Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.

+
NR
2
C
R
R
or
C
R
R
C
R
H
NR NR
NH
O
O
R
OH
R
OH
C
GLOSSARY G-9
is lower in energy than
Hückel’s rule (Section 13.6) All planar, cyclic, fully conjugated
molecules with 4n + 2 π electrons will be aromatic (especially
stable).The rule works because such molecules will have
molecular orbital systems in which all bonding molecular orbitals
are full and all antibonding molectular orbitals are empty. In such
molecules, the bonding degenerate molecular orbitals are filled.
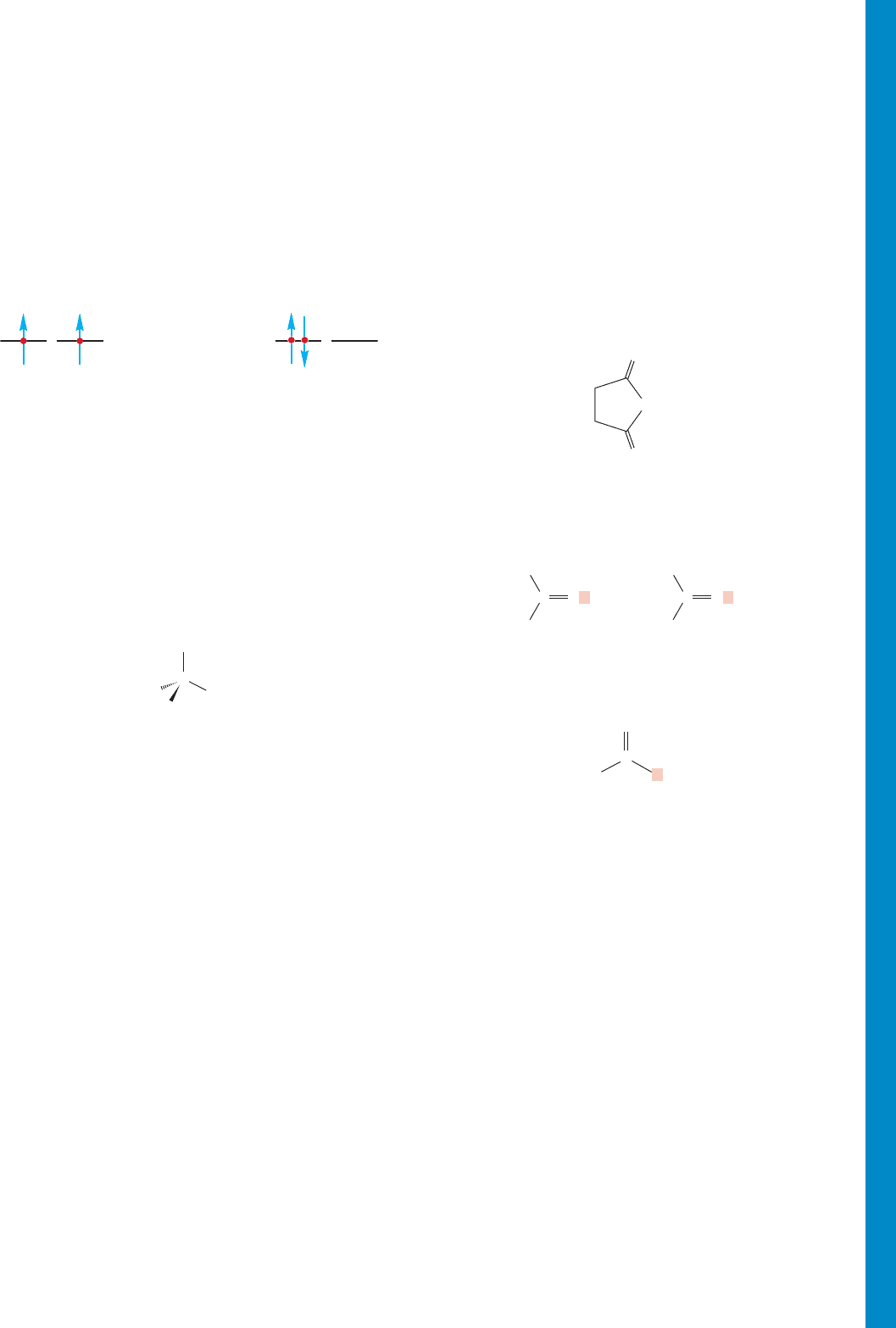
Hund’s rule (Section 1.2) For a given electronic configuration, the
state with the greatest number of parallel spins is the lowest in
energy. That is,
Hunsdiecker reaction (Section 17.7) The conversion of silver
salts of carboxylic acids into alkyl halides, usually bromides.
Hybridization (Section 2.2) A mathematical model in which
atomic orbital wave functions are combined to produce new,
combination, or hybrid orbitals. The new orbitals are made up of
fractions of the pure atomic orbital wave functions.Thus, an sp
3
hybrid is made of three parts p wave function and one part s wave
function.
Hybrid orbitals (Section 2.2) Orbitals that result from
hybridization.
Hydrate (Section 16.6) The product of the reaction of a carbonyl
compound with water.
Hydration (Section 9.7) The addition of water to a molecule.
This reaction is generally acid catalyzed.
Hydride (Section 2.4) A negatively charged hydrogen ion (H
-
)
bearing a pair of electrons.
Hydride shift (Section 9.9) The migration of hydrogen with a
pair of electrons (H
-
).
Hydroboration (Section 9.10) The addition of BH
3
(in
equilibrium with the dimer B
2
H
6
) to π systems to form
alkylboranes. These alkylboranes can be further converted into
alcohols. Addition to an unsymmetrical alkene proceeds so as to
give the less substituted alcohol.
Hydrocarbon (Section 2.1) A molecule containing only carbon
and hydrogen.
