Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.

3.2 Alkenes: Structure and Bonding 99
3.2 Alkenes: Structure and Bonding
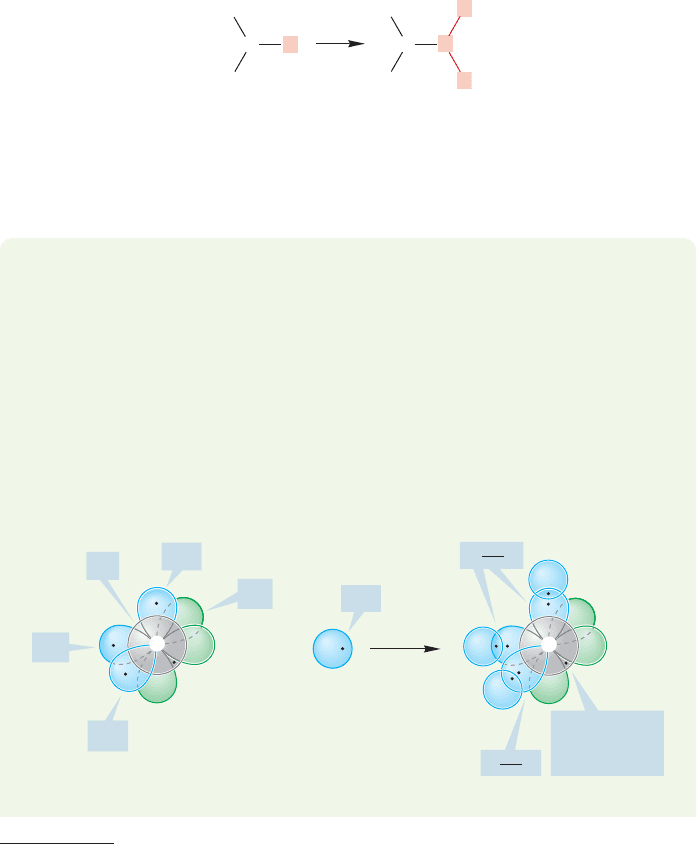
The simplest alkene is properly called ethene but is almost universally known by its
common name, ethylene.
2
Several spectroscopic measurements and chemical reactions show that ethylene
has the formula C
2
H
4
and is a symmetrical compound composed of a pair of meth-
ylene groups: H
2
CCH
2
. Earlier, when we encountered methane (CH
4
), we con-
structed a bonding scheme designed to reproduce the way four hydrogens were
attached to the central carbon.In ethylene,we have a slightly different arrangement.
Each carbon atom in ethylene is attached not to four other atoms, as is the carbon
in methane or ethane,but to three.It seems we will need a different bonding ration-
ale with which to describe Nature now. Our strategy will be to develop a bonding
scheme for the simplest trivalent compound of carbon, methyl (CH
3
), and then
extend it to ethylene, in which each carbon is attached not to three hydrogens as in
methyl, but to two hydrogens and the other methylene (CH
2
) group (Fig. 3.2).
2
We like the old names and will use many of them. The newer, systematic names are fine when complexity
develops—we obviously could not have a common, or “trivial” name for every compound. Yet some of the fla-
vor of organic chemistry is lost when the system is applied too universally. The trivial names connect to his-
tory, to the quite correct image of the bearded geezer slaving over the boiling retort, and we like that.
H
H
H
C
H
H
H
H
CC
FIGURE 3.2 Replacement of one
hydrogen in methyl (CH
3
), with a
methylene (CH
2
) group leads to the
framework of ethylene (H
2
CCH
2
).
Note that each carbon so far has only
three bonds. In this drawing the full
bonding scheme for ethylene is not
yet in place.
Three bonds are needed to make attachments to the three hydrogens in CH
3
. Using
the atomic orbitals of carbon to overlap with the three hydrogen 1s orbitals leads to
problems, just as it did in our earlier construction of methane (see Section 2.2), as
Worked Problem 3.1 shows.
WORKED PROBLEM 3.1 Produce a bonding model for neutral methyl ( CH
3
)
using the unhybridized atomic orbitals of carbon and the hydrogen 1s orbitals.
Critically discuss the shortcomings of this model. What’s wrong with it?
ANSWER There is more than one way to construct such a model. Start by deter-
mining how many electrons are available. Carbon (
6
C) supplies four (six less the
two low-energy 1s electrons), and each hydrogen supplies one, for a total of seven.
We might form three carbon–hydrogen bonds by overlap of the carbon 2p
x
,2p
y
,
and 2p
z
orbitals with three hydrogen 1s orbitals, for example.This process uses six
electrons (two in each of the three carbon–hydrogen bonds), and leaves the car-
bon 2s orbital to hold the remaining electron.
H
1s
3
2p
y
2s
2p
z
2p
x
2p
z
H
C H
C H
+
C
C
H
H
2s Orbital
containing
one electron
.
(continued)

100 CHAPTER 3 Alkenes and Alkynes
C (1s
2
) 2s
2
2p
x
2p
y
Combine 2s, 2p
x
, 2p
y
three sp
2
hybrid orbitals; 2p
z
is not used
FIGURE 3.3 The combination of
three atomic orbitals of carbon.
