Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.

23.7 Additional Problems 1219
(b) A second peptide, similarly treated, gives a compound in
which a single hydrogen appears in the high-resolution
1
H
NMR spectrum as an eight-line signal at about δ 3.5 ppm. What
information can you derive about the structure of this peptide?
PROBLEM 23.35 A tripeptide of unknown structure is
hydrolyzed in acid to one molecule of Ala, one of Cys, and one
of Met. The tripeptide reacts with phenyl isothiocyanate, fol-
lowed by treatment with acid, to give a phenylthiohydantoin
that shows only a three-hydrogen doublet at about δ 2 ppm in
the
1
H NMR spectrum. The tripeptide is not cleaved by BrCN.
Deduce the structure from these data.
PROBLEM 23.36 A dodecapeptide 1 is hydrolyzed in acid to
give 2 Arg, Asp, Cys, His, 2 Leu, Lys, 2 Phe, and 2 Val. The
Edman procedure yields a phenylthiohydantoin that shows two
3H doublets and a multiplet integrating for 1H at about
δ 4.0 ppm in the
1
H NMR spectrum. Treatment of 1 with
trypsin leads to , ,
and . Treatment of 1 with chymotrypsin leads to
, and .
Deduce the structure of 1 and explain your reasoning.
PROBLEM 23.37 In Section 23.4, we saw that the tBoc group
can be used to protect (or block) the amino group of an amino
acid during peptide synthesis. This group can be added by
allowing the amino acid to react with di-tert-butyl dicarbonate
(1). Once the amide linkage has been formed, the tBoc protect-
ing group can be removed by mild acid “hydrolysis.” Both
of these reactions are illustrated below. Provide mechanisms for
the addition of the tBoc group and its removal. Hint: One
of the products of the removal is 2-methylpropene.
Lys
.
Val
.
ArgVal
.
His
.
Phe, Leu
.
Arg
.
Asp
.
Cys
.
Leu
.
Phe
Val
.
Arg
Asp
.
Cys
.
Leu
.
Phe
.
LysVal
.
His
.
Phe
.
Leu
.
Arg
PROBLEM 23.38 In Section 23.4c (Fig. 23.49), we saw that
the acylating agent in DCC-mediated peptide bond forma-
tion is the anhydride. There is another intermediate that
could act as the acylating agent. What is it, and how can it
function as the acylating agent? Hint: This intermediate
appears in Figure 23.49.
R
O
O
COO
–
H
3
N
H
2
N
+
(H
3
C)
3
CO
+
++
C
C
O
OC(CH
3
)
3
O R
(H
3
C)
3
CO
CO
2
N
H
COOH
O
O
R
(H
3
C)
3
CO
NH—Peptide
N
H
O
(a)
(b)
1
R
NH—Peptide
1. NaOH,
(CH
3
)
3
COH/H
2
O
2. neutralize
1. CF
3
COOH
2. (CH
3
CH
2
)
3
N
CH
2
(CH
3
)
2
C
H
3
C
Ph
+
–
NH
COOH
COOCH
3
CH
3
H
3
COOC
H
3
C
H
3
C
Ph
Ph
Ph
N
N
N
N
N
NO
O
O
COOH
12
4
3
NaNO
2
/HCl
H
2
O
H
3
COOCC CCOOCH
3
xylene, 120 ⬚C
(DMAD)
Ac
2
O
PROBLEM 23.40 In Problem 23.12, you were asked to write a
mechanism for the acylation of glycine.The mechanism pre-
sented in the answer is actually incomplete, at least when the
reaction is run in the presence of excess acetic anhydride. For
example, what is the purpose of the water in the second step?
There is actually an intermediate involved in this reaction, the
2-oxazolin-5-one (1), colloquially referred to as an azlactone.
PROBLEM 23.39 Reaction of N-phenylalanine (1) with
nitrous acid affords N-nitroso amino acid (2), which on
treatment with acetic anhydride yields “sydnone” (3).
Sydnones belong to a class of compounds known as
mesoionic compounds. These compounds cannot be
satisfactorily represented by Lewis structures not involving
charge separation. The name sydnone derives from the
University of Sydney where the first examples were prepared
in 1935. Reaction of 3 and dimethyl acetylenedicarboxylate
(DMAD) gives pyrazole (4). Propose mechanisms for the
formations of 2, 3, and 4. Hint: For the formation of 4,it
may be helpful to consider the other possible Lewis
structures for sydnone 3.
1220 CHAPTER 23 Amino Acids and Polyamino Acids (Peptides and Proteins)
NH
2
COOH PhCHO
NaOCOCH
3
(NaOAc)
COOH
COOH
HN
O
N
O
H
Ph
Ph
Ph
O
2
3
H
2
O
Δ
H
2
/PtO
2
1. HCl/H
2
O/Δ
2. neutralize
O
O
O
H
3
C
CH
3
HN
COO
–
NH
3
O
+
Ph
PROBLEM 23.41 In Problem 23.40, we saw that the azlactone
(1) is an intermediate in the acetylation of glycine in the
presence of excess acetic anhydride. The generation of
azlactone 1 in this reaction lies at the heart of one of the
oldest known amino acid syntheses. For example, if glycine
(or N-acetylglycine) is treated with acetic anhydride in the pres-
ence of benzaldehyde and sodium acetate, the benzylidene
azlactone (2) is formed. Azlactone 2 can then be converted into
racemic phenylalanine (3) by the following sequence. Propose a
mechanism for the formation of 2. Hint: Azlactone 1 is still an
intermediate in this reaction.
PROBLEM 23.42 When optically active N-methyl-L-alanine
(1) is heated with an excess of acetic anhydride, followed by
treatment with water, the resulting N-acetyl-N-methylalanine
(2) is racemic. If the acetylation reaction is run in the presence
of dimethyl acetylenedicarboxylate (DMAD), the pyrrole 3 can
be isolated in good yield. Account for the racemization of 2 and
the formation of 3. Hint: There is a common intermediate
involved in these two reactions. Also, see Problem 23.39.
1
Optically active
2. H
2
O
O
O
O
O
O
1. H
3
C
CH
3
O
H
3
C
CH
3
H
3
C
H
3
C
H
3
C
H
3
C
CH
3
H
3
C
NH
COOH
H
3
COOCC CCOOCH
3
Δ
(DMAD)
Δ
COOCH
3
H
3
COOC
N
32
(racemic)
COOH
N
O
CH
3
CH
3
PROBLEM 23.43 (a) What mRNA sequence does the follow-
ing DNA sequence produce? T A C G G G T T T A T C
(b) What message does that mRNA sequence produce?
