Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.


3.14 Acidity of Alkynes 129
3.14 Acidity of Alkynes
It is generally most difficult for even a strong base ( ) to remove a proton from
a hydrocarbon to give an anion. In other words,hydrocarbons are generally very weak
acids. We have already had a brief discussion of the methyl anion in Chapter 2
(p. 62), but methane, the parent of this anion, is an extraordinarily weak acid
(Fig. 3.63).
4
B
:
-
3.13 Physical Properties of Alkynes
The physical properties of alkynes resemble those of alkenes and alkanes. Table 3.5
collects some data.
C
B
–
B
–
+
–
–
The methyl anion
(methide)
The acet
y
lide ion
HC
CH
3
HC
H
BH
+
+
CH
BH
+
CH
3
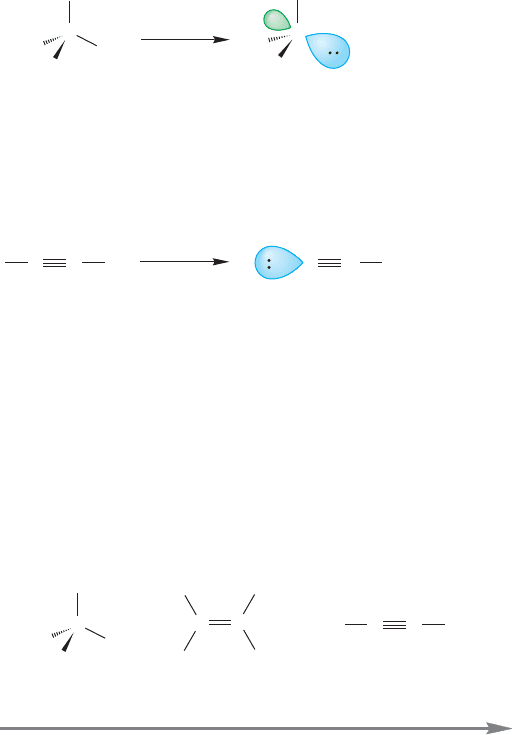
FIGURE 3.63 Strong bases ( )
can remove a terminal hydrogen
of an acetylene. Terminal acetylenes
are reasonably strong acids (for
hydrocarbons).
B
:
-
4
In Figure 3.63 you see the first use of the universal “This Does Not Happen” sign, the red crossed circle .
It will reappear many times in the rest of the book to show you when something does not happen.
A remarkable property of terminal alkynes is that they are reasonably strong
Brønsted acids (proton donors). Compared to most other hydrocarbons it is easy to
remove the terminal acetylenic hydrogen as a proton, leaving behind an anion called
an acetylide. Terminal alkynes are stronger acids than alkanes by roughly a factor
of 10
30
! Be sure you are clear on this point! Terminal alkynes are not strong acids
in the sense that traditional mineral acids such as hydrochloric acid (HCl) and
sulfuric acid (H
2
SO
4
) are, but they are certainly strong in comparison to most other
hydrocarbons.
Why should terminal alkynes be relatively strong hydrocarbon acids? To answer
this question,consider the orbital containing the residual pair of electrons in the two
cases of Figure 3.63. For the methyl anion, this orbital is hybridized approximate-
ly sp
3
, with roughly 25% s character. In the acetylide, the orbital containing the
pair of electrons is sp hybridized, and therefore has 50% s character. An electron
in an s orbital is substantially lower in energy than one in a p orbital, and so the
TABLE 3.5 Some Simple Alkynes
Name Formula mp (°C) bp (°C)
Acetylene (ethyne) 80.8 84
Propyne 101.5 23.2
1-Butyne 125.7 8.1
2-Butyne 32.2 27
1-Pentyne 106 40.2
1-Hexyne 131.9 71.3
1-Heptyne 81 99.7
1-Octyne 79.3 125.2HC
q
C
O
(CH
2
)
5
CH
3
HC
q
C
O
(CH
2
)
4
CH
3
HC
q
C
O
(CH
2
)
3
CH
3
HC
q
C
O
(CH
2
)
2
CH
3
CH
3
O
C
q
C
O
CH
3
HC
q
C
O
CH
2
CH
3
HC
q
C
O
CH
3
HC
q
CH
130 CHAPTER 3 Alkenes and Alkynes
If the hypothesis that the hybridization of the anion is critical is correct, then
alkenes should be intermediate in acidity between alkanes and alkynes.This hypoth-
esis is exactly right: alkenes are about 10
10
–10
12
more acidic than alkanes,and 10
18–20
less acidic than alkynes (Fig. 3.65).