A
n sp
2
hybrid H
1s
C H
Bonding
σ orbital
+
FIGURE 3.4 The bonding σ
orbital.
C
O
H
However, this model gives a “methyl”with carbon–hydrogen bonds 90° apart. It is
possible for a central carbon to be surrounded by three hydrogens 120° apart, and
so we might suspect that this unhybridized model with its 90° angles will be desta-
bilized by electron–electron repulsion. Moreover, overlap of a 1s orbital with a 2p
orbital is inefficient because the rear lobes of the 2p orbitals are wasted. Stronger
bonds can be constructed from directed, hybrid orbitals.
Other possible answers include making two carbon–hydrogen bonds from 2p/1s
overlap and the third from 2s/1s overlap. This model leaves the last electron in a
2p orbital and consequently has the same problems as noted previously.
H
C H Bonds
close together
Wasted lobe
Wasted lobe
Wasted lobe
90⬚
C
H
H
The new sp
2
hybrids are generally shaped like the sp
3
hybrids constructed
earlier. They are directed orbitals—there is a fat lobe and a thin lobe, so they lead
to quite efficient overlap with the hydrogen 1s orbitals (Fig. 3.4). The directed sp
2
hybrid orbital overlaps with the spherical 1s orbital on hydrogen to produce a
carbon–hydrogen σ bond.The antibonding orbital (σ*) is not shown in Figure 3.4.
What do you guess is the angle between the three hybrid orbitals? Remember
that one of the problems with using unhybridized carbon atomic orbitals is that this
process produces bonds that are too close together, and repulsions between filled
orbitals are not minimized. Hybrid orbitals produce strong bonds (good overlap) that
As in our earlier discussion of sp
2
hybridization (p. 55), we will create three new
hybrid orbitals that will do a better job of attaching the carbon atom to the three
hydrogens than do the pure atomic orbitals. Three hybrid orbitals are needed, and
our mathematical operations will therefore involve combining three wave functions
(atomic orbitals) to produce the three new hybrid orbitals. Recall that in such quan-
tum mechanical calculations the number of orbitals created always equals the num-
ber of orbitals combined in the calculation, here three. So, let’s combine the carbon
2s,2p
x
, and 2p
y
orbitals to produce three new, sp
2
hybrids.The 2p
z
orbital unused in
our calculation remains, unhybridized and waiting to be incorporated in our picture
(Fig. 3.3). Remember that the choice of 2p orbitals to be combined is arbitrary. Any
pair of p orbitals can be used, leaving the third left over.
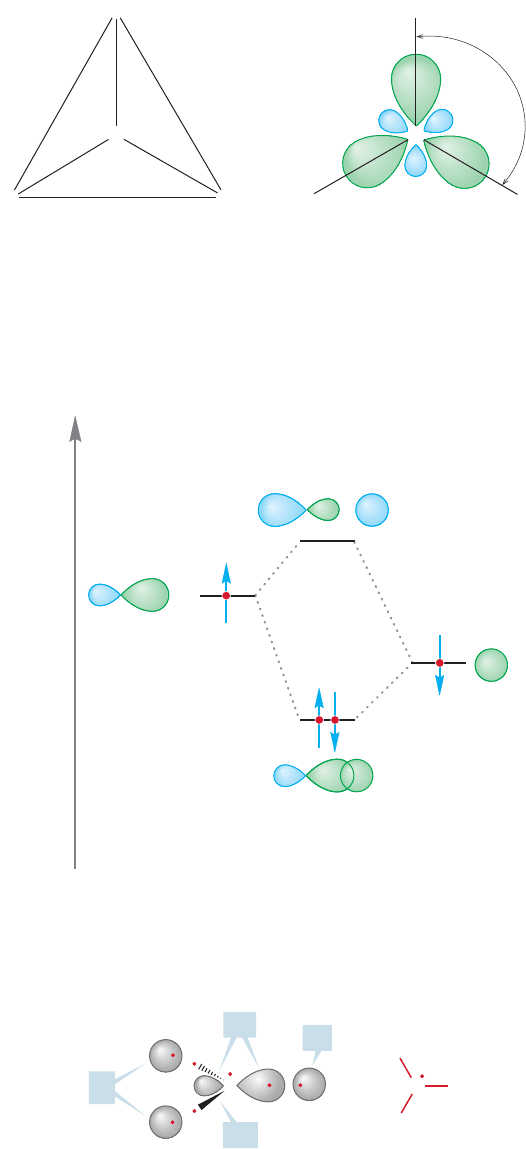
3.2 Alkenes: Structure and Bonding 101
120⬚
H
H
HH
H
H
C
C
FIGURE 3.5 In planar CH
3
, the angle
between the carbon–hydrogen bonds
is 120°. Repulsive overlap between
the filled carbon–hydrogen bonds is
minimized in this arrangement.