NH
2
COOH
COOH
HN
O
N
O
O
1
H
2
O
O
O
O
H
3
C
CH
3
CH
3
CH
3
Propose mechanisms for the formation of azlactone 1 and its
hydrolysis to N-acetylglycine.

Glossary
Absolute configuration (Section 4.3) The arrangement in space
of the atoms of an enantiomer, its stereochemical description as
(R) or (S).
Abstraction (Section 11.2) Removal of a hydrogen atom as a
result of a reaction with a radical.
Acetal (Section 16.9) The final product in the acid-catalyzed
reaction of an aldehyde or ketone with an alcohol.
Activation energy (⌬G
‡
) (Section 3.17) The difference in free
energy between the starting material and the transition state in a
reaction. It is this amount of energy that is required for a
molecule of starting material to be transformed into product.
Acyl compound (Section 18.1) A compound of the structure
C
OR
OR
R´
H
C
OR
OR
R´
R´´
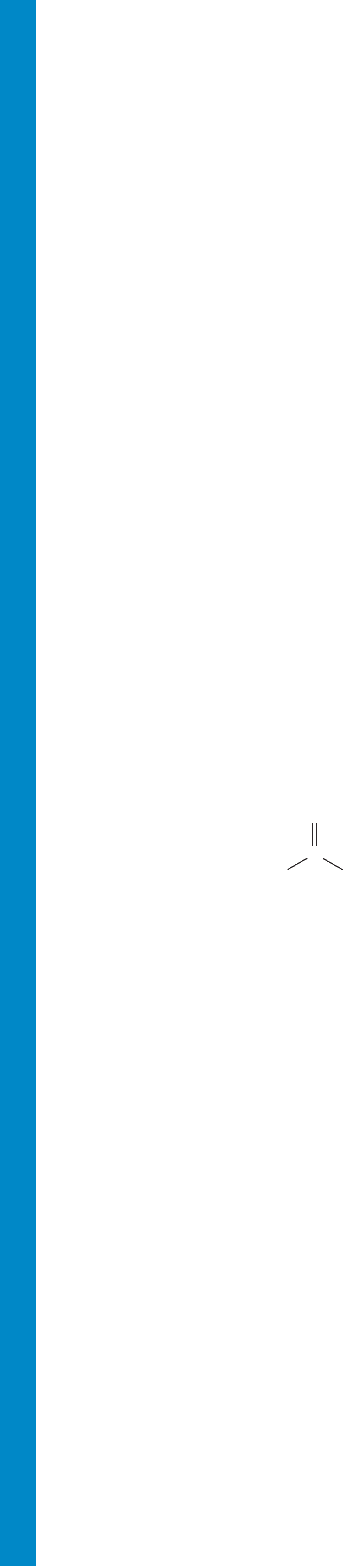
Acetylenes (Section 3.1) Hydrocarbons of the general formula
C
n
H
2n - 2
.These molecules, also called alkynes, contain
carbon–carbon triple bonds.The parent compound, ,
is called acetylene, or ethyne.
Acetylide (Section 3.14) The anion formed by removal by base of
a terminal hydrogen from an acetylene.
Achiral (Section 4.2) Not chiral.
Acid anhydride (Section 17.7) A compound formed by formal loss
of water from two molecules of a carboxylic acid.The structure is
HC
q
CH
Acid derivative (Section 18.1) One of the several functional
groups related to carboxylic acids and having the same oxidation
level.These are acid halides, acid anhydrides, esters, and amides.
We often include nitriles and ketenes in this category.
Acid halide (Section 18.2) A compound of the structure
Activating agent (Section 17.7) A reagent that converts a
carboxylic acid into a more reactive acid derivative.
Acyl group (Section 11.2) A general term for .
Acylium ion (Section 14.6) The structure , which has
the resonance form
Alcohol (Section 3.19) A molecule containing a simple hydroxyl
group, .
Aldaric acid (Section 22.4) A diacid derived from an aldohexose
by oxidation with nitric acid. It has the overall structure
. In an aldaric acid, the old
aldehyde and primary alcohol ends of the sugar have become identical.
Aldehydes (Section 10.5) Compounds containing a
monosubstituted carbon–oxygen double bond.
HOOC
O
(CHOH)
4
O
COOH
R
O
OH
R
O
C
q
O
+
.
RC
+
P
O
R
O
C
P
O
C
R
O
Cl
C
R
O
X
R = F, Cl, Br, or I
C
R
O
X
C
R
O
H
Aldohexose (Section 22.2) A hexose of the structure
.
Aldol condensation (Section 19.6) The acid- or base-catalyzed
conversion of an aldehyde or ketone into a β-hydroxy aldehyde or
β-hydroxy ketone. In acid, the enol is an intermediate; in base,
the intermediate is the enolate.The initial product often loses
water to form an α,β-unsaturated ketone or aldehyde.
Aldonic acid (Section 22.4) A monoacid derived from an
aldohexose through oxidation with bromine in water. Only the
aldehyde group is oxidized to the acid. It has the structure
Aldopentose (Section 22.2) A pentose of the structure
Aldose (Section 22.2) A sugar molecule that has an aldehyde group.
Aldotetrose (Section 22.2) A tetrose of the structure
Aldotriose (Section 22.2) A triose of the structure
O
P
CH
O
CHOH
O
CH
2
OH.
O
P
CH
O
(CHOH)
2
O
CH
2
OH.
O
P
CH
O
(CHOH)
3
O
CH
2
OH.
HOOC
O
(CHOH)
4
O
CH
2
OH.
O
P
CH
O
(CHOH)
4
O
CH
2
OH
G-1
C
R
O
C
O
O
R
Acid chloride (Section 14.6) A compound of the structure
C
RO
O
NR
2
R can be H
G-2 GLOSSARY
Alkaloid (Section 4.9) A nitrogen-containing compound, often
polycyclic and generally of plant origin.The term is more loosely
applied to other naturally occurring amines.
Alkanes (Section 2.1) The series of saturated hydrocarbons of the
general formula C
n
H
2n + 2
.
Alkene halogenation (Section 10.2) Addition of X
2
to a π bond,
giving a 1,2-dihalide.
Alkene hydrohalogenation (Section 9.2) Addition of HX to a π
bond, giving an alkyl halide.
Alkenes (Section 3.1) Hydrocarbons of the general formula,
C
n
H
2n
.These molecules, also called olefins, contain
carbon–carbon double bonds.