H
H
H
H
H
H
H
H
C
HHCC
Relatively strong acid
(for a hydrocarbon)
Very weak acid
Acidity
Intermediate
C
C
FIGURE 3.65 Alkenes are intermediate
in acidity between alkanes and alkynes.
3.15 Molecular Formulas and Degrees of
Unsaturation
How is one to determine the possible structures for a molecule of a given molecu-
lar formula? For example, what are the possibilities for C
6
H
10
? Cyclohexene,
1-hexyne, and 1,3-hexadiene are only a few of them. Before we begin to write down
the possibilities, we need to know the general possible structural types. In this case,
cycloalkenes, acyclic alkynes, and acyclic dienes are among the structures with a for-
mula C
6
H
10
. Given a formula for an unknown hydrocarbon, we are faced with the
problem of determining the number of π bonds and rings. Saturated alkanes have
the formula C
n
H
2n2
. Both alkenes and cycloalkanes have the formula C
n
H
2n
.Such
molecules are said to have “one degree of unsaturation,” which simply means that
they contain two fewer than the maximum number of hydrogens, as defined by the
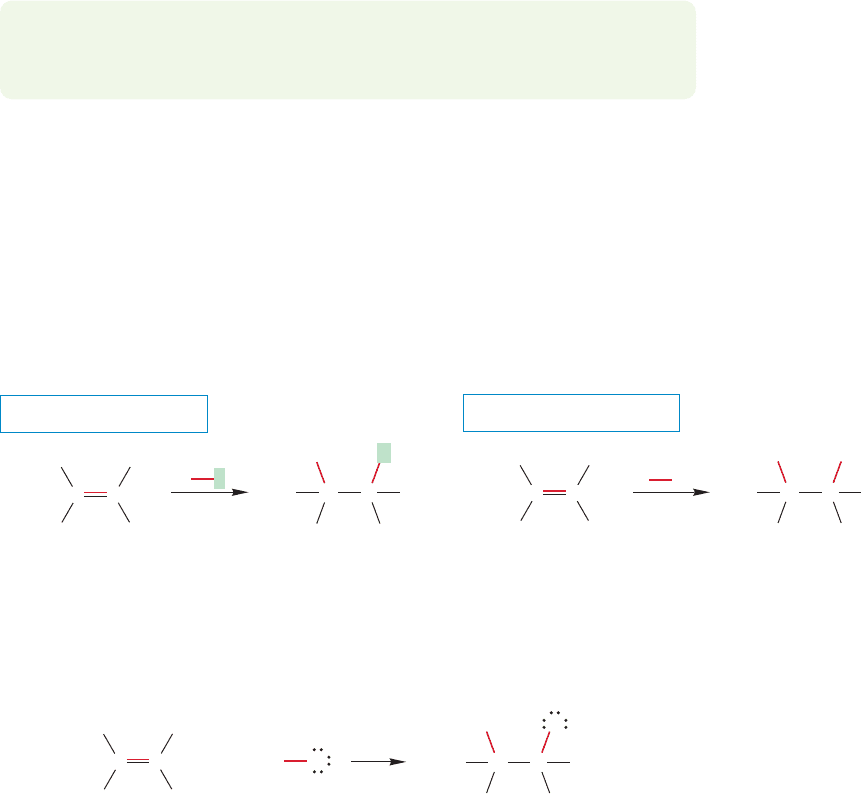
C is hybridized approximately sp
3
,
therefore the nonbonding electrons are
in an orbital of about 25% s character
Now, C is hybridized approximately sp,
and the nonbonding electrons are in an
orbital of approximately 50% s character
H
H
H
H
C
H
H
H
base, B
–
base, B
–
C
–
MethideMethane
Acetylide
Acetylene
HC
C
HC
–
CH
BH
+
BH
+
FIGURE 3.64 In an acetylide ion, the
two nonbonding electrons are
accommodated in an sp orbital.
more s character the orbital has, the lower in energy an electron in it is. The
nonbonding electrons in the acetylide ( ) are in a much lower energy
orbital than the electrons in the nonbonding orbital of the methyl anion (H
3
),
also called methide (Fig. 3.64). Therefore it is relatively easy to remove a proton
to produce the acetylide ion.