Hydrocarbon cracking (Section 11.2) The thermal treatment of
high molecular weight hydrocarbons to give lower molecular
weight fragments. Bonds are broken to give radicals that abstract
hydrogen to give alkanes, undergo β-cleavage, and
disproportionate to give alkenes and alkanes.
Hydrogenation (Section 10.2) Addition of hydrogen (H
2
) to the
π bond of an alkene to give an alkane. A soluble or insoluble
metallic catalyst is necessary. Alkynes also undergo hydrogenation
to give alkanes, or under special conditions, alkenes.
Hydrogen bonding (Section 6.4) A low-energy bond between a
pair of electrons, usually on oxygen, and a hydrogen.
:
:
Hydrophilic (Section 17.8) “Water-loving,” hence a polar group
soluble in water.
Hydrophobic (Section 17.8) “Water-hating,”hence a nonpolar
group insoluble in water.
Hyperconjugation (Section 9.5) The stabilization of carbocations
through the overlap of a filled σ orbital with an empty 2p orbital
on carbon.
Imide (Section 18.2) A compound containing the structure
such asO
P
C
O
NH
O
C
P
O,
Imine (Section 16.11) The nitrogen analogue of a ketone or
aldehyde. See Schiff base.
Iminium ion (Section 16.11) A compound of the structure
Inductive effects (Section 9.6) Electronic effects transmitted
through σ bonds. All bonds between different atoms are polar,
and thus many molecules contain dipoles.These dipoles can
affect reactions through induction.
Infrared (IR) spectroscopy (Section 15.4) In IR, absorption of
IR energy causes bonds to vibrate and rotate.The characteristic
vibrational frequencies can be used to determine the functional
groups present in a molecule.
Inhibitors (Section 11.4) Species that can react with a radical,
thus destroying it. Inhibitors interrupt chain reactions.
Initiation (Section 11.4) The first step in a radical chain reaction
in which a free radical is produced that can start the chain-
carrying or propagation steps.
Integral (Section 15.6) The determination of the relative
numbers of hydrogens corresponding to the signals in an NMR
spectrum.
Inversion of configuration (Section 7.4) The change in the
handedness of a molecule during certain reactions. Generally,
inversion of stereochemistry means that an (R) starting material
would be transformed into an (S) product.

G-10 GLOSSARY
R
NCO
C O
C
R
R
Ion (Section 1.2) A charged atom or molecule.
Ion-exchange chromatography (Section 23.4) A separation
technique that relies on differences in the affinity of molecules
for a charged substrate.
Ionic bond (Section 1.2) The electrostatic attraction between a
positively charged atom or group of atoms and a negatively
charged atom or group of atoms.
Ionization potential (Section 1.2) A measure of the tendency of
an atom or molecule to lose an electron.
Ipso attack (Section 14.12) Addition to an aromatic ring at a
position already occupied by a nonhydrogen substituent.
Isobutyl group (Section 2.8) The (CH
3
)
2
CHCH
2
group.
Isocyanate (Section 18.14) A compound of the structure
Isoelectric point (pI ) (Section 23.2) The pH at which the amino
acid has no net charge.
Isomers (Section 2.5) Molecules of the same formula but
different structures.
Isoprene (Section 12.13) 2-Methyl-1,3-butadiene.
Isoprene rule (Section 12.13) Most terpenes are composed of
isoprene units combined in “head-to-tail”fashion.
Isopropyl group (Section 2.8) The (CH
3
)
2
CH group.
Isotope effect (Section 21.4) The effect on the rate of a reaction
of the replacement of an atom with an isotope.The replacement
of hydrogen with deuterium is especially common.
Karplus curve (Section 15.6) The dependence between the
coupling constant of two hydrogens on adjacent atoms and the
dihedral angle between the carbon–hydrogen bonds.
Kekulé forms (Section 13.3) Resonance forms for benzene in
which overlap between adjacent carbons is emphasized. These
forms superficially resemble 1,3,5-cyclohexatriene.
Ketene (Section 12.3) A compound of the structure
Keto–enol tautomerization (Section 19.2) The equilibrium
between a carbonyl compound and the enol that can be formed if
the carbonyl compound has an α hydrogen.The equilibrium
almost always favors the carbonyl species.
-Keto ester or acetoacetate synthesis (Section 19.5) The α
hydrogen between two carbonyl groups can be deprotonated with
weak base.The anion formed will react with an electrophile in
this synthesis.
Ketone (Section 10.5) A compound containing a carbon–oxygen
double bond in which the carbon is attached to two other
carbons.
Ketose (Section 22.2) A sugar containing not the usual aldehyde
group, but a ketone.
Kiliani–Fischer synthesis (Section 22.3) A method of
lengthening the chain of an aldose by one carbon atom. A pair of
sugars, epimeric at the new C(1), is produced.
Kinetic control (Section 8.8) A reaction in which the product
distribution is determined by the heights of the different
transition states leading to products.
Kinetic enolate (Section 19.7) The most easily formed enolate. It
may or may not be the same as the most stable possible enolate,
the thermodynamic enolate.
Kinetic resolution (Section 23.2) A technique for separating a
pair of enantiomers based on the selective transformation of one
of them, often by an enzyme.
Kinetics (Section 8.8) The study of the rates of reactions.
Knoevenagel condensation (Section 19.6) Any of a number of
condensation reactions related to the crossed aldol condensation.
A stabilized anion, often an enolate, is first formed and then adds
to the carbonyl group of another molecule. Dehydration usually
leads to the formation of the final product.
Kolbe electrolysis (Section 17.7) The electrochemical conversion
of carboxylate anions into hydrocarbons. The carboxyl radical
produced loses CO
2
to give an alkyl radical that dimerizes.
Lactam (Section 17.7) A cyclic amide.
Lactone (Section 17.7) A cyclic ester.
Leaving group (Section 7.3) The departing group in a
substitution or elimination reaction.
Le Châtelier’s principle (Section 8.2) A system at equilibrium
adjusts so as to relieve any stress on it.