Alkoxide ion (Section 6.4) The conjugate base of an alcohol, RO
-
.
Alkyl compounds (Section 2.5) Substituted alkanes. One or more
hydrogens is replaced by another atom or group of atoms.
Alkyl halide (Section 6.2) Compound of the formula C
n
H
2n + 1
X,
where X = F, Cl, Br, or I.
Alkynes (Section 3.1) Hydrocarbons of the general formula
C
n
H
2n – 2
.These molecules contain carbon–carbon triple bonds.
Allene (Section 4.13) A 1,2-diene. A compound containing a
carbon atom that is part of two double bonds.
Allyl (Section 3.3) The common name for the
group.
Allylic halogenation (Section 11.8) Specific formation of a
carbon–halogen bond at the position adjacent to a carbon–carbon
double bond.
Amide (Sections 6.7, 17.7) A compound of the structure
H
2
C
P
CH
O
CH
2
Remember that this term also refers to the ions H
2
N
-
,RHN
-
,
R
2
N
-
,RR′N
-
.
Amine (Section 6.7) A compound of the structure R
3
N , where R
can be H, alkyl, or aryl but not acyl. Cyclic and aromatic amines
are common.
Amine inversion (Section 6.7) The conversion of one pyramidal
form of an amine into the other through a planar, sp
2
hybridized
transition state.
␣-Amino acids (Section 23.2) 2-Aminoacetic acids, the
monomeric constituents of the polymeric peptides and proteins.
Amino terminus (Section 23.4) The amino acid at the free amine
end of a peptide polymer.
Ammonia (Section 6.7) NH
3
, the simplest of all amines.
Ammonium ion (Section 6.7) R
4
N
+
,R = alkyl, aryl, or H.
Anchimeric assistance (Section 21.2) The increase in rate of a
reaction that proceeds through intramolecular displacement over
that expected of an intermolecular displacement.
Angle strain (Section 5.2) The increase in energy caused by the
deviation of an angle from the ideal demanded by an atom’s
hybridization.
Anion (Section 1.2) A negatively charged atom or molecule.
:
:
Annulene (Section 13.6) A cyclic polyene that is at least formally
fully conjugated.
Anomeric carbon (Section 22.2) The carbon in a cyclic sugar that
is the acetal carbon.
Anomers (Section 22.2) For aldoses, these are sugars differing
only in the stereochemistry at C(1).They are C(1) stereoisomers.
Antarafacial motion (Section 20.5) Migration of a group from
one side of a π system to the other in a sigmatropic shift.
Antibonding molecular orbital (Section 1.5) A molecular orbital
that is the result of mixing atomic orbitals in an out-of-phase
fashion. Occupation of an antibonding molecular orbital by an
electron destabilizes a molecule.
anti Elimination (Section 7.9) An elimination reaction in which
the dihedral angle between the breaking bonds, usually
, is 180°.
anti-Markovnikov addition (Section 9.11) Addition to a π bond
that results in the substituent being on the less substituted carbon
of what was the π bond.
Aprotic solvent (Section 6.5) A solvent that is not a proton
donor. An aprotic molecule does not bear a transferable proton.
Arene (Section 13.8) An aromatic compound containing a
benzene ring or rings.
Arndt–Eistert reaction (Section 18.14) The use of the Wolff
rearrangement to elongate the chain of a carboxylic acid by one
carbon.
Aromatic character (Section 13.5) See Aromaticity.
Aromaticity (Section 13.5) The special stability of planar, cyclic,
fully conjugated molecules with 4n + 2 π electrons. Such
molecules will have molecular orbital systems with all bonding
molecular orbitals filled and all antibonding molecular orbitals
empty. Usually, there will be filled degenerate orbitals.
Arrow formalism, curved arrow formalism, or electron pushing
(Section 1.4) A mapping device for chemical reactions.The
electron pairs (lone pairs or bond pairs) are “pushed”using curved
arrows that show the bonds that are forming and breaking in the
reaction.
Atom (Section 1.1) A neutral atom consists of a nucleus, or core
of protons and neutrons, orbited by a number of electrons equal
to the number of protons.
Atomic orbital (Section 1.1) A three-dimensional representation
of the solution of Schrödinger’s equation describing the motion
of an electron in the vicinity of a nucleus. Atomic orbitals have
different shapes, which are determined by quantum numbers.The
s orbitals are spherically symmetric, p orbitals roughly dumbbell
shaped, and the d and f orbitals are even more complicated.
Aufbau principle (Section 1.2) When adding electrons to a
system of orbitals, first fill the lowest energy orbital available
before filling any higher energy orbitals. Electron–electron
repulsion is minimized by filling systems of equi-energetic
orbitals by singly occupying all orbitals with electrons of the same
spin before doubly occupying any of them. See Hund’s rule.
Axial hydrogens (Section 5.2) The set of six straight up and down
hydrogens in chair cyclohexane. Ring flip interconverts these
hydrogens with the set of equatorial hydrogens.
and C
O
L
C
O
H
OH
C
RHN
R
R
C
HO
O
NR
2
C
RO
O
NR
2
GLOSSARY G-3
Aziridine (Section 6.7) A saturated three-membered ring
containing one nitrogen atom. An azacyclopropane.
Azo compounds (Section 11.2) Compounds of the structure,
.
Baeyer–Villiger reaction (Section 18.12) The reaction of a peroxy
acid with a carbonyl group, ultimately giving an ester.
Base pair (Section 23.5) A hydrogen-bonded pair of bases, always
adenine–thymine (A-T) in DNA or adenine–uracil (A-U) in
RNA, and cytosine–guanine (C-G) in both DNA and RNA.
Base peak (Section 15.3) The largest peak in a mass spectrum, to
which all other peaks are referred.
Beckmann rearrangement (Section 18.12) The conversion of an
imine into an amide via a 1,2 shift followed by hydrolysis of the
resulting cation.
Benzaldehyde (Section 16.3) The common (and always used) name
for the simplest aromatic aldehyde, “benzenecarboxaldehyde.”
Benzene (Section 13.1) The archetypal aromatic compound; a
planar, regular hexagon of sp
2
hybridized carbons.The six 2p
orbitals overlap to form a six-electron cycle above and below the
plane of the ring.The molecular orbital system has three fully
occupied bonding molecular orbitals and three unoccupied
antibonding orbitals.
Benzhydryl group (Section 13.12) The Ph
2
CH group.
Benzoic acid (Section 13.12) (benzenecarboxylic
acid).
Benzyl (Section 13.7) The PhCH
2
group.