C
:
-
HC
q
C
:
-
3.16 An Introduction to Addition Reactions of Alkenes and Alkynes 131
3.16 An Introduction to Addition Reactions of
Alkenes and Alkynes
Alkenes, and other molecules containing π systems such as the alkynes, undergo an
addition reaction with Brønsted acids (proton donors) of the general formula HX.
In an addition reaction, both the π bond and the sigma bond are broken,
and new bonds are formed to the carbons of the old π system. Figure 3.67 shows a
H
O
X
C
6
H
12
(C
n
H
2n
)
In C
6
H
2n + 2
, there are 14 hydrogens.
Calculation:
14 – 12 = 2/2 = 1 degree of unsaturation,
which means that there must be one π bond or one ring.
C
9
H
16
(C
n
H
2n – 2
)
In C
9
H
2n + 2
, there are 20 hydrogens.
Calculation:
20 – 16 = 4/2 = 2 degrees of unsaturation,
which means that there must be a total of two π bonds and rings.
FIGURE 3.66 Two calculations
of degrees of unsaturation.
H
3
C
H
3
C
H
Br
CH
3
CH
3
HBr
C
C
H
3
C
H
3
C
CH
3
CH
3
CC
A SPECIFIC EXAMPLE
H
3
C
H
3
C
H
CH
3
CH
3
C C
H X
X
H
3
C
H
3
C
CH
3
CH
3
CC
THE GENERAL CASE
FIGURE 3.67 Additions to alkenes.
PROBLEM 3.26 Calculate the degrees of unsaturation (Ω) for the following for-
mulas. Give two possible structures for each one. (a) C
5
H
6
(b) C
7
H
8
(c) C
10
H
10
(d) C
5
H
8
Br
2
.
general and specific reaction of this kind. In fact, there is a vast number of related
addition reactions, and we’ll see several of them in Chapter 9. We are going to look
first at the addition of hydrogen chloride to 2,3-dimethyl-2-butene (Fig. 3.68).
H
2,3-Dimeth
y
l-2-butene 2-Chloro-2,3-dimeth
y
lbutane
Cl
Cl
+
H
3
C
H
3
C
H
CH
3
CH
3
C C
H
3
C
H
3
C
CH
3
CH
3
CC
FIGURE 3.68 Addition of hydrogen
chloride to 2,3-dimethyl-2-butene.
alkane formula, C
n
H
2n2
. Alkynes and cycloalkenes have the formula C
n
H
2n2
, and
contain four fewer hydrogens than the C
n
H
2n2
number.These compounds have two
degrees of unsaturation. The degree of unsaturation (Ω) for a hydrocarbon is the
total number of π bonds and rings in the molecule. It can always be determined by
calculating the number of hydrogens for the corresponding saturated alkane (H
2n2
),
and then subtracting the number of hydrogens actually present and dividing by two.
This procedure also works if hydrogens are replaced with other monovalent atoms
such as the halogens. Such atoms are treated as if they were hydrogens. Later, when
we see more complicated molecules containing nitrogen, we will have to update this
subject. Figure 3.66 works out two examples.

132 CHAPTER 3 Alkenes and Alkynes
3.17 Mechanism of the Addition of Hydrogen Halides
to Alkenes
What is the “mechanism”of a reaction? In Chapter 8, we will find an elaborate answer
to this question.Often chemists use the word in a not-very-rigorous way,meaning only
an outline of the steps of a reaction using the arrow formalism, and an identification
of all intermediate species in a reaction leading from a starting material to a product.