Levorotatory (Section 4.4) The rotation of the plane of plane-
polarized light in the counterclockwise direction.
Lewis acid (Section 1.7) Any species that reacts with a Lewis
base. An electrophile.
Lewis base (Section 1.7) Any species with a reactive pair of
electrons. A nucleophile.
Lewis structure (Section 1.3) In a Lewis structure, every electron
(except the 1s electrons for atoms other than hydrogen or helium)
is shown as a dot. In slightly more abstract Lewis structures,
electrons in bonds are shown as lines connecting atoms.
Lithium diisopropylamide (LDA) (Section 19.2) An effective
base for alkylation reactions of carbonyl compounds.This
compound is a strong base but a poor nucleophile, and thus is
effective at removing α hydrogens but not at promoting additions
to carbonyl compounds.
Lobry de Bruijn–Alberda van Ekenstein reaction (Section 22.4)
The base-catalyzed interconversion of aldo and keto sugars.The
key intermediate is the double enol formed by protonation of an
enolate.
Lone-pair electrons (Section 1.3) Electrons in an orbital that is
not involved in binding atoms. See Nonbonding electrons.
Long-range coupling (Section 15.6) Any coupling between
nuclei separated by more than three bonds. It is usually observed
only in high-field
1
H NMR spectroscopy.

GLOSSARY G-11
LUMO (Section 3.17) Lowest unoccupied molecular orbital.
M + 1 peak (Section 15.3) The signal in a mass spectrum
that comes from a +1 isotope (usually due to
13
C) of the
molecular ion.
Magid’s second rule (Section 19.11) “Always try the Michael
reaction first.”
Magid’s third rule (Section 19.14) “In times of desperation
and/or despair, when all attempts at solution seem to have failed,
try a hydride shift.”
Malonic ester synthesis (Section 19.5) The alkylation of malonic
esters to produce substituted diesters, followed by hydrolysis and
decarboxylation of the malonic acids to give substituted acetic
acids.
Mannich reaction (Section 19.13) The reaction between a ketone
or aldehyde with an amine to give an imine, which then reacts
with a nucleophile.
Markovnikov’s rule (Section 9.5) In additions of Lewis acids to
alkenes, the Lewis acid will add to the less substituted end of the
alkene.The rule works because additions of Lewis acids in that
way produce the more substituted, more stable carbocation.
Mass spectrometry (MS) (Section 15.3) The analysis of the ions
formed by bombardment of a molecule with high-energy
electrons. High-resolution MS can give the molecular formula of
an ion, and an analysis of the fragmentation pattern can give
information about the structure.
Meerwein–Ponndorf–Verley–Oppenauer (MPVO) equilibration
(Section 19.14) A method of oxidizing alcohols to carbonyl
compounds, or, equivalently, of reducing carbonyls to alcohols,
that uses an aluminum atom to clamp the partners in the reaction
together. A hydride shift is involved in the crucial step.
Meisenheimer complex (Section 14.12) The intermediate in
nucleophilic aromatic substitution formed by the addition of a
nucleophile to an aromatic compound activated by electron-
withdrawing groups, often NO
2
.
Mercaptan (Section 6.9) A thiol, RSH.The sulfur counterpart of
an alcohol.
Mercaptide (Section 6.9) The sulfur counterpart of an alkoxide,
RS
-
.
Meso compound (Section 4.8) An achiral compound containing
stereogenic atoms.
Messenger RNA (mRNA) (Section 23.5) A polynucleotide
produced from DNA whose base sequence codes for amino acid
assembly.
Meta (Section 13.7) 1,3-Substitution on a benzene ring.
Methane (Section 2.1) The simplest stable hydrocarbon, CH
4
.
Methine group (Section 2.8) The CH group.
Methyl anion (Section 2.4)
-
CH
3
.
Methyl cation (Section 2.4)
+
CH
3
.
Methyl compounds (Section 2.3) Substituted methanes,
compounds.
Methylene group (Section 2.7) The CH
2
group.
Methyl radical (Section 2.4)
Micelle (Section 17.8) An aggregated group of fatty acid salts (or
other molecules) in which a hydrophobic center consisting of the
.
CH
3
.
CH
3
O
X
:
hydrocarbon chains is protected from an aqueous environment by
a spherical skin of polar hydrophilic groups.
Michael reaction (Section 19.6) The addition of a nucleophile,
often an enolate, to the β position of a double bond of an α,β-
unsaturated carbonyl group.
Microscopic reversibility (Section 8.6) The notion that the
lowest-energy path for a reaction in one direction will also be the
lowest-energy path in the other direction.
Molecular ion (Section 15.3) The charged species obtained by
removing an electron from a molecule.
Molecular orbital (Section 1.1) An orbital not restricted to the
region of space surrounding an atom, but extending over several
atoms in a molecule. Molecular orbitals are formed through the
overlap of atomic or molecular orbitals. Molecular orbitals can be
bonding, nonbonding, or antibonding.
Molozonide (Section 10.5) The initial product of the reaction of
ozone and an alkene.This molecule contains a five-membered
ring with three oxygen atoms in a row. See Primary ozonide.
Monosaccharide (Section 22.6) A simple six-carbon sugar.
Mutarotation (Section 22.4) The interconversion of anomeric
sugars in which an equilibrium mixture of α and β forms is
reached.
n + 1 rule (Section 15.6) The number of lines for a hydrogen will
be n + 1, where n is the number of equivalent adjacent hydrogens.
Natural product (Section 2.12) A product formed in Nature,
which does not include compounds made in the lab.
Neighboring group effect (Section 21.1) A general term for the
influence of an internal nucleophile (very broadly defined) on the
rate of a reaction, or on the structures of the products produced.
Newman projection (Section 2.5) A convention used to draw
what one would see if one could look down a bond.The groups
attached to the front atom are drawn in as three lines. The back
atom is represented as a circle to which its attached bonds are
drawn. Newman projections are enormously useful in seeing
spatial (stereochemical) relationships in molecules.