Energy
sp
2
1s
*
sp
2
– 1s (antibonding
molecular orbital)
sp
2
+ 1s (bonding
molecular orbital)
FIGURE 3.6 The overlap of sp
2
and 1s orbitals can occur in a
bonding, stabilizing way (sp
2
1s),
or an antibonding, destabilizing way
(sp
2
1s).
are directed so as to minimize repulsions. As Figure 3.5 shows, the best way to
arrange three things in space (the hydrogens in this case) surrounding a central object
(here, the carbon) so they are as far from one another as possible, is to put them at
the corners of an equilateral triangle. And indeed, the angles between the three sp
2
hybrids are exactly 120°. Recall the discussion of BH
3
in Section 2.2c.
Each of the three sp
2
/1s overlapping systems produces a bonding (σ sp
2
1s)
and an antibonding (σ* sp
2
1s) molecular orbital. Stabilization is maximized
if we form two-electron bonds, as the stabilized bonding molecular orbital
will be filled and the antibonding, high-energy molecular orbital is empty
(Fig. 3.6).
Each hydrogen supplies a single electron.Therefore,in order to form three two-
electron bonds we need a single electron from carbon for each of the three sp
2
hybrids
(Fig. 3.7).
H
CH
H
H
H
H
C
=
sp
2
sp
2
1s
1s
FIGURE 3.7 Three two-electron
carbon–hydrogen bonds can be
formed using one electron from each
hydrogen and three electrons from
carbon (all electrons are shown in
red).This leaves one electron on
carbon (shown in red) left over. In
this figure, two of the sp
2
orbitals are
shown schematically as wedges.
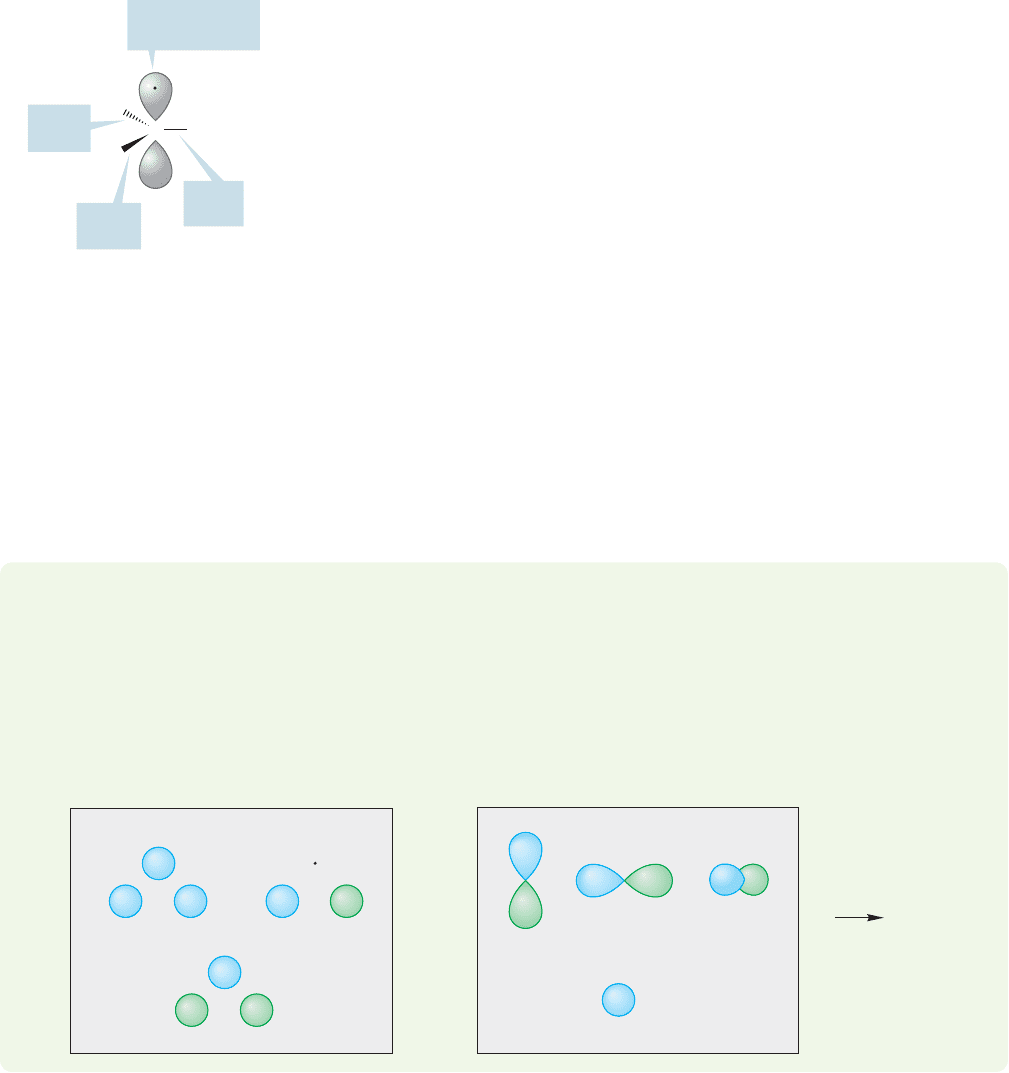
102 CHAPTER 3 Alkenes and Alkynes
H
H
H
C
sp
2
/1s
Bond
sp
2
/1s
Bond
sp
2
/1s
Bond
Singly occupied
2p
z
orbital
FIGURE 3.8 A view of sp
2
hybridized
CH
3
.