In the following discussion of the addition reaction to alkenes, we will be content with
this less formal “mechanism.”Addition to a symmetrical alkene begins with a straight-
forward Brønsted acid–Brønsted base reaction, in which HX donates a proton to one
of the two equivalent carbons of the π bond. In this reaction, the HX molecule acts
as the Brønsted acid and the alkene acts as the Brønsted base. The first step in the
δ
+
δ
–
Cl
H
Cl
H
=
FIGURE 3.70 The dipole in .H
O
Cl
different atoms. However, these are partial charges and are not fully developed. In
such a bond, the electrons will be polarized toward the more electronegative atom,
the atom with the greater attraction for the electrons in the shared orbital. As first
mentioned in Chapter 1 (p. 15), electronegativity is the tendency of an atom in a
bond to attract electrons.
Notice that the most electronegative elements are in the upper right of the peri-
odic table. The ability to attract electrons is related to the effective nuclear charge,
which in turn depends on the effectiveness of the electrons surrounding the nucle-
us in shielding an additional negatively charged electron from the nucleus and its
positive charge.In any unsymmetrical covalent bond, there will be a dipole with the
negative end on the more electronegative atom. A dipole in a polarized bond is
indicated with an arrow pointing toward the more electronegative element and
partial charges are shown as δ
and δ
(Fig. 3.70).
Molecules such as hydrogen chloride and hydrogen bromide are very polar, even
though they are covalently, not ionically, bonded.The electrons in the bond joining
the hydrogen and the halogen are not shared equally, and the dipole results from
that unequal sharing. Hydrogen chloride has a dipole moment of 1.08 D. Although
neither hydrogen chloride nor hydrogen bromide is an ionic compound, each read-
ily donates a proton, the positive end of its dipole. This familiar property is what
makes hydrogen chloride and hydrogen bromide good Brønsted acids. Now let’s
apply these ideas to the reaction of alkenes with these acids.
In this reaction, the π bond of the alkene and the σ bond of have been
converted into a pair of σ bonds attaching H and Cl to carbon atoms. We must
lead into this reaction with a look at the structure and properties of molecules
such as hydrogen chloride that contain bonds between two very different kinds
of atoms.
Recall that all bonds between different atoms are polar—the electrons in the bond
cannot be shared equally in a covalent bond between different atoms. The limit of
this phenomenon is an ionic bond in which two oppositely charged species are held
together by the electrostatic attraction between them. Potassium chloride and sodi-
