Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.

3.19 A Catalyzed Addition to Alkenes: Hydration 139
is “no,” and in fact, we can never know for certain that we are absolutely right.
A mechanistic hypothesis survives only pending the next clever test.Someone—you
perhaps—may develop a new test of the ideas outlined in this section that reveals
an error, or at least an area that needs further thinking and working out of details.
It’s sad but true: It is easy to disprove a scientific hypothesis—just find some con-
tradictory data. It is impossible ever to prove a mechanistic hypothesis in chemistry
beyond a shadow of a doubt.
We all have to get used to this ambiguity—it is part of the human condition.
We can be quite certain we are right in our mechanistic ideas, and the more time
that goes by, and the more tests our ideas pass, the more confident we can be that
we are basically right. However, there have been some big surprises in science, as
conventional wisdom is reversed every once in a while, and there will be more. To
be honest, we sort of like it when that happens.
PROBLEM 3.30 Predict the products of the following reactions:
CC
H
H
H
3
C
H
3
C
HBr
?
?
H
2
SO
4
(HO SO
2
OH)
PROBLEM SOLVING
All “which direction does HX add” problems can be approached—and solved—
in the same way. Always draw out the two possible carbocations formed by initial
protonation of the double bond. For an unsymmetrical (ends different) double
bond, there are always two and only two possibilities. Next evaluate those two
carbocations—ask yourself which of them is more stable? It is that one that will
lead to the major product. The answer to Problem 3.30 is typical. Use it as a
prototype, a template for answering all similar questions.
GO
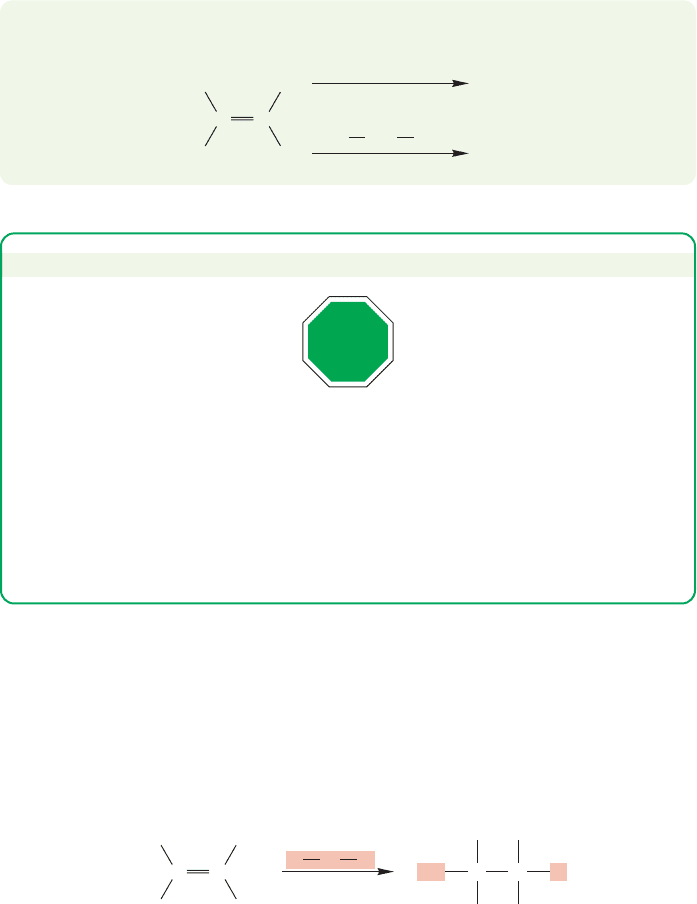
3.19 A Catalyzed Addition to Alkenes: Hydration
Not every HX is a strong enough acid to protonate an alkene. Water, HOH
(here X OH), is an example of such a weak acid. Yet it would be very useful to be
able to add the elements of water to an alkene,to “hydrate”an alkene as in Figure 3.80,
CC
CH
3
CH
3
H
3
C
H
3
C
CC
CH
3
CH
3
CH
3
CH
3
H
HO
AlcoholAlkene
????
H O H
FIGURE 3.80 The highly desirable
addition of water to an alkene.
140 CHAPTER 3 Alkenes and Alkynes
because the products ( , called alcohols) are useful in themselves and
are also versatile starting materials for the synthesis of other molecules. The
solution to this problem is to use a small amount of an acid that is strong
enough to protonate the alkene. The added acid acts as a catalyst for the addi-
tion. A catalyst is a material that increases the rate of a reaction without being
consumed.
