in motion by treat-
ment with a carboxypeptidase. We need a method that unzips the protein from one
end or the other, revealing at each step the terminal amino acid.
Methods have now been developed that allow just such a step-by-step sequenc-
ing of peptides. One effective way is called the Edman degradation, after Pehr
Edman (1916–1977), and uses the molecule phenylisothiocyanate (Fig. 23.34).
Isocyanates have appeared before (p. 918), and an isothiocyanate is just the sulfur
analogue of an isocyanate.
1198 CHAPTER 23 Amino Acids and Polyamino Acids (Peptides and Proteins)
O
..
..
C
R
R
Curtius
rearrangement
Acyl azide
h ν
N
3
N
An isocyanate
Phenylisothiocyanate
CO
..
..
..
Ph
NCS
..
..
..
WEB 3D
FIGURE 23.34 An isothiocyanate
is just the sulfur version of an
isocyanate, the product of a Curtius
rearrangement.
PROBLEM 23.19 Write a mechanism for the isocyanate formation in Figure 23.34.
Like isocyanates, isothiocyanates react rapidly with nucleophiles. Reactions
of isocyanates with ammonia or amines yield ureas. In a similar fashion,
isothio
cyanates give a thiourea as the first formed intermediate (Fig. 23.35).
WEB 3D
O
..
..
O
..
..
R
R
NH
3
NH
2
N
A urea
Phenylisothiocyanate
A thiourea
CO
..
..
..
Ph
NCS
..
..
..
H
N
..
..
S
..
..
S
..
..
R
R
NH
3
NH
2
N
A thiourea
CS
..
..
..
H
N
..
..
..
..
N
H
R
H
2
N
..
+
..
H
N
..
N
H
H
R
..
N
..
H
N
..
H
N
..
O
..
..
Ph
R
R
O
..
..
O
..
..
FIGURE 23.35 Isothiocyanates react
with amines to give thioureas.
Phenylisothiocyanate can be used
to label the amino terminus of a
polypeptide.