Ninhydrin (Section 23.3) The hydrate of indan-1,2,3-trione,
a molecule that reacts with amino acids to give the purple dye
used in quantitative analysis of amino acids.
Nitrene (Section 18.14) A reactive intermediate containing a
neutral, monovalent nitrogen atom.The nitrogen counterpart of a
carbene.
Nitrile (Section 18.2) A compound of the structure RCN, also
commonly called a cyanide.
NMR spectrum (Section 2.14) The result of nuclear magnetic
resonance spectroscopy. A spectrum has ppm as the x-axis and
signal intensity as the y-axis.
Node (Section 1.2) The region of zero electron density separating
regions of opposite sign in an orbital. At a node the sign of the
wave function is zero.
Nonbonding electrons (Section 1.3) Electrons in an orbital that
is not involved in binding atoms. See Lone-pair electrons.
Nonbonding orbital (Section 1.5) An orbital that is neither
bonding nor antibonding. An electron in a nonbonding orbital
neither helps to hold the molecule together nor tears it apart.
G-12 GLOSSARY
OR
OR
OR
C
R
Nonreducing sugar (Section 22.6) A carbohydrate containing no
amount of an oxidizable aldehyde group.
Norbornyl system (Section 21.3) The bicyclo[2.2.1]heptyl
system.
Nuclear magnetic resonance (NMR) spectroscopy (Section
2.14) Spectroscopy that detects the absorption of energy as the
lower-energy nuclear spin state in which the nuclear spin is
aligned with an applied magnetic field flips to the higher-energy
spin state in which it is aligned against the field. See also
Chemical shift (δ), Coupling constant ( J), and Integral.
Nucleic acid (Section 23.5) The polynucleotides DNA and RNA.
Nucleophile (Section 1.7) A Lewis base. A strong nucleophile
has a high affinity for a carbon 2p orbital.
Nucleophilic aromatic substitution (Section 14.12) The
substitution reaction that occurs when a nucleophile replaces a
group on an aromatic ring, usually a halogen.
Nucleophilicity (Section 7.4) The strength of a nucleophile.
Nucleoside (Section 23.5) A sugar, either ribose or deoxyribose,
bonded to a heterocyclic base at its 1′ position.
Nucleotide (Section 23.5) A phosphorylated nucleoside; one of
the monomers of which DNA and RNA are composed.
Nucleus (Section 1.1) The positively charged core of an atom
containing the protons and neutrons.
Octet rule (Section 1.2) The notion that special stability attends
the filling of the 2s and 2p atomic orbitals to achieve the
electronic configuration of neon, a noble gas.
Off-resonance decoupling (Section 15.7) A technique used in
13
C NMR in which coupling between
13
C and
1
H is restricted to
the hydrogens directly attached to the carbon.The number of
directly attached hydrogens can be determined from the
multiplicity of the observed signal for the carbon.
Olefins (Section 3.8) Hydrocarbons of the general formula,
C
n
H
2n
.These molecules are systematically called alkenes, and
they contain carbon–carbon double bonds.
Optical activity (Section 4.4) The rotation by a molecule of the
plane of plane-polarized light.
Orbital (Section 1.1) A three-dimensional representation of the
solution to the Schrödinger equation. See Wave function (ψ).
Orbital interaction diagram (Section 1.5) A way of analyzing the
interaction between atoms that leads to bonding.
Organocuprate (Section 10.4) An organometallic reagent
(R
2
CuLi) notable for its ability to react with primary or
secondary halides ( ) to generate hydrocarbons, ,
and to add to the β position of α,β-unsaturated carbonyl
compounds.
Organolithium reagent (Section 6.3) A strongly basic
organometallic reagent, , formed from a halide and
lithium. A characteristic reaction is addition to carbonyl groups.
Organometallic reagent (Section 6.3) Molecule that contains
both carbon and a metal. Usually carbon is at least partially
covalently bonded to the metal. Examples are Grignard reagents,
organolithium reagents, and lithium organocuprates.
Ortho (Section 13.7) 1,2-Disubstituted on a benzene ring.
R
O
Li
R
O
R¿R¿
O
X
Orthogonal orbitals (Section 1.5) Two noninteracting orbitals.
The bonding interactions are exactly balanced by antibonding
interactions.
Osazone (Section 22.4) A 1,2-phenylhydrazone formed by
treatment of a sugar with three equivalents of phenylhydrazine.
Oxaphosphetane (Section 16.17) The four-membered
intermediate in the Wittig reaction that contains two carbons, a
phosphorus, and an oxygen.
Oxirane (Section 7.10) A three-membered ring containing
oxygen, also called an epoxide or oxacyclopropane.
Oxonium ion (Section 3.19) A molecule containing a trivalent,
positively charged, oxygen atom, R
3
O
+
.
Oxymercuration (Section 10.3) The mercury-catalyzed
conversion of alkenes into alcohols. Addition is in the
Markovnikov sense, and there are no rearrangements. A three-
membered ring containing mercury is an intermediate in the
reaction. Alkynes also undergo oxymercuration to give enols that
are rapidly converted into carbonyl compounds under the
reaction conditions.
Ozonide (Section 10.5) The product of rearrangement of the
primary ozonide formed on reaction of ozone with an alkene.
C
C
O
O
O
Ozonolysis (Section 10.5) The reaction of ozone with π systems.
The intermediate products are ozonides that can be transformed
into carbonyl-containing compounds of various kinds.
Paired spin (Section 1.2) Two electrons with opposite spins have
paired spins.
Para (Section 13.7) 1,4-Disubstituted on a benzene ring.
[n]Paracyclophane (Section 14.3) A benzene ring bridged 1,4
(para) with a chain of atoms.
Parallel spin (Section 1.2) Two electrons with the same spin have
parallel spins.These electrons can not occupy the same orbital.