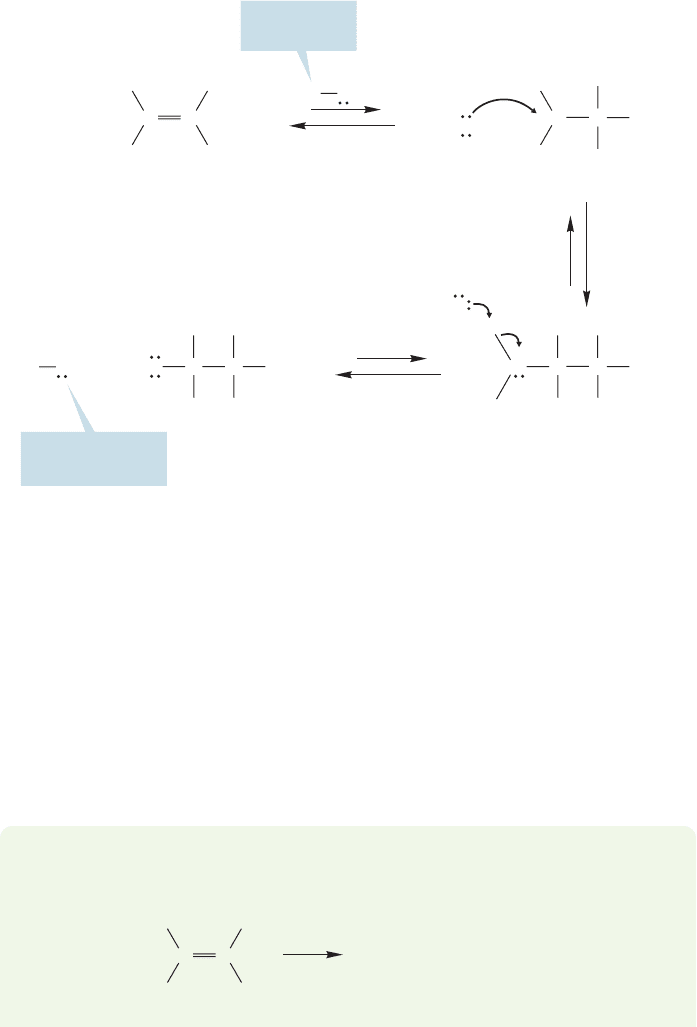
Let’s work through the mechanism for the hydration of alkenes.The first step
is protonation of the alkene by the catalyst, H
3
O
. As in the reaction of HCl, this
addition leads to a carbocation intermediate (Fig. 3.81). Now there is no halide
R
O
OH
CC
CH
3
Catalyst is
used up here
1. Protonation of
the alkene
CH
3
H
3
C
H
3
C
CC
CH
3
CH
3
H
3
C
H
3
C
H
H
H
Carbocation intermediate
H OH
2
+
H OH
2
+
3. Deprotonation
by water
2. Addition
of water
H
2
O
H
2
O
CC
CH
3
CH
3
H
3
C
H
3
C
H
CH
3
CH
3
H
3
C
H
3
C
Oxonium ion
intermediate
O
+
CCH
Alcohol
HO
Catalyst is
regenerated here
+
FIGURE 3.81 The acid-catalyzed
addition of water to 2,3-dimethyl-
2-butene—a hydration reaction.
ion (I
,Br
,Cl
,F
) to capture the carbocation, but there are lots of water mol-
ecules available. Capture by water leads not to the final product, the alcohol, but
to an intermediate oxonium ion, in which a trivalent oxygen bears a positive
charge. Water then deprotonates the oxonium ion to give the alcohol, and regen-
erate the catalyst, H
3
O
.
You can see from this last step why only a catalytic amount of acid is
necessary. Although it is consumed in the first protonation step, it is regener-
ated in the last step and can recycle to start the hydration reaction again
(Fig. 3.81).
WORKED PROBLEM 3.31 Only one alcohol is produced in the following reaction.
What is it, and why is only one alcohol, not two, produced?
H
3
O
+
H
2
O
C C A single alcohol product
H
H
H
3
C
H
3
C
(continued)
3.20 Synthesis: A Beginning 141
ANSWER Formation of the more stable tertiary carbocation rather than the much
less stable primary carbocation inevitably leads to the single product observed,
tert-butyl alcohol.