Benzyne (Section 14.14) 1,2-Dehydrobenzene, C
6
H
4
.
Binding site (Section 23.4) The location where a substrate resides
in an enzyme.
Birch reduction (Section 13.11) The conversion of aromatic
compounds into 1,4-cyclohexadienes through treatment with
sodium in liquid ammonia–ethyl alcohol. Radical anions are the
first formed intermediates.
tBoc (Section 23.4) A protecting group for the amino end of an
amino acid that works by transforming the amine into a less basic
carbamate.
Boltzmann distribution (Section 8.4) The range of energies of a
set of molecules at a given temperature.
Bond dissociation energy (BDE) (Section 1.6) The amount of
energy that must be applied to break a bond into two neutral
species. See Homolytic bond cleavage.
Bonding molecular orbital (Section 1.5) A molecular orbital that
is the result of mixing atomic orbitals in an in-phase fashion.
Occupation of a bonding molecular orbital by an electron
stabilizes a molecule.
Bredt’s rule (Section 3.7) Bredt noticed that there were no
examples of bicyclic molecules with double bonds at the
bridgehead position.
Bridged (Section 5.7) In a bridged bicyclic molecule, two rings
share more than two atoms.
Bridgehead position (Section 3.7) The bridgehead positions are
shared by the rings in a bicyclic molecule. In a bicyclic molecule,
the three bridges emanate from the bridgehead positions.
Ph
O
COOH
R
O
N
P
N
O
R
¿
Bromonium ion (Section 10.2) A three-membered ring
containing bromine that is formed by the reaction of an alkene
with Br
2
.The bromine atom in the ring is positively charged.
Brønsted acid (Section 2.15) A proton donor.
Brønsted base (Section 2.15) A proton acceptor.
Bullvalene (Section 20.7) Bullvalene is the only known neutral
organic molecule with a fluxional structure. Every carbon of this
(CH)
10
compound is bonded on time average to each of the other
nine carbons.
Butyl group (Section 2.8) The group CH
3
CH
2
CH
2
CH
2
.
sec-Butyl group (Section 2.8) The group CH
3
CH
2
CH(CH
3
).
tert-Butyl group (Section 2.8) The group (CH
3
)
3
C.
Cahn–Ingold–Prelog priority system (Section 3.4) An arbitrary
system for naming stereoisomers. It determines a priority system
for ordering groups.
Cannizzaro reaction (Section 19.14) The redox reaction of an
aldehyde containing no α hydrogens with hydroxide ion.
Addition of hydroxide to the aldehyde is followed by hydride
transfer to another aldehyde. Protonation generates a molecule of
the carboxylic acid and the alcohol related to the original
aldehyde.
Carbamates (Section 17.7) Esters of carbamic acid.These
molecules do not decarboxylate (lose CO
2
) easily.
Carbamic acid (Section 17.7) A compound of the structure
These acids easily decarboxylate to give amines.
Carbanion (Section 2.4) A compound containing a negatively
charged carbon atom. A carbon-based anion.
Carbene (Section 10.4) A short-lived neutral intermediate
containing a divalent carbon atom. See also Singlet carbene and
Triplet carbene.
Carbinolamine (Section 16.11) The initial intermediate in the
reaction between a carbonyl-containing molecule, , and
an amine. It is analogous to a hemiacetal.
R
2
C
P
O
Carbocation (Section 2.4) The compromise and currently widely
used name for a molecule containing a trivalent, positively
charged carbon atom.
Carbohydrate (Section 22.1) A molecule whose formula can be
factored into C
x
(H
2
O)
y
. A sugar or saccharide.
Carbonyl compound (Section 10.5) A compound containing a
carbon–oxygen double bond.

G-4 GLOSSARY
O
O
R
C
–
O
O
R
C
–
O
OH
R
C
Carboxy terminus (Section 23.4) The amino acid at the free
carboxylic acid end of a peptide polymer.
Carboxylate anion (Section 17.2) The resonance-stabilized anion
formed on deprotonation of a carboxylic acid.
Carboxylic acid (Section 10.4) A compound of the structure
Catalyst (Section 3.19) A catalyst functions to increase the rate of
a chemical reaction. It is ultimately unchanged by the reaction
and functions not by changing the energy of the starting material
or product but by providing a lower energy pathway between
them.Thus, it operates to lower the energies of the transition
states involved in the reaction.
Cation (Section 1.2) A positively charged atom or molecule.
Cationic polymerization (Section 9.8) A reaction in which an
initially formed carbocation adds to an alkene that in turn adds to
another alkene. Repeated additions can lead to polymer
formation.
Cbz (Section 23.4) A protecting group for the amino end of an
amino acid that works by transforming the amine into a less basic
carbamate.
Cellulose (Section 22.6) A polymer of glucose in which C(4) of
one glucose is linked in β fashion to C(1) of another.
Chain reaction (Section 11.1) A cycling reaction in which the
species necessary for the first step of the reaction is produced in
the last step. This intermediate then recycles and starts the
process over again.
Chemical shift (δ) (Section 15.6) The position, on the ppm scale,
of a peak in an NMR spectrum. The chemical shift for
1
H and
13
C is given relative to a standard, TMS, and is determined by the
chemical environment surrounding the nucleus.
Chichibabin reaction (Section 14.12) Nucleophilic addition of
an amide ion to pyridine (or a related heteroaromatic compound)
leading to an aminopyridine. A key step involves hydride transfer.
Chiral (Section 4.1) A chiral molecule is not superimposable on
its mirror image.
Chirality (Section 4.1) The ability of a molecule to exist in two
nonsuperimposable mirror-image forms; handedness.
cis (Section 2.12) Hydrogens “on the same side.”Applied to
specify stereochemical (spatial) relationships in ring compounds
and alkenes.
s-cis (Section 12.6) The less stable, coiled form of a 1,3-diene.
Claisen condensation (Section 19.8) A condensation reaction of
esters in which an ester enolate adds to the carbonyl group of
another ester.The result of this addition–elimination process is a
β-keto ester.
Claisen–Schmidt condensation (Section 19.7) A crossed aldol
condensation of an aldehyde without α hydrogens with a ketone
that does have at least one α hydrogen.
β
β
Cleavage (Section 11.2) The fragmentation of a radical into a
new radical and an alkene through breaking of the α–β bond.
Codon (Section 23.5) A three-base sequence in a polynucleotide
that directs the addition of a particular amino acid to a growing
chain of amino acids. Some codons also give directions: “start
assembly” or “stop assembly.”