Remember that we did not use the carbon 2p
z
orbital in creating the sp
2
hybrids.
That’s where the last electron of carbon goes. So our sp
2
bonding scheme leads to the
picture in Figure 3.8: a carbon atom surrounded by a planar array of three hydrogen
atoms, with the unhybridized 2p
z
orbital extending above and below the plane of the
four atoms.The carbon–hydrogen bonds are familiar two-electron bonds (don’t forget
the empty antibonding orbitals,though) and there is a single electron in the 2p
z
orbital.
Don’t be confused by the electron shown in only one lobe of the 2p orbital. The two
lobes are not separate, and the electron occupies the whole orbital, not just one lobe.
We have seen this molecule, CH
3
, before! It is nothing more than a methyl radical.
In the text so far, we have built up a picture that emphasizes localized bonds. In
methyl, for example, electrons occupy three bonding orbitals made up of overlap-
ping sp
2
and 1s orbitals. In Problem 3.2, we develop a picture of methyl in which
the delocalization of electrons throughout the molecule is emphasized. Delocalizing
electrons,spreading them out over several atoms rather than localizing them between
two atoms,is almost always energetically favorable.But there are advantages to both
schemes. In one sense the delocalized picture is probably more “real,” because elec-
trons are not limited to the regions of space the hybridization scheme suggests.
However, we do not make horrible energetic mistakes if we ignore the delocaliza-
tion that the orbitals you develop in Problem 3.2 show so clearly, and the hybridiza-
tion scheme is excellent for “bookkeeping” purposes. It helps us to keep track of
electrons in the chemical reactions that follow in later chapters, for example.
Molecular orbitals for
2p
x
2p
y
2s
2p
z
?
HH
H
Atomic orbitals for carbon
+
–
BA
C
It is important that we do not view the molecular orbital and hybridization schemes
as being in conflict or as giving substantially different pictures of the bonding in CH
3
.
Note, for example, that the geometry is exactly the same in the two schemes. We are
humans, stuck with our inability to apprehend the properties of electrons easily, and
needing approximations in order to represent Nature. Different approximate bonding
schemes have been developed that emphasize different properties of the molecules.The
molecular orbital picture does an excellent job of showing the distribution of electrons
throughout the molecule.The hybridization picture sacrifices an ability to show this delo-
calization for the advantages of clarity, and ease of following the course of chemical reac-
tions. We need to keep both representations in mind as we study chemical reactions.
PROBLEM 3.2 Construct the bonding molecular orbitals for planar methyl (CH
3
).
Use the molecular orbitals for cyclic H
3
(A, B, and C) given below. The molecu-
lar orbitals for H
3
are shown in the drawing, but if they are unfamilar, take a look
now at Problems 1.62 and 1.63. Allow these orbitals to interact with the appro-
priate atomic orbitals of a carbon atom placed at the center of the triangle of
hydrogens. Next, place the bonding molecular orbitals in order of energy, lowest
first. The dot in B shows the position of the third hydrogen atom.
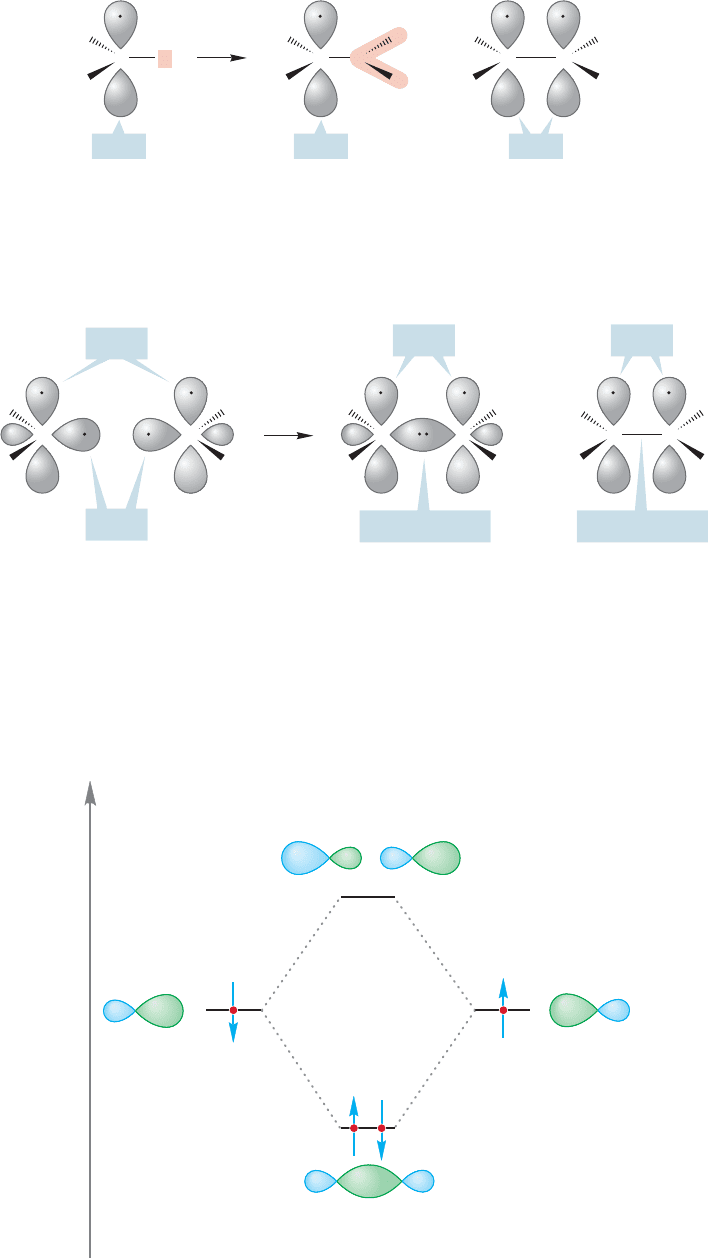
3.2 Alkenes: Structure and Bonding 103
=
H
H
C
C
H
H
C C
2p
z
2p
z
2p
z
H
H
H
H
H
H
C
H
FIGURE 3.9 Our first orbital picture
of ethylene.