H
3
O
+
CC
H
H
H
3
C
H
3
C
CC
H
H
H
3
C
H
3
C
H
2
O
H
2
O
H
CC
CH
3
H
CH
3
H
H
tert-Butyl alcohol
HO
CCH
3
CH
3
CH
3
CH
3
CH
3
H
CCH
2
OH
Much less stable Not formed
More stable
+
+
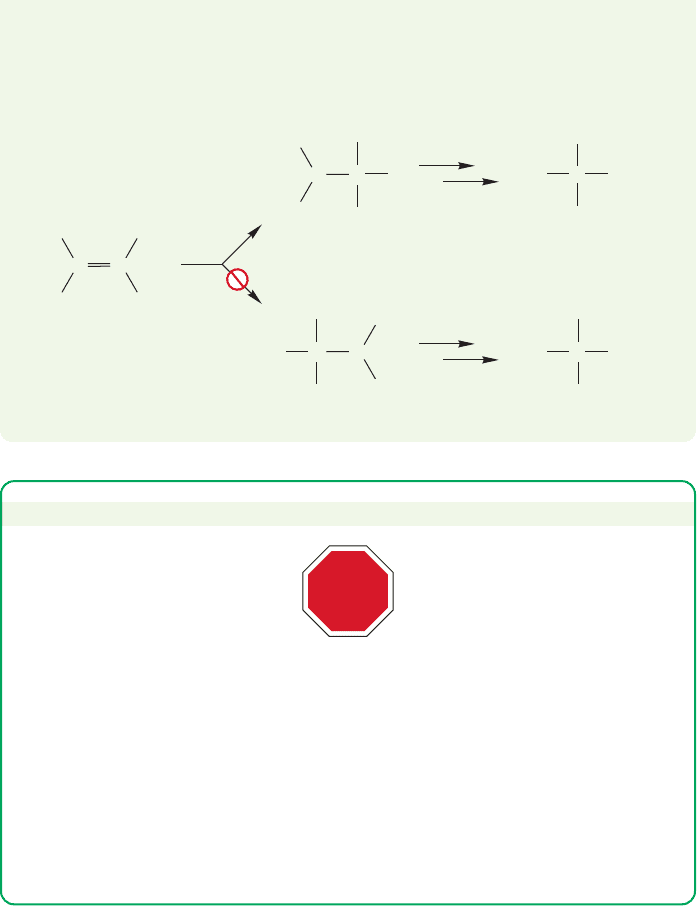
PROBLEM SOLVING
The order of carbocation stability is: tertiary (3°, R
3
C
) more stable than secondary
(2°, R
2
HC
), more stable than primary (1°, RH
2
C
), more stable than methyl
(H
3
C
). We’ll explain this stability order later, but you should remember that
simple primary and methyl carbocations are too high in energy to be formed in
common organic reactions. A primary or methyl carbocation is a mechanistic stop
sign. Nothing you write after it can be correct. Don’t go through the stop sign!
What does “simple” mean? It means that no special factors such a resonance
stabilization can be present—a “simple” carbocation is one that is not
significantly delocalized by resonance.
STOP
3.20 Synthesis: A Beginning
Organic chemistry is not only understanding how reactions occur. A very impor-
tant part of chemistry is the use of those reactions to make molecules.The construc-
tion of target molecules from smaller pieces is called synthesis, and we are now able
to do a surprising amount of this kind of thing. Let’s just take stock of what kinds
of transformations we can do—of what kinds of molecules we can make out of other
kinds of molecules.
The addition of hydrogen halides to alkenes gives us a way to make alkyl bro-
mides, chlorides, and iodides (fluorides don’t work very well) as long as we have the
appropriate starting alkene. We will have to be careful when using unsymmetrical
alkenes, because there is a choice to be made here. As you saw in Section 3.18, the
addition of HX will only lead to the more substituted alkyl halide, as the regiochem-
istry of the addition is controlled by the stability of the intermediate carbocation.
142 CHAPTER 3 Alkenes and Alkynes
We can also make alcohols through the acid-catalyzed addition of water to
alkenes (Section 3.19).
In each chapter, the synthetic methods developed and discussed are collected on
the Summary page. In this chapter, these reactions appear on pages 143 and 144.
PROBLEM 3.32 Devise syntheses of the following three compounds, starting in
each case with any alkene that contains four carbons or fewer. You do not have to
write mechanisms, although at this point it may be very helpful for you to do so.