Parent ion (p) (Section 15.3) An ion formed in the mass
spectrometer through loss of a single electron. It can undergo
fragmentation reactions before detection.
Pauli principle (Section 1.2) No two electrons in an atom or
molecule may have the same values of the four quantum
numbers.
Pentose (Section 22.2) A five-carbon sugar.
Peptide (Section 6.1) A polyamino acid in which the constituent
amino acids are linked through amide bonds.They are
distinguished from proteins only by the length of the polymer.
Peptide bond (Section 23.2) The amide bond that links amino
acids.
Ortho esters (Section 17.7) Compounds of the structure
H
N
..
..
N
C
C
O
O
O
GLOSSARY G-13
Pericyclic reaction (Section 20.1) A reaction in which the
maintenance of bonding overlap between the lobes of the orbitals
involved in bond making and breaking is controlling. All single-
barrier (concerted) reactions can be regarded as pericyclic reactions.
Phenonium ion (Section 21.3) The benzenonium ion produced
through intramolecular displacement of a leaving group by the π
system (not the σ system) of an aromatic ring.
Phenyl (Ph) (Section 13.7) A common and systematic name for
the benzene ring as substituent,
Pi () orbitals (Section 3.2) Molecular orbitals made from the
overlap of p orbitals.
pK
a
(Section 6.4) The negative logarithm of the acidity constant.
A strong acid has a low pK
a
, a weak acid has a high pK
a
.
Plane-polarized light (Section 4.4) Light that has been passed
through a polarizing filter.
-Pleated sheet (Section 23.4) One of the common forms of
secondary peptide structure in which hydrogen bonding holds
chains of amino acids roughly in parallel lines.
Polar covalent bond (Section 1.3) Any shared electron bond
between two different atoms must be polar. Two nonidentical
atoms must have different electronegativities and will attract the
shared electrons to different extents, creating a dipole.
Polarimeter (Section 4.5) A device for measuring the amount of
rotation of the plane of plane-polarized light.
Polyenes (Section 3.4) Any molecule containing multiple double
bonds.
Polynuclear aromatic compound (Section 13.10) An aromatic
molecule composed of two or more fused aromatic rings.
Polysaccharide (Section 22.6) A carbohydrate that is composed
of multiple saccharides.The complex carbohydrates are
polysaccharides.
␣ Position (Section 19.2) The position next to a functional group.
For example, the α position in a ketone is the position adjacent to
the carbon–oxygen double bond.
ppm Scale (Section 15.5) The chemical shift scale commonly
used in NMR spectroscopy in which the positions of absorptions
are quoted as parts per million (ppm) of applied magnetic field.
On the ppm scale, the chemical shift is independent of the
magnetic field.
Primary amine (Section 6.7) An amine bearing only one R group
and two hydrogens.
Primary carbon (Section 2.8) A carbon atom attached to one
other carbon.
Primary ozonide (Section 10.5) The initial product of the
reaction of ozone and an alkene.This molecule contains a five-
membered ring with three oxygen atoms in a row. See
Molozonide.
C
6
H
5
O
X.
Primary structure (Section 23.4) The sequence of amino acids in
a protein.
Product-determining step (Section 7.6) The step in a multistep
reaction that determines the structure or structures of the products.
Propagation (Section 11.4) The product-producing steps of a
chain reaction. In the last propagation step, a molecule of product
is formed along with a new radical that can carry the chain.
Propargyl (Section 3.11) The common name for the
group.
Propyl group (Section 2.7) The CH
3
CH
2
CH
2
group.
Protecting group (Section 16.10) Sensitive functional groups in a
molecule can be protected by a reaction that converts them into a
less reactive group, called a protecting group. A protecting group
must be removable to regenerate the original functionality.
Protein (Section 6.1) A polyamino acid in which the constituent
amino acids are linked through amide bonds.They are
distinguished from peptides only through the length of the
polymer.
Protic solvent (Section 6.5) A solvent containing a hydrogen
easily lost as a proton. Examples are water and most alcohols.
Pseudo-first-order reaction (Section 8.4) A bimolecular reaction
in which the concentration of one reagent (usually the solvent)
does not change appreciably.
Pyranose (Section 22.2) A sugar containing a six-membered
cyclic ether.
Pyranoside (Section 22.4) A pyranose in which the anomeric OH
at C(1) has been converted into an OR group.
Pyridine (Section 13.9) Any compound containing the following
“azabenzene” ring system:
HC
q
C
O
CH
2
Pyrolysis (Section 11.2) The process of inducing chemical change
by supplying heat energy. See Thermolysis.
Pyrrole (Section 13.6) Any compound containing the following
ring structure:
Quantum numbers (Section 1.3) These numbers evolve from the
Schrödinger equation and characterize the various solutions to it.
They may have only certain values, and these values determine
the distance of an electron from the nucleus (n), the shape (l) and
orientation (m
l
) of the orbitals, and the electron spin (s).
Quaternary carbon (Section 2.8) A carbon attached to four other
carbons.
Quaternary structure (Section 23.4) The structure of a self-
assembled aggregate of two or more protein units.
Racemic mixture (racemate) (Section 4.4) A mixture containing
equal amounts of two enantiomeric forms of a chiral molecule.
Radical anion (Section 10.11) A negatively charged molecule
containing both a pair of electrons and an odd, unpaired electron.

G-14 GLOSSARY
Radical cation (Section 15.3) A species that is positively charged
yet contains a single unpaired electron. These are commonly
formed by ejection of an electron when a molecule is bombarded
with high-energy electrons in a mass spectrometer.
Random coil (Section 23.4) Disordered portions of a chain of
amino acids.
Raney nickel (Section 6.9) A good reducing agent composed of
finely divided nickel on which hydrogen has been adsorbed.
Rate constant (k) (Section 8.5) A fundamental property of a
reaction that depends on the temperature, pressure, and solvent,
but not on the concentrations of the reactants.