We could easily imagine exchanging one of the three hydrogens of CH
3
for another
atom or group. In constructing ethylene (H
2
CCH
2
), this other group would be another
methylene (CH
2
; Fig. 3.9). This transformation gives us our first orbital picture of
ethylene although the carbon–carbon double bond is not yet complete-
ly drawn in Figure 3.9. So our first picture of ethylene is derived from joining a pair of
methylene groups.The two sp
2
hybrids not involved in the carbon–hydrogen bonds over-
lap to make an sp
2
/sp
2
σ bond joining the two carbons of ethylene.The two 2p
z
orbitals
remain, and extend above and below the plane defined by the six atoms (Fig. 3.10).
(H
2
C
P
CH
2
),
H
H
CC
H
H
H
H
C CCC
H
H
H
H
H
H
=
2p
z
2p
z
2p
z
sp
2
sp
2
/sp
2
σ Bond sp
2
/sp
2
σ Bond
FIGURE 3.10 Another orbital picture
of ethylene.
The structure of ethylene shown in Figure 3.10 is quite analogous to that pro-
duced in the construction of ethane from a pair of sp
3
hybridized carbons (Section
2.5). In ethane, two sp
3
hybrids overlapped to form a bonding σ orbital and an anti-
bonding σ* orbital (p. 68). Construction of the carbon–carbon sigma bond system
in ethylene begins with the similar overlap of a pair of sp
2
hybrids. Once more, a
bonding σ orbital and antibonding σ* orbital are produced, this time by the con-
structive and destructive overlap of a pair of sp
2
hybrids (Fig. 3.11). Because there
are only two electrons, only the low-energy, bonding molecular orbital is filled.
Energy
σ*
σ
sp
2
+ sp
2
(bonding molecular orbital)
sp
2
– sp
2
(antibonding molecular orbital)
sp
2
sp
2
FIGURE 3.11 Overlap of the two sp
2
hybrids to form a bonding and
antibonding pair of molecular
orbitals.
104 CHAPTER 3 Alkenes and Alkynes
So far, we have ignored the 2p
z
orbital on each carbon. It is time to take them
into account. These two carbon 2p
z
orbitals are only the distance of a single bond
apart and will interact quite strongly. Moreover, they are of equal energy and that
too contributes to a strong interaction. Orbitals of equal energy interact most
strongly (p. 34). We know what happens when atomic orbitals overlap—bonding
and antibonding molecular orbitals are created. The new molecular orbitals
formed from overlap of a pair of 2p orbitals are shown schematically in Figure 3.12.
Energy
2p – 2p = π* (antibonding)
2p + 2p = π (bonding)
Symmetry plane
contains all four
hydrogens and
both carbons
66 kcal/mol
2p
2p
Stabilization
H
H
H
H
C C
2p/2p Overlap gives a π bond
sp
2
/sp
2
Overlap gives a σ bond
FIGURE 3.12 Overlap of a pair of 2p orbitals results in
a lower-energy, bonding π orbital and a higher-energy,
antibonding π* orbital.The stabilization amounts to
about 66 kcal/mol when the π orbital is filled.
WEB 3D
=
H
H
H
H
C
C
H
H
H
H
CC
The coded representation for a double
bond; note that there is no distinction
made between the
σ and π bonds; a
typical C
C bond distance is 1.33 A
⬚
FIGURE 3.13 The π and σ bonds
of a carbon–carbon double bond are
not differentiated in the coded
representation of an alkene.