(CH
3
)
3
COH (CH
3
)
2
CHOH CH
3
CH
2
CHOHCH
3
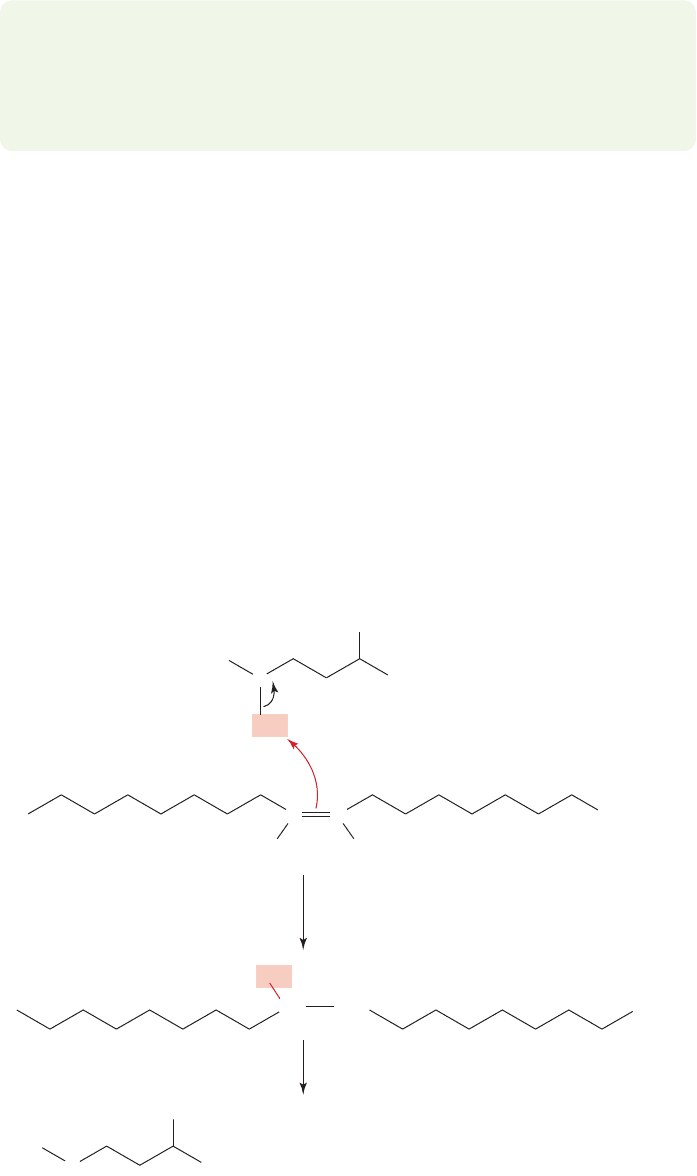
3.21 Special Topic: Alkenes and Biology
In sharp contrast to the alkanes, the unsaturated alkenes are highly active biologi-
cally. As we saw in Section 3.2,alkenes contain π bonds that are substantially weak-
er than almost all σ bonds of alkanes. Accordingly, their reactivity is higher, and
Nature “uses” this reactivity to accomplish things. One thing that can be accom-
plished is the formation of carbon–carbon single bonds. It is extraordinarily fortu-
nate for us that Nature is able to do that reaction easily, because our tissues are
composed mainly of carbon–carbon single bonds. We’ll just sketch one example of
alkene-mediated carbon–carbon single bond formation here.We will return to more
details of this and related transformations in later chapters.
For its own purposes, the tuberculosis-causing organism Mycobacterium tubercu-
losis needs a molecule called 10-methylstearate.It makes this compound from a mod-
ified amino acid, S-adenosylmethionine, and oleate, a salt of oleic acid (Fig. 3.82).
S
+
+
R
CH
3
NH
3
Oleate
S-Adenosylmethionine
C
HH
carbon–carbon bond making
and
carbon–sulfur bond breaking
Further reactions lead to 10-methylstearate
C COO
–
CH
+
+
+
CH
COO
–
R
NH
3
COO
–
COO
–
S
CH
3
FIGURE 3.82 The reaction of a
nucleophilic alkene (Lewis base) to
produce a carbon–carbon single
bond, shown in red.

3.22 Summary 143
The first interesting step in what turns out to be, ultimately, a reaction chock full of
interesting steps is the transfer of the methyl group to the alkene in oleate.The dou-
ble bond acts as the trigger for the whole sequence. Note also that here the π bond
acts as a Lewis base, much as it does in its reaction with HCl (p. 132), where it acts
as a Brønsted base.
This chapter deals mainly with the structural consequences of
sp
2
and sp hybridizations. The doubly bonded carbons in alkenes
are hybridized sp
2
and the triply bonded carbons in alkynes are
hybridized sp. In these molecules, atoms are bound not only by
the σ bonds we saw in Chapter 2 but by π bonds as well.
These π bonds, composed of overlapping 2p orbitals, have
substantial impact upon the shape (stereochemistry) of the mol-
ecule. Alkenes can exist in cis (Z) or trans (E) forms—they have
sides. Unlike the alkanes, which contain only σ bonds which
have very low barriers to rotation, there is a substantial barrier
(⬃66 kcal/mol) to rotation about a π bond. The linear alkynes
do not have cis/trans isomers and the question of rotation does
not arise.
Double and triple bonds can be contained in rings if the
ring size is large enough to accommodate the strain incurred by
the required bond angles.The smallest trans cycloalkene stable
at room temperature is trans-cyclooctene. The smallest stable
cycloalkyne is cyclooctyne.