um fluoride are examples (Fig. 3.69). Charges also exist in covalent bonds between
H
O
Cl
1s
2
2s
2
2p
6
3s
2
3p
6
1s
2
2s
2
2p
6
3s
2
3p
6
17
Cl
–
KCl NaF
19
K
+
1s
2
2s
2
2p
6
1s
2
2s
2
2p
6
9
F
–
11
Na
+
FIGURE 3.69 Two examples of
charge-separated, ionic bonding.

3.17 Mechanism of the Addition of Hydrogen Halides to Alkenes 133
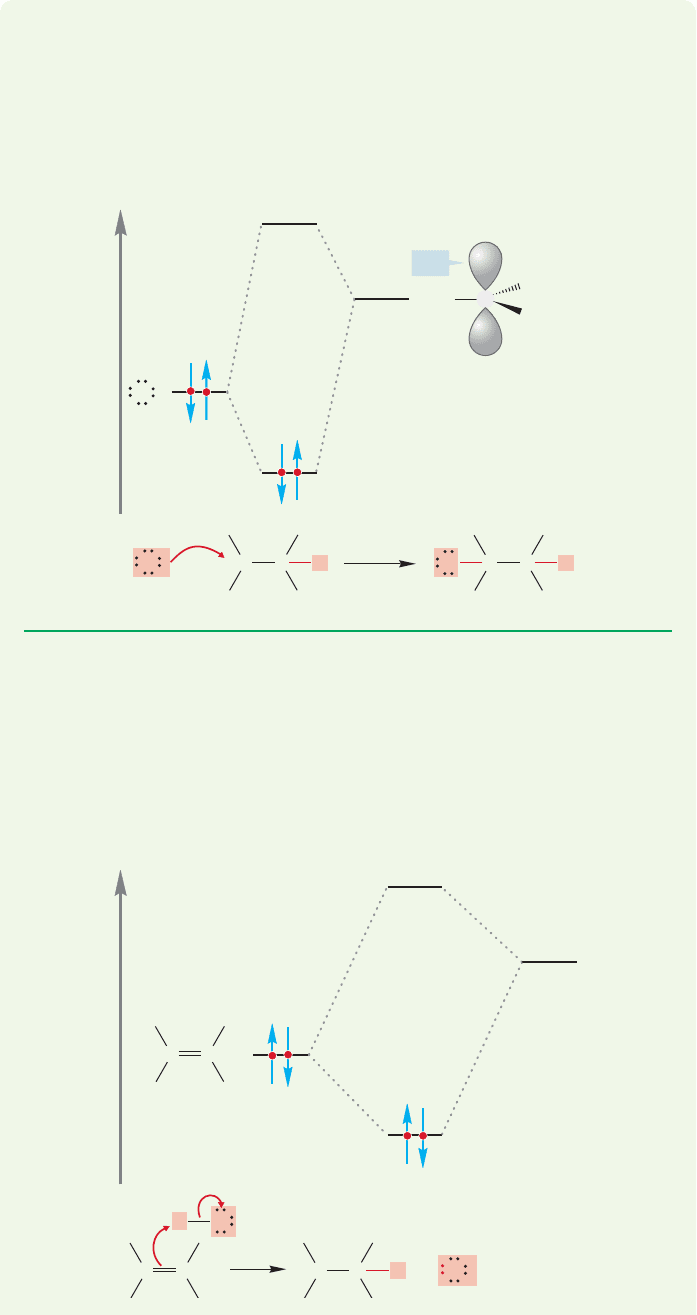
No great structural differences appear, however. The trigonal (attached to three
groups) carbon is hybridized sp
2
, and the angles are roughly 120°.The
empty carbon 2p
z
orbital extends above and below the plane of the central carbon
and the three carbons attached to it (Fig. 3.72).
Let’s verify that the central carbon is positively charged (Fig. 3.73). In a neutral car-
bon atom,the six positive nuclear charges are balanced by six electrons.The central car-
bon in this cation has a pair of 1s electrons and a half-share in the electrons in the three
covalent bonds to the alkyl groups, for a total of five. The six positive charges in the
nucleus are balanced by only five electrons,and so the carbon atom is positively charged.
Reactions such as these that involve polar molecules are best understood in terms
of Highest Occupied Molecular Orbital–Lowest Unoccupied Molecular Orbital
(HOMO–LUMO) orbital interactions. As we saw in Section 1.7,p. 41, when a filled
occupied orbital overlaps an empty orbital, the two electrons are stabilized in the new,
lower energy molecular orbital. The words “Lewis bases react with Lewis acids” are
essentially equivalent to saying, “The interaction of a filled and empty orbital is sta-
bilizing.” Indeed, this notion is one of the central unifying themes of organic reactiv-
ity, as essentially all reactions involving polar molecules can be understood this way.
We will revisit the addition reaction to alkenes in detail in Chapter 9, but see
right now if you can identify the HOMOs and LUMOs in the two steps of this
reaction, that is, do Problems 3.27 and 3.28.
C
O
C
O
C
H
3
C
H
3
C
H
3
C
H
3
C
Cl
Cl
H
C
+
H
CH
3
CH
3
C C
H
CH
3
CH
3
C
–
Cl
Protonation
1
Addition of Cl
–
2
H
3
C
H
3
C
CH
3
CH
3
CC
FIGURE 3.71 The first step in this two-step reaction is the protonation of the alkene to give a carbocation.
In the second step, a chloride ion adds to the cation to give the final product.
CH
3
H
3
C
Cl
H
C
–
Cl
2p
z
2p
z
CH(CH
3
)
2
H
H
The methyl cation is
flat; the central carbon
is hybridized sp
2
This cation is also planar;
the central carbon
is approximately sp
2
+
C
H
+
H
3
C
H
3
C
CH
3
CH
3
CC
FIGURE 3.72 Protonation of
2,3-dimethyl-2-butene gives a planar
carbocation closely related to the
methyl cation. The central carbon of
each species is sp
2
hybridized.