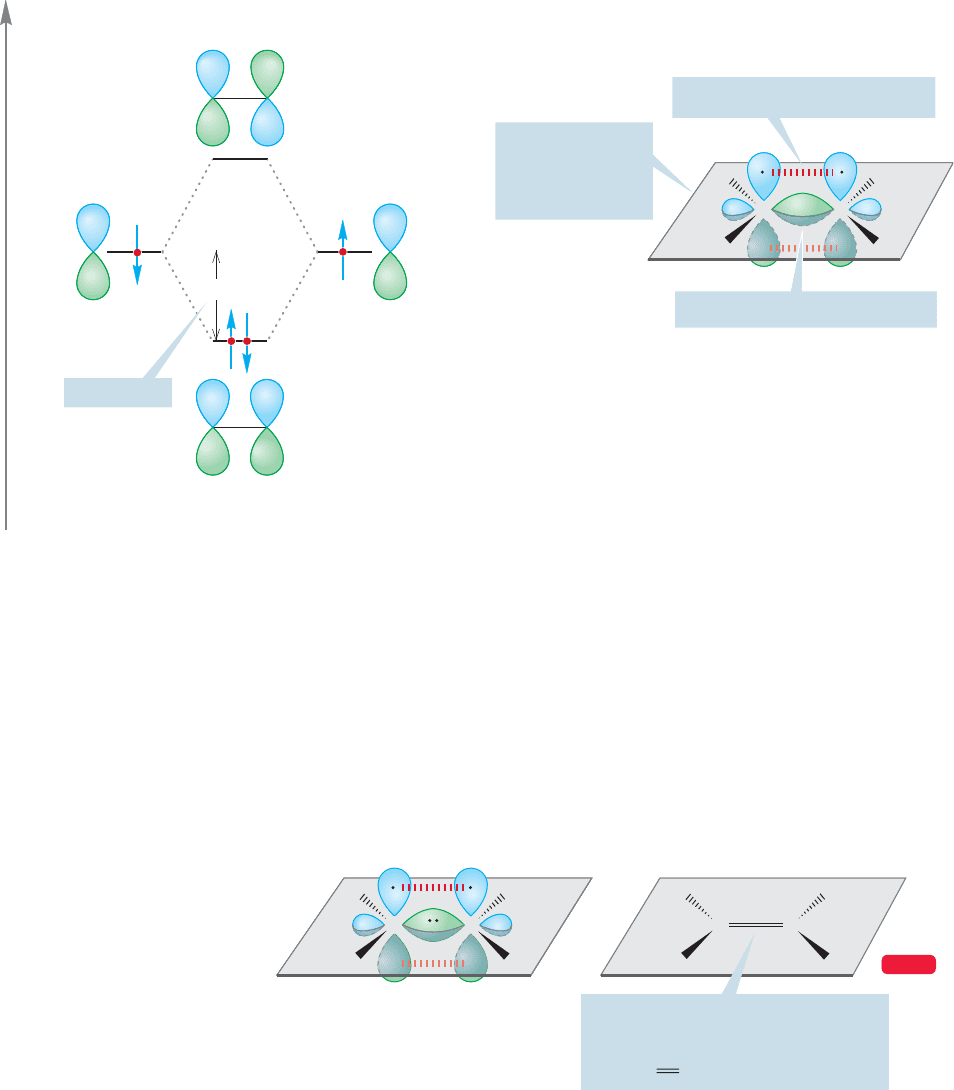
These new orbitals are not σ orbitals because they do not have cylindrical symme-
try.There is a plane of symmetry instead, and this makes them pi () orbitals, here
called π (bonding) and π* (antibonding).There are only two electrons to be put into
the new system of two molecular orbitals, one from each carbon 2p
z
orbital. These
are accommodated very nicely in the highly stabilized bonding π molecular orbital.
So the two carbons of ethylene are held together by two bonds. One is made up
of sp
2
/sp
2
σ overlap and the other of 2p/2p π overlap.There is a double bond between
the two carbons made up of the σ and π bonds. The convention is to simply draw
two lines between the atoms, making no distinction between the two bonds,
although, as you have just seen, this approximation is far from justified (Fig. 3.13).
3.2 Alkenes: Structure and Bonding 105
The overall bonding scheme for a carbon–carbon double bond includes both σ and
π bonds (and their empty antibonding counterparts) and is also shown in Figure 3.13.
Carbon–carbon double bonds are quite short, with a typical bond distance
being 1.33 Å.
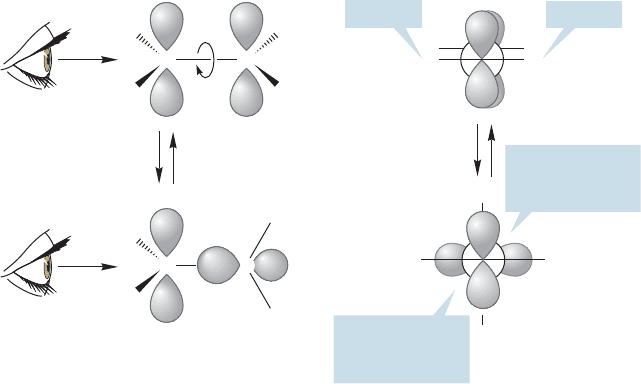
In ethane, there is nearly free rotation about the carbon–carbon bond
(⬃3 kcal/mol, p. 65). Is the same true for ethylene? Actually, the argument is
a bit more subtle. There isn’t really free rotation about the carbon–carbon
bond in ethane. There is a 3-kcal/mol barrier, produced by the need to pass
through a structure in which the carbon–hydrogen bonds are eclipsed. Our real
question should be, How high is the barrier to rotation about the carbon–carbon
double bond in ethylene? To begin the answer we construct Newman projections
by sighting along the carbon–carbon bond (Fig. 3.14).