More substituted alkenes and alkynes are more stable than
their less substituted relatives.
3.22 Summary
New Concepts
acetylenes (p. 98)
acetylide (p. 129)
activation energy (ΔG
‡
) (p. 136)
alcohol (p. 140)
alkenes (p. 98)
alkynes (p. 98)
allyl (p. 108)
Bredt’s rule (p. 122)
bridgehead position (p. 121)
Cahn–Ingold–Prelog priority system (p.111)
catalyst (p. 140)
cycloalkenes (p. 110)
degree of unsaturation (Ω) (p. 131)
double bond (p. 98)
ethene (p. 99)
ethylene (p. 99)
heat of formation (p. 115)
HOMO (p. 133)
LUMO (p. 133)
olefins (p. 123)
oxonium ion (p. 140)
pi (π) orbitals (p. 104)
polyenes (p. 110)
propargyl (p. 127)
regiochemistry (p. 137)
triple bond (p. 114)
vinyl (p. 107)
1¢H °
f
2
Key Terms
The Cahn–Ingold–Prelog priority system is introduced. It is
used in the (Z/E) naming system. We will encounter it again
very soon in Chapter 4.
In this chapter, we continue the idea of constructing larger
hydrocarbons out of smaller ones by replacing the different
available hydrogens with an X group. When X is methyl (CH
3
),
a larger hydrocarbon is produced from a smaller one.
Addition reactions between alkenes and acids, , are
introduced. Many acids ( , , , HOSO
2
OH,
generally, ) add directly to alkenes. The first step is addi-
tion of a proton to give the more stable carbocation. In the sec-
ond step of the reaction, the negative end of the original H
O
X
H
O
X
H
O
IH
O
ClH
O
Br
H
O
X
dipole usually adds to complete the addition process. The regio-
chemistry of the addition is determined by the formation of the
more stable carbocation in the original addition.
Other molecules are not strong enough acids to
protonate alkenes. Water (HOH) is an excellent example.
However, such molecules will add if the reaction is acid-cat-
alyzed. Enough acid catalyst is added to give the protonated
alkene, which is attacked by water. The catalyst is regenerated in
the last step and recycles to carry the reaction further.
The removal by a base of a hydrogen from the terminal
position of acetylenes to give acetylides is mentioned. Terminal
alkynes are moderately acidic molecules.
H
O
X
Reactions, Mechanisms, and Tools
1. Acetylides
B
–
Base
C
–
CH
3
C CH+ +BHCH
3
C
Syntheses

144 CHAPTER 3 Alkenes and Alkynes
2. Alkyl Halides
X = Cl, Br, I, sometimes F
Addition gives the compound
with the halide, X, on the
more substituted carbon
CC
HX
CC
H
X
CC
H
H
HX
CCH
H
H
X
The concept of cis/trans (Z/E) isomerism is a continuing
problem for students. Errors of omission (the failure to find a
possible isomer, for example) and of commission (failure to
recognize that a certain double bond, so easily drawn on
paper, really cannot exist) are all too common. Unlike σ
bonds, π bonds have substantial barriers to rotation
(⬃66 kcal/mol). The barrier arises because of the shape of the
p orbitals making up the π bond. Rotation decreases overlap
and raises energy. Accordingly, double bonds have sides,
which are by no means easily interchanged. Substituents
cannot switch sides, and are locked in position by the high
barrier to rotation. It is vital to see why there are two isomers
of 2-butene, cis- and trans-2-butene, but only one isomer of
2-methyl-2-butene (Fig. 3.83).
This relatively easy idea has more complicated implications.
For example, the need to maintain planarity (p/p overlap) in a π
bond leads to the difficulty of accommodating a trans double
bond in a small ring, or at the bridgehead position of bridged
bicyclic molecules. In each case, the geometry of the molecule
results in a twisted (poorly overlapping) pair of p orbitals.
Common Errors
In this chapter, we encounter the determination of molecular
formulas. The following three review problems (problems
3.33–3.35) deal with this subject.
PROBLEM 3.33 Alkanes combine with oxygen to produce car-
bon dioxide and water according to the following scheme:
This process is generally referred to as combustion. An impor-
tant use of this reaction is the quantitative determination of ele-
mental composition (elemental analysis). Typically, a small sam-
ple of the compound is completely burned and the water and
carbon dioxide produced are collected and weighed. From the
weight of water the amount of hydrogen in the compound can
be determined. Similarly, the amount of carbon dioxide formed
allows us to determine the amount of carbon in the original
compound. Oxygen, if present, is usually determined by differ-
ence. The determination of the relative molar proportions of
C
n
H
2n + 2
+ (3n + 1)/2 O
2
U
(n + 1)H
2
O + nCO
2
carbon and hydrogen in a compound is the first step in deriving
its molecular formula.