In looking at Figure 3.71, don’t forget the curved arrow formalism (p. 23). The
double-barbed arrows track the movements of pairs of electrons. The color-coding
should help. Notice also the red and green equilibrium arrows.The different lengths
reflect the exothermicity or endothermicity of each step.The first step is endother-
mic, and the second is exothermic.
Let’s look at the intermediate carbocation in Figure 3.71. In Chapter 2 (p. 62),
we described the methyl cation (
CH
3
). The cation formed by protonation of
2,3-dimethyl-2-butene is related to the methyl cation,but the three hydrogens of
CH
3
have been replaced with three alkyl groups: two methyls and an isopropyl (Fig. 3.72).
Neutral (no charge)
6
C (1s
2
2s
2
2p
2
)
C
+
R
RR
This
6
C has only five
electrons surrounding it:
1s
2
, and three shared in
the bonds to the three R
groups; therefore, it has
a single positive charge
FIGURE 3.73 The determination of
the charge on carbon in a
carbocation.
reaction is this protonation.It is followed by an addition step,in which the newly formed
chloride ion captures the newly formed carbocation. The carbocation is an intermedi-
ate in this reaction. Figure 3.71 shows this sequence for 2,3-dimethyl-2-butene.
CONVENTION ALERT
134 CHAPTER 3 Alkenes and Alkynes
WORKED PROBLEM 3.27 Identify the HOMO and LUMO for the second step of
the reaction in Figure 3.71.
ANSWER In the second step of the reaction, chloride ion acts as the Lewis base
(nucleophile) and reacts through one of the filled orbitals (the HOMO) contain-
ing the nonbonding pairs of electrons. The LUMO (electrophile) is easy to find;
it must be the empty 2p orbital of the carbocation.
WORKED PROBLEM 3.28 In this much harder problem, identify the HOMO and
LUMO in the first step of the reaction shown in Figure 3.71.
ANSWER The first step of the reaction is much more difficult to deal with. Part
of it is easy: The alkene is the nucleophile and supplies the HOMO. More pre-
cisely, the nucleophile is the filled π orbital of the alkene. What empty orbital acts
as the electrophile, the LUMO? Focus on the requirement for an empty orbital.
What can it be? There is only one, the empty σ* orbital of HCl.
First step
Energy
Cl
H
π
σ
*
H
HCl
–
Cl
+
HOMO
LUMO
CCCC
CC
+
–
Cl
Energy
H
–
Cl
CH
3
H
3
C
+
C
CH(CH
3
)
2
2p
z
Cl
H
HOMO
LUMO
Second step
CC
CC
+
(continued)

3.17 Mechanism of the Addition of Hydrogen Halides to Alkenes 135
If you got this problem right, feel really good. If it was a struggle, that is alright
as well. Not all problems are easy! If they were, Nature would be trivial to under-
stand, and life would be boring indeed. In any case, try to learn from these
in-chapter answered problems. There is a “take-home lesson” intended in each
of them.
Now let’s try to estimate qualitatively the overall change in energy as this reac-
tion proceeds. We will plot energy on the vertical axis against something we might
call “reaction progress”on the horizontal axis. Roughly speaking, we are asking how
the energy changes as the reaction takes place.Notice in Figure 3.74 that two bonds
CC
CH
3
Bond broken
(103.2 kcal/mol)
Bond broken
(66 kcal/mol)
Overall: Bonds broken (103
+ 66) = 169 kcal/mol
Bonds made (96
+ 85) = 181 kcal/mol
Net: 181
– 169 = about 12 kcal/mol exothermic
Cl
–
CH
3
H
3
C
H
3
C
HCl
CC
CH
3
CH
3
H
H
3
C
H
3
C
CC
Cl
CH
3
H
H
3
C
H
3
C
CH
3
Bond made
(85 kcal/mol)
Bond made
(96 kcal/mol)
+
FIGURE 3.74 An estimation of the energetics of the addition of HCl to 2,3-dimethyl-2-butene.
must be broken in the first step of the reaction.These are the carbon–carbon π bond
(66 kcal/mol), and the bond in HCl (103.2 kcal/mol). In the final product, two
bonds have been made, the new carbon–hydrogen bond (⬃96 kcal/mol), and the
new carbon–chlorine bond (⬃85 kcal/mol). It requires almost 170 kcal/mol to break
the bonds in this reaction, but that quantity is more than fully compensated by the
roughly 181 kcal/mol released by the new bonds formed (Fig. 3.74).