HH
H
H
=
=
CC
In this form, the two 2p
z
orbitals do not overlap
90⬚ Form of ethylene
H
H
H
H
C
Eclipsing
(torsional strain)
minimized
Eclipsing
(torsional strain)
minimized
90⬚ rotation
0⬚ Form of ethylene Newman projections
90⬚ rotation
EclipsedEclipsed
H
H
H
H
HH
H
H
C
FIGURE 3.14 Although rotation
about the carbon–carbon bond
relieves torsional strain, it also
destroys the overlap between the two
2p
z
orbitals on the adjacent carbons.
This loss of orbital overlap leads to a
high barrier to rotation. When one
2p orbital is rotated 90°, orbital
overlap is completely lost.
Note that in the 0° conformation of ethylene there is torsional strain
induced by the eclipsing of the carbon–hydrogen bonds. This strain is removed
in the 90° rotated arrangement because now the electrons are as far apart
as possible. In ethane, each pair of eclipsed hydrogens induces approximately
1 kcal/mol of destabilization. Therefore our initial guess might be that the
0° form of ethylene would be about 2 kcal/mol higher in energy than the 90°
form. That estimate would be approximately right if torsional strain were
the whole story. But it is not, as we have ignored the pair of 2p
z
orbitals,
and their interaction completely overwhelms the small amount of torsional
strain.
In the 0° form, the two p orbitals overlap; in the 90° form they do not
(Fig. 3.14).When rotation occurs, the 2p/2p overlap declines and so does the sta-
bilization derived from occupying the new bonding molecular orbital with two
electrons.There is an enormous stabilizing effect in the 0° arrangement that over-
comes the relatively minor effects of torsional strain.
ETHYLENE: A PLANT HORMONE
106 CHAPTER 3 Alkenes and Alkynes
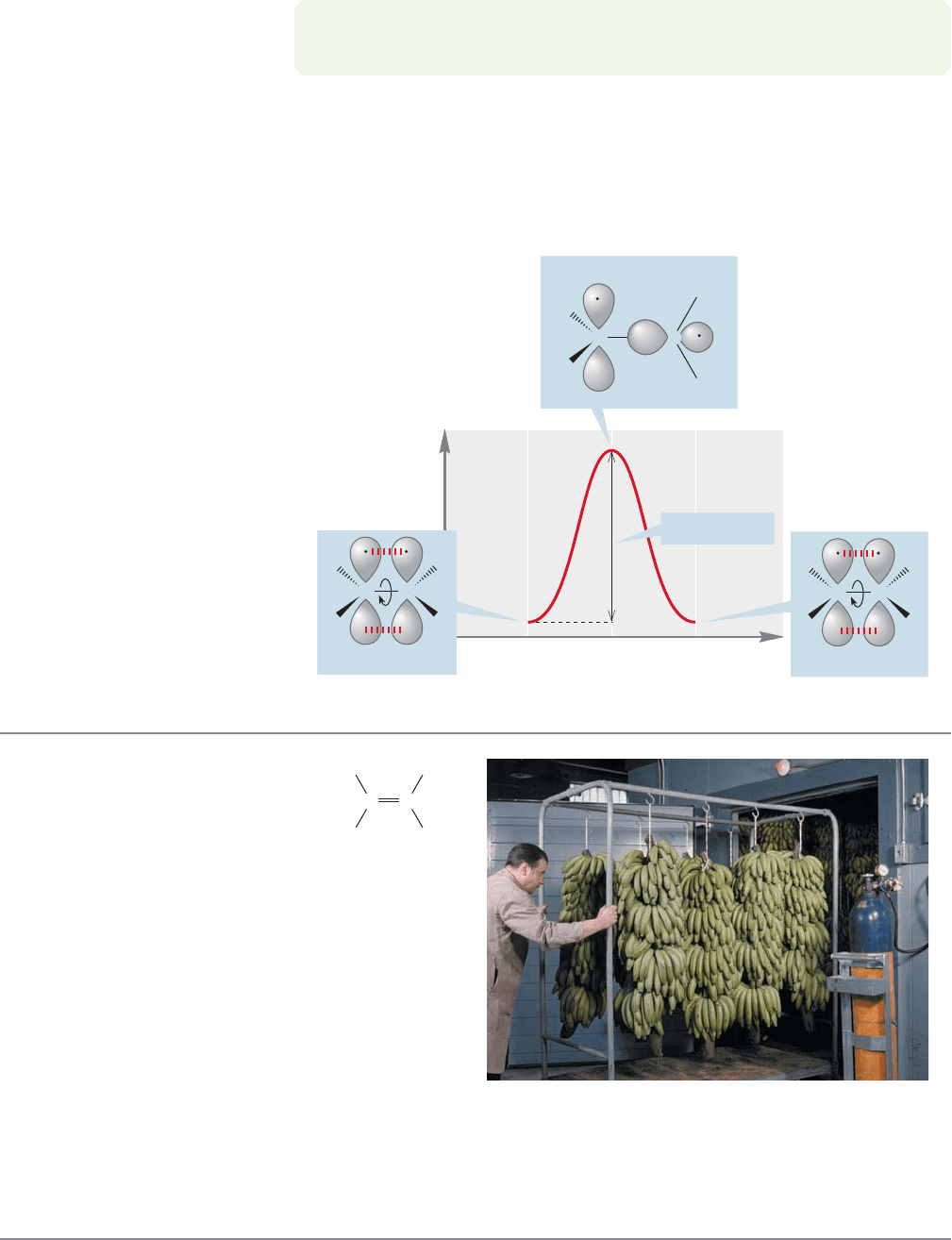
Figure 3.15 plots the energy change as rotation occurs. The barrier to rota-
tion here is no paltry 3 kcal/mol as it is in ethane but 65.9 kcal/mol, an amount
far too high to be available under normal conditions. Alkenes are “locked” in a
planar conformation by this amount of energy. We will soon see some conse-
quences of this locking.