Coenzyme (Section 16.18) A molecule able to carry out a
chemical reaction with another molecule only in cooperation with
an enzyme.The enzyme’s function is often to bring the substrate
and the coenzyme together.
Concerted reaction (Section 9.9) A single-barrier process. In a
concerted reaction, starting material is converted into product
with no intermediate structures.
Configurational carbon (Section 22.2) The stereogenic carbon of
a carbohydrate that is furthest from C(1) or the carbonyl carbon.
This is the carbon whose configuration determines whether the
sugar is of the
D or L family.
Conformation (Section 2.5) The three-dimensional structure of a
molecule. Conformations are interconverted by rotations about
single bonds.
Conformational analysis (Section 2.8) The study of the relative
energies of conformational isomers.
Conformational enantiomers (Section 4.7) Enantiomers
interconvertible through (generally easy) rotations around bonds
within the molecule.
Conformational isomers (Section 2.5) Molecules that can be
interconverted by rotation about one or more single bonds.
Conjugate acid (Section 6.4) Some atom or molecule plus a
proton. Conjugate acids and bases are related by the gain and loss
of a proton.
Conjugate base (Section 6.4) Some molecule less a proton.
Conjugate acids and bases are related by the gain and loss of a
proton.
Conjugated double bonds (Section 12.1) Double bonds in a
1,3-relationship are conjugated.
Conrotation (Section 20.3) In a conrotatory process, the end p
orbitals of a polyene rotate in the same sense (both clockwise or
both counterclockwise).
Constitutional isomers (Section 4.11) Molecules of the same
formula but with different connectivities among the constituent
atoms.
Cope rearrangement (Section 20.6) This [3,3] sigmatropic shift
converts one 1,5-diene into another.
Coupling constant ( J) (Section 15.6) The magnitude (in hertz)
of J, the measure of the spin–spin interaction between two nuclei.
Covalent bond (Section 1.2) A bond formed by the sharing of
electrons through the overlap of atomic or molecular orbitals.

–
..
OH
C
NC
R
R
GLOSSARY
G-5
C
O
CHN
2
R
Crossed (mixed) aldol condensation (Section 19.7) An aldol
condensation between two different carbonyl compounds.This
reaction is not very useful unless strategies are employed to limit
the number of possible products.
Crossed (mixed) Claisen condensation (Section 19.9) A Claisen
condensation between two different esters.
Crown ether (Section 6.10) A cyclic polyether often capable of
forming complexes with metal ions.The ease of complexation
depends on the size of the ring and the number of heteroatoms in
the ring.
Cryptand (Section 6.10) A three-dimensional, bicyclic
counterpart of a crown ether. Various heteroatoms (O, N, S) act
to complex metal ions that fit into the cavity.
Cumulated alkene (cumulene) (Section 12.1) Any molecule
containing at least three consecutive double bonds,
.The parent cumulene is butatriene.
Curtius rearrangement (Section 18.14) The thermal or
photochemical decomposition of an acyl azide to give an
isocyanate.
Cyanogen bromide (BrCN) (Section 23.4) A reagent able to
cleave peptide chains after a methionine residue.
Cyanohydrin (Section 16.8) The product of addition of hydrogen
cyanide to a carbonyl compound.
R
2
C
P
C
P
C
P
CR
2
Cycloaddition reaction (Section 20.4) A reaction in which two π
systems are converted into a ring. The Diels–Alder reaction and
the 2 + 2 reaction of a pair of ethylenes to give a cyclobutane are
examples.
Cycloalkanes (Section 2.1) Cyclic alkanes.
Cycloalkenes (Section 3.4) Ring compounds containing a double
bond within the ring.
Cycloheptatrienylium ion (Section 13.6) See Tropylium ion.
Cyclopentadienyl anion (Section 13.6) A five-carbon aromatic
anion containing 6 π electrons (4n + 2, n = 1).
Daughter ion (Section 15.3) In MS, an ion formed by the
fragmentation of the first-formed parent ion.
Decarboxylation (Section 17.7) The loss of carbon dioxide, a
common reaction of 1,1-diacids and β-keto acids.
Decoupling (Section 15.6) The removal of coupling between
hydrogens or other nuclei (see Coupling constant) through either
chemical exchange or electronic means.
Degenerate reaction (Section 20.1) In a degenerate reaction, the
starting material and product have the same structure.
Degree of unsaturation (Ω) (Section 3.15) In a hydrocarbon, this
is the total number of π bonds and rings.
Delocalization (Section 1.4) The ability of electrons to move
between several atoms.
Delocalization energy (Section 13.5) The energy lowering
conferred by the delocalization of electrons. In benzene, this is
the amount by which benzene is more stable than the
hypothetical 1,3,5-cyclohexatriene containing three localized
double bonds. See Resonance energy.
Denaturing (Section 23.4) The destruction of the higher order
structures of a protein, sometimes reversible, sometimes not.
Deoxyribonucleic acid (DNA) (Section 23.5) A polymer of
nucleotides made up of deoxyribose units connected by
phosphoric acid links. Each sugar is attached at C(1′) to one of
the bases, A,T, G, or C.
DEPT (distortionless enhancement with polarization transfer)
(Section 15.7) A spectroscopic method of determining the
number of hydrogens attached to a carbon.
Detergent (Section 17.8) A long-chain alkyl sulfonic acid salt.
Dewar benzene (Section 13.2) The unstable C
6
H
6
molecule
bicyclo[2.2.0]hexa-2,5-diene.
Dewar forms (Section 13.3) Resonance forms for benzene in
which overlap between 2p orbitals on two para carbons is
emphasized. These forms superficially resemble Dewar benzene
(bicyclo[2.2.0]hexa-2,5-diene).
Dextrorotatory (Section 4.4) The rotation of the plane of plane-
polarized light in the clockwise direction.
Dial (Section 16.3) A molecule containing two aldehyde groups,
a dialdehyde.
Diastereomers (Section 4.8) Stereoisomers that are not mirror
images.
Diastereotopic (Section 15.6) Diastereotopic hydrogens (or
groups) are different both chemically and spectroscopically under
all circumstances.
Diazo compounds (Section 10.4) Compounds of the structure
.
Diazo ketone (Section 18.14) A compound of the structure
R
2
C
P
N
2
Diazonium ion (Section 14.7) The group N
2
+
as in RN
2
+
.
Dicyclohexylcarbodiimide (DCC) (Section 23.4) A dehydrating
agent effective in the coupling of amino acids through the
formation of amide bonds.
Dieckmann condensation (Section 19.9) An intramolecular, or
cyclic, Claisen condensation.