As we have seen,in the chemistry trade,this kind of reaction, in which the prod-
ucts are more stable than the starting materials, is called an exothermic reaction.But
this reaction actually takes place in two steps—we do not just suddenly arrive at a
product from the starting material.There is an intermediate carbocation in the reac-
tion, a transient charged species relatively high in energy. Not only is the carbocat-
ion charged—a bad deal in terms of energy—but the carbon does not have an octet
of electrons, which is another factor contributing to its high energy. There are two
factors that make this first step difficult. First, the bonds to be broken, the
carbon–carbon π bond and the σ bond between hydrogen and chlorine, are collec-
tively stronger than the bond made, the new carbon–hydrogen bond shown in red
in Figure 3.74. Second, charged species such as carbocations are high in energy, and
are not usually easily formed. Thus, the first step in this reaction is surely “uphill”
in energy, and thus is an endothermic reaction.
By contrast, the second step of this reaction, capture of the carbocation by chlo-
ride, is sure to be very energy-releasing—an exothermic reaction. Charges are annihi-
lated and a new carbon–chlorine bond is made, at no bond-breaking cost. If we plot

136 CHAPTER 3 Alkenes and Alkynes
these estimated energy changes,we get Figure 3.75, the first of many such energy dia-
grams to appear in this book as we estimate the energetics of other reactions.The over-
all reaction is exothermic—the products are more stable than the starting materials,
PROBLEM 3.29 Generalize from the discussion of HCl addition to predict the
products of the following reactions:
CC
CH
3
CH
3
H
3
C
H
3
C
HBr
H
2
SO
4
(HO SO
2
OH)
HI
?
?
?
Reaction pro
g
ress
Energy
+
–
+
Cl
Cl
Cl
H
H
H
CC
CC
CC
H
3
C
H
3
C
CH
3
CH
3
Cl
H
C
δ
+
δ
–
C
H
3
C
H
3
C
Transition state for the
first step
CH
3
CH
3
H
3
C
H
3
C
CH
3
CH
3
H
3
C
H
3
C
CH
3
CH
3
FIGURE 3.75 In this simple addition
of hydrogen chloride to an alkene, the
first step, the endothermic formation
of a carbocation, is the slow step.The
developing partial charges are shown
as δ
and δ
.
but an initial endothermic step, the formation of the intermediate carbocation, must
occur before product can be formed.There is an energy hill—a barrier—to be passed.
The tops of the hills in Figure 3.75 are transition states—the high points in ener-
gy separating starting material and product. We encountered transition states ear-
lier when we discussed the barrier to rotation in ethane and other alkanes (p. 67).
Although these energy maxima cannot be isolated, we can describe their structures.
In Figure 3.75, the making and breaking bonds are shown as dashed partial bonds.
The developing partial positive and negative charges appear as δ
and δ
.
With just a little thought we have a very clear picture of the addition reaction. An
unstable carbocationic intermediate is formed and then destroyed as the chloride ion adds
to give the final product. Notice two important things about this reaction. First, it can-
not proceed unless enough energy is supplied so that the first transition state can be
reached. This amount of energy—that required to pass over the highest-energy point,
the highest transition state—is called the activation energy (ΔG
‡
) for the reaction.
Second, we get a bonus from this analysis. If we understand the pathway from
left to right, we also understand the reverse reaction—the one that runs from right
to left. It follows exactly the same energy curve, only in the opposite direction.

3.18 The Regiochemistry of the Addition Reaction 137
3.18 The Regiochemistry of the Addition Reaction
In the reaction introduced in Figure 3.68, the addition of HCl to 2,3-dimethyl-
2-butene, the two ends of the alkene are the same, which means that there is no
choice to be made in the protonation step. Addition to either end of the alkene
gives exactly the same carbocation. Let’s extend our discussion just a bit and exam-
ine an alkene in which the two ends are different. We’ll do more of this sort of
thing in Chapter 9, when we take a long look at many kinds of addition reactions,
but there is much to be learned from even a small extension of the symmetrical
addition reaction.