If combustion of 5.00 mg of a hydrocarbon gives 16.90 mg
of carbon dioxide and 3.46 mg of water, what are the weight
percents of carbon and hydrogen in the sample?
PROBLEM 3.34 Calculate the weight percents for each
element in the following compounds: (a) C
5
H
10
and
(b) C
9
H
6
ClNO.
PROBLEM 3.35 A compound containing only carbon,
hydrogen, and oxygen was found to contain 70.58% carbon
and 5.92% hydrogen by weight. Calculate the empirical
formula for this compound. If the compound has a molecular
weight of approximately 135 g/mol, what is the molecular
formula?
PROBLEM 3.36 How many degrees of unsaturation are
there in compounds of the formula C
5
H
8
? Write at least eight
isomers including ones with no π bonds and ones with
no rings.
3.23 Additional Problems
These are different molecules
These are the same molecule
=
trans-2-Butene
cis-2-Butene
2-Methyl-2-butene
H
H
H
H
CH
3
CH
3
H
3
C
H
H
CH
3
CH
3
H
3
C
H
3
C
H
3
C
H
3
C
H
3
C
FIGURE 3.83
3. Alcohols
CC
H
2
O
H
3
O
+
H
2
O
H
3
O
+
CC
H
OH
C
The addition reaction of water
is catalyzed by acid
C
H
H
CCH
H
H
OH
3.23 Additional Problems 145
PROBLEM 3.37
Determine the degrees of unsaturation for the
following compounds.
PROBLEM 3.43 Write systematic names for the following
compounds:
PROBLEM 3.44 Draw structures for the following compounds:
(a) (Z)-3-fluoro-2-methyl-3-hexene
(b) 2,6-diethyl-1,7-octadien-4-yne
(c) trans-3-bromo-7-isopropyl-5-decene
(d) 5-chloro-4-iodo-6-methyl-1-heptyne
PROBLEM 3.45 Although you should be able to draw structures
for the following compounds, they are named incorrectly. Give
the correct systematic name for each compound:
(a) 5-chloro-2-methyl-6-vinyldecane
(b) 3-isopropyl-1-pentyne
(c) 4-methyl-6-hepten-1-yne
(d) (E)-3-propyl-2,5-hexadiene
PROBLEM 3.46 Find all the compounds of the formula
C
4
H
6
Br
2
in which the dipole moment is zero. It is easiest to
divide this long problem into sections. First, work out the num-
ber of degrees of unsaturation so that you can see what kinds of
molecules to look for. Then find the acyclic molecules. Next
work out the compounds containing a ring.
PROBLEM 3.47 Find all the compounds of the formula C
4
H
6
in which there are four different carbon atoms. There are only
nine compounds possible, but two of them may seem quite
strange at this point. Hint: One of these molecules contains a
carbon that is part of two double bonds, and the other is a com-
pound containing only three-membered rings.
PROBLEM 3.48 Find all the isomers of the formula C
4
H
5
Cl.
As in Problem 3.46, it is easiest to divide this problem into sec-
tions. First, find the degrees of unsaturation so that you know
what types of molecule to examine. There are 23 isomers to
find. Hint: See the hint for Problem 3.47.
(a)
(b)
(c)
(d)
I
Cl
Br
(a) (b) (c) (d)
PROBLEM 3.38 Provide the IUPAC name for the following
compounds:
PROBLEM 3.39 Are the two structures below the same
molecule? Draw the Newman projection for each looking down
the bond. Which form do you think is more
stable? Why?
PROBLEM 3.40 Draw and write the systematic names for all
of the acyclic isomers of 1-heptene (C
7
H
14
). There are 36 iso-
mers, including 1-heptene.
PROBLEM 3.41 Draw and write the systematic names for all of
the acyclic isomers of 1-octyne (C
8
H
14
) containing a triple
bond. There are 32 isomers, including 1-octyne.
PROBLEM 3.42 Draw and write the systematic names for all
of the isomers of dichlorobutene (C
4
H
6
Cl
2
). There are 27 iso-
mers, according to usually reliable sources.
(a) (b)
C(2)
O
C(3)
(a) (b) (c)
(d) (e)
Br
I
Cl
146 CHAPTER 3 Alkenes and Alkynes
PROBLEM 3.49 Find the compound of the formula C
10
H
16
,
composed of two six-membered rings, that has only three dif-
ferent carbons.
PROBLEM 3.50 Develop a bonding scheme for the compound
H
2
CO in which both carbon and oxygen are hybridized sp
2
.