Diels–Alder reaction (Section 12.12) The concerted reaction of
an alkene or alkyne with a 1,3-diene to form a six-membered
ring.
NCN
..
..
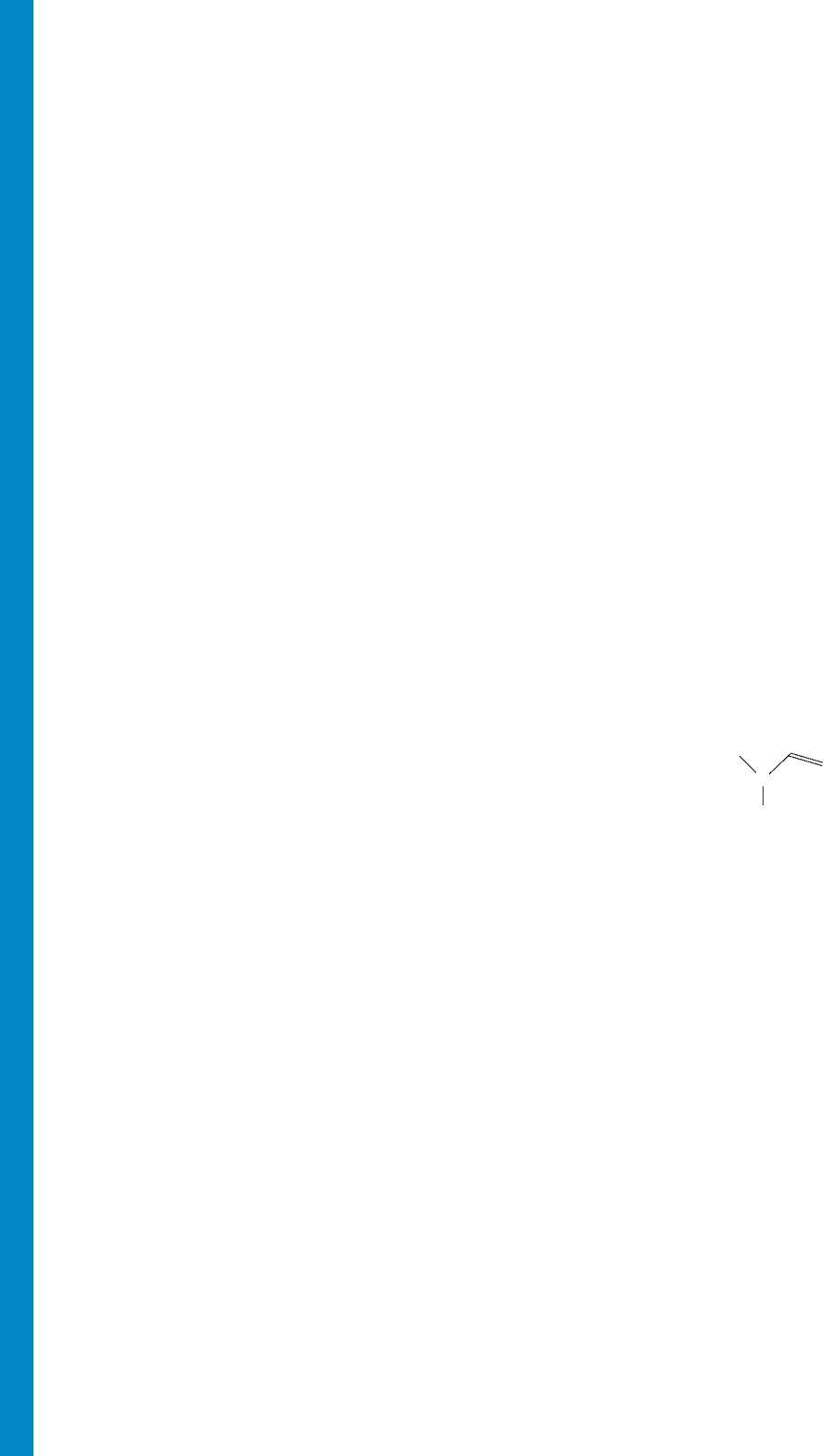
H
R
N
G-6 GLOSSARY
Dienophile (Section 12.12) A molecule that reacts with a diene
in a Diels–Alder fashion.
Dihedral angle (Section 2.5) The torsional, or twisting, angle
between two bonds. In an system, the dihedral
angle is the angle between the
planes.
Diol (Section 6.6) A molecule containing two OH groups. Also
called a glycol.
Dione (Section 16.3) A compound containing two ketone groups,
a diketone.
1,3-Dipolar reagents, 1,3-dipoles (Section 10.5) Molecules for
which a good neutral structure cannot be written. Ozone is a
typical example.These species undergo addition to π systems to
give five-membered rings.
Dipole moment (Section 1.3) A dipole moment in a molecule
results when two opposite charges or partial charges are
separated.
Diradical (Section 10.4) A species containing two unpaired
electrons, usually on different atoms.
Disaccharide (Section 22.6) A molecule that is composed of two
monosaccharides. The formula for a disaccharide is C
12
H
22
O
11
.
Disproportionation (Section 11.2) The reaction of a pair of
radicals to give a saturated and unsaturated molecule by
abstraction of a hydrogen by one radical from the position
adjacent to the free electron of the other radical.
Disrotation (Section 20.3) In a disrotatory process, the end p
orbitals of a polyene rotate in opposite senses (one clockwise and
the other counterclockwise).
Disulfide bridges (Section 23.4) The attachment of amino acids
through sulfur–sulfur bonds formed from the oxidation of
cysteine CH
2
SH side chains. Disulfide bridges can be formed
within a single peptide or between two peptides.
Dithiane (Section 19.12) Six-membered ring containing a pair of
sulfur atoms, usually in the 1,3 positions.
Double bond (Section 3.1) Two atoms can be attached by a
double bond composed of one σ bond and one π bond.
E1cB Reaction (Section 7.9) An elimination reaction in which
the first step is loss of a proton to give an anion.The anion then
internally displaces the leaving group in a second step.The
product has a new π bond.
E1 Reaction (Section 7.8) The unimolecular elimination reaction.
The ionization of the starting material is followed by the loss of a
proton to base.The product has a new π bond.
E2 Reaction (Section 7.9) The bimolecular elimination reaction.
The proton and leaving group are lost in a single, base-induced
step. The product has a new π bond.
Eclipsed ethane (Section 2.5) The conformation of ethane in
which all carbon–hydrogen bonds are as close as possible.This
conformation is not an energy minimum, but the top of the
barrier separating two molecules of the stable, staggered
conformation of ethane.