So, let’s allow HCl to react with 2-methylpropene, an alkene in which the two
ends are certainly very different. Now two different protonations are possible, and
addition of chloride to the two carbocations will give two products (Fig. 3.76). This
kind of distinction is called the regiochemistry of the reaction. In fact, only one of
the two possible products is formed. Let’s see if we can guess why.
Each potential reaction pathway in Figure 3.76 involves a different carbocation-
ic intermediate.Perhaps formation of a tertiary carbocation requires less energy than
CC
H
H
H
3
C
H
3
C
Cl
–
Cl
–
CC
H
H
3
C
H
3
C
CH
3
Cl
H
CC
H
H
CH
3
CH
3
Cl
H
H
3
C
Cl
H
CCH
H
3
C
This product
is not formed
Primary carbocation
2-Methylpropene
isobutene
CCH
2
H
3
C
The product
of the reaction
Tertiary carbocation
+
+
H H
H H
FIGURE 3.76 Two possible additions
of HCl to 2-methylpropene.
formation of a primary carbocation. If that idea were correct, the energy hill to
climb to give the observed product (formed from the tertiary carbocation) would
be lower than that leading to the product that is not observed (Fig. 3.77).
Energy
Tertiary
carbocation—
lower energy?
Primary
carbocation—
higher energy?
Cl
–
Cl
–
CH
3
H
3
C
H
3
C
CCH
2
HCl+
H
3
C
H
3
C
C
H
H
3
C
CH
3
CH
2
+
C
+
FIGURE 3.77 Relative stability of the
tert-butyl cation (tertiary) and the
isobutyl cation (primary).

138 CHAPTER 3 Alkenes and Alkynes
This idea leads to a prediction. If our surmise that the stability of carbocations
increases with substitution is correct, that is, if a tertiary carbocation is more stable
than a primary carbocation, presumably a tertiary carbocation will also be more
stable than a secondary carbocation, and a secondary carbocation will be more sta-
ble than a primary carbocation. The complete order of cationic stability would
be tertiary secondary primary methyl (Fig. 3.78).
Tertiary
C
+
CH
3
H
3
CCH
3
Primary
C
+
CH
3
HH
Methyl
More
stable
than
More
stable
than
More
stable
than
C
+
H
HH
Secondary
C
+
H
H
3
CCH
3
FIGURE 3.78 Predicted stability order
for carbocations.
If the order of carbocation stability shown in Figure 3.78 is correct, we should
always get the product resulting from formation of the more stable carbocation
(Fig. 3.79).
5
We can test this idea. Just allow HCl to add to 2-methyl-2-butene
or propene, each an unsymmetrical alkene. If we are right in our ideas about carbo-
cation stability, the products will be 2-chloro-2-methylbutane and isopropyl chlo-
ride, respectively. When these experiments are run, those are exactly the products
observed (Fig. 3.79).
CC
CH
3
H
H
3
C
H
3
C
Cl
H
CC
CH
3
H
H
3
C
Cl
–
Tertiary
carbocation
Secondary
carbocation
H
3
C
H
Cl
2-Chloro-2-methylbutane
100%
not
C
C
C
H
CH
3
H
3
C
H
3
C
H
CH
H
CH
3
CH
3
H
CC
H
H
H
3
C
H
Cl
H
CC
H
HH
Cl
–
Secondary
carbocation
Primary
carbocation
H
3
C
H
Cl
Isopropyl chloride
100%
not
C
C
C
H
H
H
3
C
H
H
C
H
CH
3
H
H
H
+
+
+
+
2-Methyl-2-butene
Propene
FIGURE 3.79 Addition of HCl to two
unsymmetrical alkenes.
5
We hope you are wondering why this order exists. We will get to that question in Chapter 9.
The results shown in Figure 3.79 are in accord with our mechanistic ideas; our
proposed reaction mechanism is supported by the new data. Do we now know how
this addition of HCl to alkenes works? Is our mechanism proved? Sadly, the answer