PROBLEM 3.51 (a) Develop a bonding scheme for a ring made
up of six CH groups, (CH)
6
. Each carbon is hybridized sp
2
. (b) This
part is harder.The molecule you built in (a) exists, but all the car-
bon–carbon bond lengths are equal. Is this consistent with the struc-
ture you wrote in (a), which almost certainly requires two different
carbon–carbon bond lengths? How would you resolve the problem?
PROBLEM 3.52 In this chapter, we developed a picture of the
methyl cation (
CH
3
), a planar species in which carbon is
hybridized sp
2
. In Chapter 2, we briefly saw compounds formed
from several rings, bicyclic compounds. Explain why it is possi-
ble to form carbocation (a) in the bicyclic compound shown
below, but very difficult to form carbocation (b). It may be
helpful to use models to see the geometries of these molecules.
PROBLEM 3.53 Show the curved arrow formalism (electron
pushing or arrow pushing) for the following reactions. Which side
of the equilibrium is favored in each reaction? Use the pK
a
table in
the endpaper of the book to answer this part of the problem.
(
a
)(
b
)
H
C
+
+
C
PROBLEM 3.55 Explain the formation of two products in the
following reaction:
OH
..
..
..
–
+
RCC
H
HOH
..
..
..
–
+
RCC
CH
3
..
–
+
RCCH
H
CH
4
..
–
+
RCC
OH
2
..
+
+
OH
2
..
..
+
+
CC
H
H
H
R
CC
H
R
H
H
H
PROBLEM 3.54 Predict the products of the following reactions:
HF
CH
3
OH
CH
3
OH
2
CC
H
H
H
3
C
H
3
C
+
?
?
HCl
C +
CH
3
Cl
H
H
2
C
CC
CH
2
CH
H
H
H
2
C
CC
CH
3
H
H
H
2
C
Cl
PROBLEM 3.56 Explain the formation of the two products in
the reaction shown.
PROBLEM 3.57 In principle, there is a second “double addi-
tion” possible in Problem 3.56. Explain why only one compound
is formed. Hint: Think “resonance.”
Use Organic Reaction Animations (ORA) to answer the
following questions:
PROBLEM 3.58 Choose the reaction titled “Alkene hydration”
and click on the HOMO button. Observe the calculated high-
est occupied molecular orbital for 2-methylpropene. One hydro-
gen on each methyl group is not involved in the HOMO. Why
is that the case?
PROBLEM 3.59 In the “Alkene hydration” reaction, click on
Play and observe the last step of the reaction, which is removal
of an acidic hydrogen. It is shown in the following figure:
Show the electron pushing for the reaction going from left to
right. Show the electron pushing for the reaction going from
right to left. Which direction is favored? How can the neutral
alcohol be obtained from this reaction?
PROBLEM 3.60 Choose the reaction “Acetylide addition” and
click on the HOMO button and then play the reaction. Stop
the reaction before the acetylide reacts with the bromoethane.
Observe the calculated highest occupied molecular orbital of
the propyne anion. Are the π orbitals involved in the anion?
Why or why not?
H
2
O
+
++
OH
2
+
RO ROH
H
H
H
HCl
HCl
This compound is formed
CC
CH
3
H
3
C
H
Cl
H
3
C
C CH
2
CH
3
Cl
Cl
This compound
is not formed
H
3
C
C CCH
3
Cl
H
Cl
H
H
3
CCCCH
3
C +C
CH
3
H
Cl
H
3
C
H
3
C
C CH
2
CH
3
Cl
Cl
HCl
H
3
CCCCH
3

Stereochemistry
147
4.1 Preview
4.2 Chirality
4.3 The (R/S) Convention
4.4 Properties of Enantiomers:
Physical Differences
4.5 The Physical Basis of Optical
Activity
4.6 Properties of Enantiomers:
Chemical Differences
4.7 Interconversion of
Enantiomers by Rotation
about a Single Bond:
gauche-Butane
4.8 Diastereomers and Molecules
Containing More than One
Stereogenic Atom
4.9 Physical Properties of
Diastereomers: Resolution, a
Method of Separating
Enantiomers from Each Other
4.10 Determination of Absolute
Configuration (R or S)