Edman degradation (Section 23.4) The phenyl
isothiocyanate–induced cleavage of the amino acid at the amino
terminus of a peptide. Successive applications of the Edman
technique can determine the sequence of a peptide.
X
O
C
O
C and C
O
C
O
X
X
O
C
O
C
O
X
Electrocyclic reaction (Section 20.3) The interconversion of a
polyene and a ring compound. The end p orbitals of the polyene
rotate so as to form the new σ bond of the ring compound.
Electron (Section 1.1) A particle of tiny mass (1/1845 of a
proton) and a single negative charge.
Electron affinity (Section 1.2) A measure of the tendency for an
atom or molecule to accept an electron.
Electronegativity (Section 1.3) The tendency for an atom to
attract electrons.
Electronic spectroscopy (Section 12.7) The measurement of the
absorption of energy when electromagnetic radiation of the
proper energy is provided. An electron is promoted from the
HOMO to the LUMO.
Electrophile (Section 1.7) A lover of electrons, a Lewis acid.
Electrophilic aromatic substitution (Section 14.4) The classic
substitution reaction of aromatic compounds with Lewis acids.
A hydrogen attached to the benzene ring is replaced by the Lewis
acid and the aromatic ring is retained in the overall reaction.
Electrophoresis (Section 23.2) A technique for separating amino
acids or chains of amino acids that takes advantage of the
different charge states of different amino acids (or their
polymers) at a given pH.
Elimination reaction (Section 7.8) A reaction that results in a
new π bond.
Enamine (Section 16.11) The nitrogen analogue of an enol, a
vinyl amine.These compounds are nucleophilic and useful in
alkylation reactions.
Enantiomers (Section 4.2) Nonsuperimposable mirror images.
Enantiotopic (Section 15.6) Enantiotopic hydrogens are
chemically and spectroscopically equivalent except in the
presence of optically active (single enantiomer) reagents.
Endergonic (Section 8.2) A reaction in which the products are
less stable than the starting materials.
endo (Section 12.12) Aimed “inside” the cage in a bicyclic
molecule. In a Diels–Alder reaction, the endo product generally
has the substituents aimed toward the newly produced double
bond.
Endothermic reaction (Section 1.6) A reaction in which the
bonds in the products are higher energy than those in the starting
materials. In an endothermic reaction the product is less stable
than the starting material.
Enol (Section 10.8) A vinyl alcohol.These compounds usually
equilibrate with the more stable keto forms.
Enolate (Section 19.2) The resonance-stabilized anion formed on
treatment of an aldehyde or ketone containing an α hydrogen
with base.
Enone (Section 19.6) A molecule with an alkene and a ketone or
aldehyde. Usually the π systems are conjugated.
Enthalpy change (⌬H°) (Section 1.6) The difference in total
bond energies between starting material and product in their
standard states.

GLOSSARY G-7
Entropy change (⌬S°) (Section 8.2) The difference in disorder
between the starting material and product in their standard
states.
Epimers (Section 22.2) Stereoisomers that differ at a single
stereogenic atom.
Episulfonium ion (Section 21.2) A three-membered ring
containing a trivalent, positively charged sulfur atom.
Epoxidation (Section 10.4) The reaction between a π bond and a
peroxy acid to give an epoxide.
Epoxide (Section 7.10) A saturated three-membered ring
containing a single oxygen atom. See Oxirane.
Equatorial hydrogens (Section 5.2) The set of six hydrogens
more or less in the plane of the ring in chair cyclohexane. These
hydrogens are interconverted with the set of axial hydrogens
through ring “flipping” of the chair.
Equilibrium constant (K) (Section 8.2) The equilibrium constant
is related to the difference in energy between starting material
and products (ΔG°) in the following way: K = e
-ΔG °/RT
.
Essential amino acid (Section 23.2) Any of the 10 amino acids
that cannot be synthesized by humans and must be ingested
directly.
Ester hydrolysis (Section 18.8) The conversion of an ester into an
acid through treatment with an acid catalyst in excess water.The
reverse of Fischer esterification. This reaction also occurs in base,
and is called saponification in that case.
Ethene (Section 3.2) The simplest alkene is . Its
common name is ethylene.
Ether (Section 6.8) A compound of the general structure ROR or
ROR′.
Ethyl compounds (Section 2.5) Substituted ethanes;
compounds.
Ethylene (Section 3.2) The simplest alkene, It is
more properly known as ethene, a name that is rarely used.
Exergonic (Section 8.2) A reaction in which the products are
more stable than the starting materials.
exo (Section 12.12) Aimed “outside”the cage in a bicyclic
molecule. In a Diels–Alder reaction, the exo product generally has
the substituents aimed away from the newly produced double
bond.
Exothermic reaction (Section 1.6) A reaction in which the bonds
in the products are lower energy than those in the starting
materials. In an exothermic reaction, the product is more stable
than the starting meerial.
Extinction coefficient (Section 12.7) The proportionality
constant e in Beer’s law, A = log I
0
/I = elc.
Fatty acids (Section 17.8) Long-chain carboxylic acids generated
by the hydrolysis of fats. Fatty acids are derived from acetic acid
and always contain an even number of carbons.
First-order reaction (Section 8.4) A reaction for which the rate
depends on the product of a rate constant and the concentration
of a single reagent.
First-order spectrum (Section 15.6) An NMR spectrum whose
coupling and chemical shifts can be interpreted directly. In a
first-order spectrum the coupling between hydrogens follows the
n + 1 rule.
H
2
C
P
CH
2
.
CH
3
CH
2
O
X
H
2
C
P
CH
2
Fischer esterification (Section 17.7) The conversion of a
carboxylic acid into an ester by treatment with an acid catalyst in
excess alcohol.The reverse of ester hydrolysis.
Fischer projection (Section 22.2) A schematic stereochemical
representation. In sugars, the aldehyde group is placed at the top
and the primary alcohol at the bottom. Horizontal bonds are
taken as coming toward the viewer and vertical bonds as
retreating.
Fluxional structure (Section 20.7) In a molecule with a fluxional
structure, a given atom does not have fixed nearest neighbors.
Instead, the framework atoms move about over time, each being
bonded on time average to all the others.
Force constant (Section 15.4) A property of a bond related to the
bond strength: to the stiffness of the bond. Bonds with high force
constants absorb at high frequency in the IR.
Formal charge (Section 1.3) The charge an atom has when the
number of electrons of that atom does not match the number of
protons for that atom.