Rate-determining step (Section 7.6) The step in a reaction with
the highest-energy transition state.
Reaction mechanism (Section 7.2) Loosely speaking, How does
the reaction occur? How do the reactants come together? Are
there any intermediates? What do the transition states look like?
More precisely, a determination in terms of structure and energy
of the stable molecules, reaction intermediates, and transition
states involved in the reaction, along with a consideration of how
the energy changes as the reaction progresses.
Reactive intermediates (Section 2.4) Molecules of great
instability, and hence fleeting existence under normal conditions.
Most carbon-centered anions, cations, and radicals are examples.
Rearrangement (Section 9.9) The migration of an atom or group
of atoms from one place to another in a molecule.
Rearrangements are exceptionally common in reactions involving
carbocations.
Reducing sugar (Section 22.2) A sugar containing some amount
of an oxidizable free aldehyde group.
Regiochemistry (Section 3.18) The orientation of a reaction
taking place on an unsymmetrical substrate.
Regioselective reaction (Section 7.9) If a reaction may produce
one or more isomers and one predominates, the reaction is called
regioselective.
Resolution (Section 4.9) The separation of a racemic mixture
into its constituent enantiomeric molecules.
Resonance arrow (Section 1.4) A specific way of indicating that
two structures are resonance forms.
Resonance energy (Section 13.5) The energy lowering conferred
by the delocalization of electrons. In benzene, this is the amount
by which benzene is more stable than the hypothetical 1,3,5-
cyclohexatriene containing three localized double bonds. See
Delocalization energy.
Resonance forms (Section 1.4) Many molecules cannot be
represented adequately by a single Lewis form. Instead, two or
more different electronic representations must often be combined
to give a good description of the molecule.These different
representations are called resonance forms.
Retention of configuration (Section 7.4) The preservation of the
handedness of a molecule in a reaction. Generally, retention of
configuration means that an (R) starting material would be
transformed into an (R) product.
Retrosynthetic analysis (Section 16.15) A way of figuring out
how a compound might be synthesized by looking at what
compound was its immediate precursor. This process is applied
step-by-step until readily available starting materials are reached.
Ribonucleic acid (RNA) (Section 23.5) A polymer of nucleotides
made up of ribose units connected by phosphoric acid links. Each
sugar is attached at C(1′) to one of the bases, A, U, G, or C.
Robinson annulation (Section 19.11) A classic method for
construction of six-membered rings using a ketone and methyl
vinyl ketone. It involves a two-step sequence of a Michael
reaction between the enolate of the original ketone and methyl
vinyl ketone, followed by an intramolecular aldol condensation.
Rosenmund reduction (Section 18.6) The reduction of acid
chlorides to aldehydes using a poisoned catalyst and hydrogen.
Ruff degradation (Section 22.3) A method for shortening the
carbon backbone of a sugar by one carbon.The aldehyde carbon
at C(1) is lost and a new aldehyde created at the old C(2).
Saccharide (Section 22.1) A molecule whose formula can be
factored into C
x
(H
2
O)
y
. A sugar or carbohydrate.
Sandmeyer reaction (Section 14.7) The reaction of an aromatic
diazonium ion with cuprous salts to form substituted aromatic
compounds.
Sanger degradation (Section 23.4) A method for determining the
amino acid at the amino terminus of a chain using 2,4-
dinitrofluorobenzene. Unfortunately, the Sanger degradation
hydrolyzes, and thus destroys, the entire peptide.
Saponification (Section 17.8) Base-induced hydrolysis of an
ester, usually a fatty acid.
Saturated hydrocarbons (Section 2.12) Alkanes of the molecular
formula C
n
H
2n + 2
.
Saytzeff elimination (Section 7.8) Formation of the more
substituted alkene in an elimination reaction.
Schiff base (Section 16.11) The nitrogen analogue of a ketone or
aldehyde. See Imine.
Secondary amine (Section 6.7) An amine bearing one hydrogen
and two R groups.
Secondary carbon (Section 2.8) A carbon attached to two other
carbons.
Secondary structure (Section 23.4) Ordered regions of a protein
chain.The two most common types of secondary structure are
the α-helix and the β-pleated sheet.
Second-order reaction (Section 8.4) A reaction for which the
rate depends on the product of a rate constant and the
concentrations of two reagents.
Side chain (Section 23.2) The group attached to the α carbon of
an amino acid.
Sigma bond (Section 2.2) Any bond with cylindrical symmetry.
Sigmatropic shift (Section 20.5) The migration of an atom or
group, under orbital symmetry control, along a π system.
Silyl ether (Section 16.10) A molecule of the general form
R—O—SiR
3
.
Singlet carbene (Section 10.4) A singlet carbene contains only
paired electrons. In a singlet carbene, the two nonbonding
electrons have opposite spins and occupy the same orbital.
S
N
1 Reaction (Section 7.6) Substitution, nucleophilic,
unimolecular. An initial ionization is followed by attack of the
nucleophilic solvent.
S
N
2 Reaction (Section 7.4) Substitution, nucleophilic,
bimolecular. In this reaction, the nucleophile dispaces the leaving

–
O
S
R
R
+
GLOSSARY
G-15
Nu
..
–
R+
L
Nu
..
–
R+
L
group by attack from the rear.The stereochemistry of the starting
material is inverted.
Soap (Section 17.8) The sodium salt of a fatty acid.
Solvated electron (Section 10.11) The species formed when
sodium is dissolved in ammonia.The product is a sodium ion and
an electron surrounded by ammonia molecules. This electron can
add to alkynes (and some other π systems) in a reduction step.
Solvation (Section 6.4) The stabilizing effects of a solvent,
typically the stabilization of ions by a polar solvent.
Solvolysis reaction (Section 7.6) In such S
N
1 and S
N
2 reactions,
the solvent acts as the nucleophile.