4.11 Stereochemical Analysis
of Ring Compounds
(a Beginning)
4.12 Summary of Isomerism
4.13 Special Topic: Chirality without
“Four Different Groups
Attached to One Carbon”
4.14 Special Topic: Stereochemistry
in the Real World:
Thalidomide, the
Consequences of Being
Wrong-Handed
4.15 Summary
4.16 Additional Problems
4
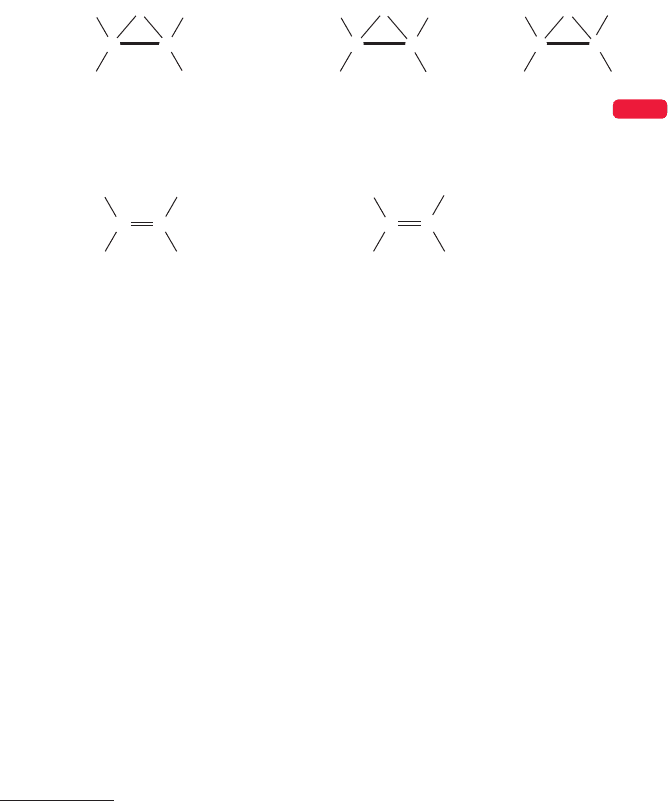
MIRROR IMAGES This photo shows a woman and her mirror image. In this chapter
we will see that some molecules exist in mirror image forms.
148 CHAPTER 4 Stereochemistry
cis-1,2-Dimethylcyclopropane
Two representations of
trans-1,2-dimethylcyclopropane
H
3
C
CH
3
H
H
cis-2-Butene
or (Z )-2-butene
H
3
C
CH
3
HH
CC
trans-2-Butene
or (E)-2-butene
H
3
C
H
H
CH
3
CC
H
CH
3
H
3
C
H
C C
H
3
C
H
H
CH
3
C C
CH
2
CH
2
CC
CH
2
WEB 3D
FIGURE 4.1 cis- and trans-1,2-
Dimethylcyclopropane and cis-
and trans-2-butene are pairs of
stereoisomeric molecules. In the two
cyclopropanes, the thicker bond
indicates the side of the ring closest
to you.
I held them in every light. I turned them in every attitude. I surveyed their
characteristics. I dwelt upon their peculiarities. I pondered upon their
conformation.
—EDGAR ALLAN POE,
1
BERENICE
1
Edgar Allan Poe (1809–1849) was an American poet and writer probably most famous for his macabre
stories and poems. As the quotation shows, he was also an early devotee of stereochemistry.
4.1 Preview
If you worked Problem 2.29 (p. 85), you saw that there are two isomers of
trans-1,2-dimethylcyclopropane. We will see in this chapter why two trans-1,2-
dimethylcyclopropanes exist and continue with a detailed discussion of stereochemistry,
the structural and chemical consequences of the arrangement of atoms in space.We have
already seen several examples of stereoisomeric molecules, compounds that differ
only in the spatial arrangement of their constituent parts. The compounds cis-
and trans-1,2-dimethylcyclopropane are stereoisomers (as are all cis/trans pairs).
Substituents on both ring compounds and alkenes can be attached in two ways: cis
and trans (Fig. 4.1). We will soon see that the two trans-1,2-dimethylcyclopropanes
are stereoisomers of a different kind.
In this chapter, we will examine subtle questions of stereoisomerism. Most mol-
ecules of Nature are chiral. The word “chiral”is derived from the Greek and means
“handed.” Chiral molecules are related to their mirror images in the same way that
your left hand is related to your right. Your hands are not identical—they cannot be
superimposed. They are mirror images of one another. Many molecules of Nature
(for example, the amino acids) are handed in the same way: One might say that our
amino acids are all left-handed. The source of the ubiquity of left-handedness of
the amino acids is one of the great remaining questions in chemistry.Was it chance?
Did an accidental left-handed start determine all that followed? When we encounter
extraterrestrial civilizations will their amino acids (assuming they have any) also be
left-handed or will we find right-handed amino acids? As it now appears we will
find no cohabitants within our own solar system, and as physical contact with our
galactic neighbors is unlikely, you might consider how you would convey to an invis-
ible resident of the planet Altair 4 our concepts of left and right.
Chirality, or handedness, is a complicated but important subject that is
absolutely essential to organic and biological chemistry. When we return to our
examination of reaction mechanisms—of the way molecules come together to