Fragmentation pattern (Section 15.3) The characteristic
spectrum of ions formed by decomposition of a parent ion
produced in a mass spectrometer when a molecule is bombarded
by high-energy electrons.
Free radical (Section 6.3) A neutral molecule containing an odd,
unpaired electron. Also simply called radical.
Friedel–Crafts acylation (Section 14.6) The electrophilic
substitution of aromatic molecules with acyl chlorides facilitated
by strong Lewis acids, usually AlCl
3
.
Friedel–Crafts alkylation (Section 14.5) The electrophilic
substitution of aromatic molecules with alkyl chlorides catalyzed
by strong Lewis acids, usually AlCl
3
.
Frost circle (Section 13.6) A device used to find the relative
energies of the molecular orbitals of planar, cyclic, fully
conjugated molecules. A polygon corresponding to the ring size
of the molecule is inscribed in a circle, vertex down. The
intersections of the polygon with the circle give the relative
positions of the molecular orbitals.
Functional group (Section 1.4) An atom or group of atoms that
generally reacts the same way no matter what molecule it is in.
Furan (Section 13.9) An aromatic five-membered ring compound
containing four CH units and one O atom.
Furanose (Section 22.2) A sugar containing a five-membered
cyclic ether.
Furanoside (Section 22.4) A furanose in which the anomeric OH
has been converted into an acetal.
Fused (Section 5.7) Two rings sharing only two carbons.
Gabriel synthesis (Section 23.2) A synthesis of amino acids
that uses phthalimide as a source of the amine nitrogen.
Overalkylation is avoided by decreasing the nucleophilicity of the
nitrogen in this way.
..
O
..

Y
X
.
.
X
+ Y
Y
X
..
–
+
X + Y
G-8 GLOSSARY
R
OH
H
OR
C
Gas chromatogaphy (GC) (Section 15.2) A method of separation
in which molecules are forced to equilibrate between the moving
gas phase and a stationary phase packed in a column.The less
easily a molecule is adsorbed in the stationary phase, the faster it
moves through the column.
GC/IR (Section 15.2) The combination of gas chromatography
and infrared spectroscopy in which the molecules separated by a
gas chromatograph are led directly into an infrared spectrometer
for analysis.
GC/MS (Section 15.2) The combination of gas chromatography
and mass spectrometry in which the molecules separated by a gas
chromatograph are led directly into a mass spectrometer for
analysis.
Gel-filtration chromatography (Section 23.4) A
chromatographic technique that relies on polymeric beads
containing molecule-sized holes. Molecules that fit easily into the
holes pass more slowly down the column than larger molecules,
which fit less well into the holes.
Gem-diol (Section 16.6) A diol with both OH groups on the
same carbon.
Geminal (Section 10.6) A disubstituted compound with both
substituents on the same carbon.
Gibbs free energy change (⌬G°) (Section 8.2) The difference in
free energy during a reaction.The parameter ΔG° is composed
of an enthalpy (ΔH°) term and an entropy (ΔS°) term. ΔG° =
ΔH° - T ΔS°.
Glycol (Section 6.6) A dialcohol. See Diol.
Glycolysis (Section 22.3) The process of breaking carbohydrates
into smaller carbohydrates.
Glycoside (Section 22.4) A sugar in which the anomeric OH at
C(1) has been converted into an OR group.
Grignard reagent (Section 6.3) A strongly basic organometallic
reagent formed from a halide and magnesium in an ether solvent.
An important and characteristic reaction is the addition to
carbonyl groups. The simplest formulation is RMgX.
Haloform (Section 19.4) A compound of the structure HCX
3
,
where X = F, Cl, Br, or I.
Haloform reaction (Section 19.4) The conversion of a methyl
ketone into a molecule of a carboxylic acid and a molecule of
haloform. The trihalo carbonyl compound is formed, base adds to
the carbonyl group, and the trihalomethyl anion (
-
CX
3
) is
eliminated.The reaction works for X = Cl, Br, or I.
Halohydrin (Section 10.2) A molecule with a halogen and a
hydroxy group. Halohydrins are formed when an alkene reacts
with X
2
in H
2
O.
Hammond postulate (Section 8.8) The transition state for an
endothermic reaction will resemble the product. It can be
equivalently stated as, The transition state for an exothermic
reaction will resemble the starting material.
Haworth form (Section 22.2) The representation of sugars in
which they are shown as planar rings with the ring perpendicular
to the plane of the paper.
:
Heat of formation (⌬H
f
°) (Section 3.6) The heat evolved or
required for the formation of a molecule from its constituent
elements in their standard states.The more negative the heat of
formation, the more stable the molecule.
Heisenberg uncertainty principle (Section 1.1) For an electron,
the uncertainty in position times the uncertainty in momentum
(or speed) is a constant. We cannot know the exact position and
momentum (speed) of an electron at the same time.
␣-Helix (Section 23.4) A right-handed coiled form adopted by
many proteins in their secondary structures.
Hell–Volhard–Zelinsky (HVZ) reaction (Section 19.4) The
conversion of a carboxylic acid into either the α-bromo acid or
the α-bromo acid bromide through reaction with PBr
3
/Br
2
.
Hemiacetal (Section 16.9) The initial product when an alcohol
adds to an aldehyde or ketone.
Heteroaromatic compound (Section 13.9) See Heterobenzene.
Heterobenzene (Section 13.6) A benzene ring in which one (or
more) ring carbons is replaced with another heavy (nonhydrogen)
atom.
Heterogeneous catalysis (Section 10.2) A catalytic process in
which the catalyst is insoluble.
Heterolytic bond cleavage (Section 1.6) The breaking of a bond
to produce a pair of oppositely charged ions.
Hexose (Section 22.2) A six-carbon sugar.
High-performance liquid chromatography (HPLC) (Section
15.2) An especially effective version of column chromatography
in which the stationary phase consists of many tiny spheres that
provide an immense surface area for absorption.
Hofmann elimination (Section 7.9) The formation of the less
substituted alkene in an elimination reaction.
Hofmann rearrangement (Section 18.14) The formation of
amines through the treatment of amides with bromine and base.
An intermediate isocyanate is hydrolyzed to a carbamic acid that
decarboxylates.
HOMO (Section 3.17) Highest occupied molecular orbital.
Homogeneous catalysis (Section 10.2) A catalytic process in
which the catalyst is soluble.
Homolytic bond cleavage (Section 1.6) The breaking of a bond
to form two neutral species.
Homotopic (Section 15.6) Homotopic hydrogens are identical,
both chemically and spectroscopically, under all circumstances.