Specific rotation (Section 4.5) The rotation of plane-polarized
light induced by a solution of a molecule of concentration
1 g/mL in a tube 10 cm long.
Spectroscopy (Section 2.14) The study of the interactions
between electromagnetic radiation and atoms and molecules.
sp Hybrid (Section 2.2) A hybrid orbital made by the
combination of one s and one p atomic orbital.
sp
2
Hybrid (Section 2.2) A hybrid orbital made by the
combination of one s orbital and two p orbitals.
sp
3
Hybrid (Section 2.2) A hybrid orbital made by the
combination of one s orbital and three p orbitals.
Spiro (Section 5.7) A structural motif in which two rings share a
single carbon.
Staggered ethane (Section 2.5) The energy minimum
conformation of ethane in which the carbon–hydrogen bonds
(and the electrons in them) are as far apart as possible.
Starch (Section 22.6) A nonlinear polymer of glucose containing
amylose, an α-linked linear polymer of glucose.
Stereochemistry (Section 4.1) The physical and chemical
consequences of the arrangement in space of the atoms in
molecules.
Stereogenic atom (Section 4.3) An atom, usually carbon, of such
nature and bearing groups of such nature that it can have two
nonequivalent configurations.
Steric requirements (Section 2.9) The space required by an atom
or group of atoms.
Steroid (Section 12.14) A class of four-ring compounds, always
containing three six-membered rings and one five-membered
ring.The ring system can be substituted in many ways, but the
rings are always connected in the following fashion:
Strecker synthesis (Section 23.2) A synthesis of amino acids
using an aldehyde, cyanide, and ammonia, followed by hydrolysis.
Structural isomers (Section 2.7) Molecules of the same formula
that differ structurally.
Substituent (Section 2.3) Any atom or group of atoms other than
hydrogen attached to a molecule.
Substitution reaction (Section 7.1) The replacement of a leaving
group in by a nucleophile, Nu
-
.Typically,
:
R
O
L
Sugar (Section 22.1) A molecule whose formula can be factored
into C
x
(H
2
O)
y
. A saccharide or carbohydrate.
Sulfide (Section 6.9) The sulfur counterpart of an ether, RSR′.
See Thioether.
Sulfone (Section 16.16) , the final product of
oxidation of a sulfide with hydrogen peroxide.
Sulfonium ion (Section 7.10) A positively charged sulfur ion,
R
3
S
+
.
Sulfoxide (Section 16.16) A compound of the structure
R
O
SO
2
O
R
Superacid (Section 14.13) A number of highly polar, highly
acidic, but weakly or nonnucleophilic solvent systems. Superacids
are often useful in stabilizing carbocations, at least at low
temperature.
Suprafacial motion (Section 20.5) Migration of a group in a
sigmatropic shift in which bond breaking and bond making take
place on the same side of the π system.
syn Addition (Section 9.10) The addition of two groups to a π
bond in which the two groups end up being on the same side.
syn Elimination (Section 7.9) An elimination reaction in which
the dihedral angle between the breaking bonds, usually
, is 0°.
Tautomerization (Section 10.8) The process through which
molecules related through the change of position of a single
hydrogen interconvert.
Tautomers (Section 1.4) Molecules related through the change of
position of a single hydrogen.
Termination (Section 11.4) The mutual annihilation of two
radicals. Termination steps destroy chain-carrying radicals and
end chains through bond formation.
Terpenes (Section 12.13) Compounds whose carbon skeletons
are composed of isoprene units.
Tertiary amine (Section 6.7) An amine bearing no hydrogens and
three R groups.
Tertiary carbon (Section 2.8) A carbon attached to three other
carbons.
Tertiary structure (Section 23.4) The structure of a protein
induced by its folding pattern.
Tetrahedral intermediate (Section 17.7) The first intermediate
formed when a nucleophile attacks a carbonyl carbon.
Tetrahydropyranyl (THP) ether (Section 16.10) Protecting
groups for alcohols of the structure
C
O
H and C
O
L
..
..
O
..
..
OR
G-16 GLOSSARY
S
C
RHN
NHR
..
....
..
O
NH
2
H
2
N
C
S
SR
RO
C
Tetramethylsilane (TMS) (Section 15.5) The standard “zero
point” on the ppm scale in NMR spectroscopy. The chemical
shift of a nucleus is quoted in parts per million of applied
magnetic field relative to the position of TMS.
Te t ro s e (Section 22.2) A four-carbon sugar.
Thermodynamic control (Section 8.8) In a reaction under
thermodynamic control, the product distribution depends on the
energy differences between the products.
Thermodynamic enolate (Section 19.15) The most stable
enolate. It may or may not be the same as the most rapidly
formed enolate, the kinetic enolate.
Thermodynamics (Section 8.8) The study of energetic
relationships.
Thermolysis (Section 11.2) The process of inducing chemical
change by supplying heat energy. See Pyrolysis.
Thioether (Section 6.9) The sulfur counterpart of an ether, RSR′.
A sulfide.
Thiol (Section 6.9) The sulfur counterpart of an alcohol, RSH.
Thionyl chloride (Section 7.4) SOCl
2
, an effective reagent for
converting alcohols into chlorides and carboxylic acids into acid
chlorides.
Thiourea (Section 23.4) A compound of the structure
Three-center, two-electron bonding (Section 21.3) A bonding
system in which only two electrons bind three atoms.This
arrangement is common in electron-deficient molecules such as
boranes and carbocations.
Torsional strain (Section 5.2) Destabilization caused by the
proximity of bonds (usually eclipsing) and the electrons in them.
trans (Section 2.12) Hydrogens on opposite sides. Used to specify
stereochemical (spatial) relationships in ring compounds and
alkenes.
s-trans (Section 12.6) The more stable, extended form of a
1,3-diene:
Transesterification (Section 18.8) The formation of one ester
from another. The reaction can be catalyzed by either acid or
base.
Transition state (TS) (Section 2.5) The high point in energy
between starting material and product. The transition state is an
energy maximum and not an isolable compound.
Triple bond (Section 3.5) Two atoms can be attached by a triple
bond composed of one σ bond and two π bonds.
Triplet carbene (Section 10.4) In a triplet carbene, the two
nonbonding electrons have the same spin and must occupy
different orbitals.
Tropylium ion (Section 13.6) The 1,3,5-cycloheptatrienylium
ion.This ion has 4n + 2 π electrons (n = 1) and is aromatic.
Ultraviolet/visible (UV/vis) spectroscopy (Section 12.7)
Electronic spectroscopy using light of wavelength 200–400 nm.
Unpaired spin (Section 1.2) Two electrons with the same spin
quantum number have unpaired spin.
Unsaturated hydrocarbons (Section 2.12) Molecules containing
π bonds. For example, alkenes or alkynes. This term does not
include cycloalkanes.
Urea (Section 17.7)
Valence electrons (Section 1.3) The outermost, or most loosely
held, electrons.
van der Waals forces (Section 2.13) Attractive or repulsive
intermolecular forces in or between molecules caused by induced
dipole–induced dipole interactions.
van der Waals strain (Section 5.4) Repulsive forces in or between
molecules caused by induced dipole–dipole interactions.
Vicinal (Section 10.2) Groups on adjacent carbons are vicinal.
Vinyl (Section 3.3) The common name for the
group.
Vinylic hydrogen (Section 15.6) A hydrogen on an alkene.
Wagner–Meerwein rearrangement (Section 9.9) The 1,2-
migration of an alkyl group in a carbocation.
Wave function (ψ) (Section 1.2) A solution to the Schrödinger
equation, another word for orbital.
Wavenumber (Section 15.4) The wavenumber (ν
−
) equals 1/λ
or ν/c.
Weighting factor (Section 1.4) The relative contribution of a
particular resonance structure to the weighted sum of all
resonance forms.
Williamson ether synthesis (Section 7.10) The S
N
2 reaction of
an alkoxide with to give an ether.
Wittig reaction (Section 16.17) The reaction of an ylide with a
carbonyl group to give, ultimately, an alkene.
Wolff rearrangement (Section 18.14) The formation of a ketene
through the thermal or photochemical decomposition of a diazo
ketone.
Woodward–Hoffmann theory (Section 20.1) The notion that
one must take account of the phase relationships between orbitals
in order to understand reaction mechanisms. In a concerted
reaction, bonding relationships must be maintained at all times.
Xanthate ester (Section 18.13) A compound of the structure
R
O
L
H
2
C
P
CH
Ylide (Section 16.17) A compound containing opposite charges
on adjacent atoms.
Zwitterion (Section 23.2) A dipolar species in which full plus and
minus charges coexist within the same molecule.

Credits
C-1
CHAPTER 1
p. 1: Mark Garlick/Photo Researchers,Inc.; p. 32: Fundamental Photographs.
CHAPTER 2
p. 50: NASA/JPL-Caltech/STScI; p. 60: Courtesy of Katey Walter.
CHAPTER 3
p. 97: Paul A. Souders/CORBIS; p. 106: B. Anthony Stewart/National
Geographic/Getty Images.
CHAPTER 4
p. 147: Rob Wilkinson/Alamy.
CHAPTER 5
p. 185: Sandro Campardo/epa/Corbis; p. 219: James Noble/CORBIS.
CHAPTER 6
p. 223: Tamara_k/Dreamstime.com; p. 252: Beverly Logan/Photodisc
RF/Getty Images.
CHAPTER 7
p.261: Enigma/Alamy; p.312: Taylor S.Kennedy/National Geographic/Getty
Images.
CHAPTER 8
p. 331: Dreamstime.com; p. 358 (right): Visuals Unlimited/Corbis;
p. 358 (left): Jubalharshaw19/Dreamstime.com.
CHAPTER 9
p. 363: Chris Windsor/Riser/Getty Images; p. 386: Kip Peticolas/
Fundamental Photographs.
CHAPTER 10
p. 409: Robert Marien/Corbis; p. 456: Raven Regan/Design Pics/Corbis.
CHAPTER 11
p. 467: NASA/Corbis; p. 505: Ole Graf/zefa/Corbis.
CHAPTER 12
p.511: Paul Katz/Photodisc RF/Getty Images; p. 558: David Frazier/Corbis.
CHAPTER 13
p. 571: Justin Sullivan/Getty Images; p. 604: Altrendo/Getty Images.
CHAPTER 14
p. 623: Matthew Peters/Manchester United via Getty Images; p. 647: Paule
Seux/Hemis/Corbis.
CHAPTER 15
p. 694: Yasuhide Fumoto/Digital Vision/Getty Images; p. 713: Tim
O’Hara/Corbis.
CHAPTER 16
p.762: Michael W. Davidson/Photo Researchers, Inc; p.765: D.Hurst/Alamy.
CHAPTER 17
p. 828: Ira Block/National Geographic/Getty Images; p. 864: Last
Resort/Photodisc RF/Getty Image.
CHAPTER 18
p.876: Wendibcr/Dreamstime.com; p.898: J. Burgess/Photo Researchers, Inc.;
p. 921: Xmasbaby/Dreamstime.com.
CHAPTER 19
p.931: Strmko/Dreamstime.com; p. 1009: Norbert Wu/Science Faction/Getty
Images.
CHAPTER 20
p. 1030: John Lund/Blend Images/Getty Images; p. 1071: Duomo/Corbis.
CHAPTER 21
p. 1080: Michael Dwyer/Alamy; p. 1096: Brian Hagiwara/JUPITERIM-
AGES/Brand X/Alamy.
CHAPTER 22
p. 1124: AgStock Images/Corbis; p. 1164: Richard Levine/Alamy.
CHAPTER 23
p.1173: Corbis Premium RF/Alamy; p. 1177: Courtesy of Dr. Daniel Janzen.