Energy
Rotation angle
0⬚ 180⬚90⬚
90⬚
65.9 kcal/mol
H
H
C
H
H
C
0⬚
HH
H
H
CC
180⬚
HH
H
H
CC
FIGURE 3.15 A plot of potential
energy versus rotation angle in
an alkene.
to the discovery of ethylene as a plant hormone. Trees sur-
rounding gas lamps were often defoliated when gas leaks
occurred. A principal component of the illuminating gas was
ethylene, and a little investigation showed that this chemical
was the cause of the defoliation.
It’s amazing, but the simplest of all
alkenes, ethylene, is an important plant
hormone. Among other functions, ethyl-
ene acts to promote ripening of fruit. Moreover, produc-
tion of ethylene is autocatalytic; that is, a little ethylene
induces the formation of more from the amino acid
methionine, and its effects are magnified. Accordingly,
fruits such as tomatoes and bananas are now typically
shipped green in well-ventilated containers so they will
arrive unspoiled. Ripening can then be started by exposure
to ethylene.
Ethylene also induces abscission; the falling of leaves or
flowers, by promoting the formation of the enzyme cellulase
that weakens cell walls by destroying the cellulose from
which they are made. A weakened abscission layer forms at
the base of the leaf or flower, and wind or rain then breaks
the stem. Indeed, it was this phenomenon that indirectly led
H
HH
H
CC
PROBLEM 3.3 Show that in the 90° rotated form of an alkene there is zero over-
lap between the pair of carbon 2p
z
orbitals.
3.3 Derivatives and Isomers of Alkenes 107
Summary
Two carbons joined by a double bond are held together by a σ bond made up of
overlapping sp
2
orbitals and a π bond made from 2p orbitals overlapping side to
side.There is no free rotation about a carbon–carbon double bond, because such
rotation diminishes overlap between the 2p orbitals making up the π bond and
costs energy. A full 90° rotation requires about 66 kcal/mol of energy. Therefore,
alkenes are planar.
In the hybridization model, we have constructed alkenes with both relatively
strong σ bonds and weaker π bonds. The weaker, higher-energy π bonds will be
more reactive than the stronger, lower-energy σ bonds.This point is critical and is
often a source of confusion. A bond with lower energy is more stable and less reac-
tive than a higher-energy bond. The weaker π bonds are likely to be the locus of
reactivity of alkenes. In fact, alkenes and alkynes undergo all sorts of reactions that
are unknown for alkanes with their exclusive set of strong, unreactive σ bonds. As
soon as we work a bit more on nuts and bolts (nomenclature, isomers, and some
thermochemistry), we’ll get to what is usually the most fun in organic chemistry—
reactions—and they will be triggered by the relatively weak π bonds in alkenes.
3.3 Derivatives and Isomers of Alkenes
After we developed a structure for ethane (p. 68), we examined substituted
alkanes.Here we do the same thing for ethene,and discover a new kind of isomerism.
There is but one ethylene (or ethene, if you insist). We can make derivatives of
this molecule by mentally replacing a hydrogen with some “X” group. Figure 3.16
shows a few such compounds. These are not called “ethenyl” or even “ethylenyl”
compounds but instead bear the delightfully trivial name, so far untampered with,
of vinyl.
If we now let X CH
3
(methyl), we get a compound, C
3
H
6
(propene or, com-
monly, propylene, Fig. 3.17). Notice how the root prefix prop- has been retained for
this three-carbon species, and only the vowel changed to note the difference between
saturated propa
ne and unsaturated propene.
H
H
H
X
F
X =
Br
Cl
I
OH
CN
SiH
3
NH
2
Vinyl fluoride
Vinyl bromide
Vinyl chloride
Vinyl iodide
Vinyl alcohol
Vinyl cyanide
Vinyl silane
Vinylamine
Ethylene Vinyl X
CC
H
HH
H
CC
WEB 3D
FIGURE 3.16 Some substituted
alkenes called vinyl compounds.
WEB 3D
X = CH
3
C
CH
3
H
H
H
X
CC
H
H
H
Propene
C
H
HH
H
CC
FIGURE 3.17 When X CH
3
(methyl), we get propene (C
3
H
6
).
Note the change of vowel from
propane to propene.
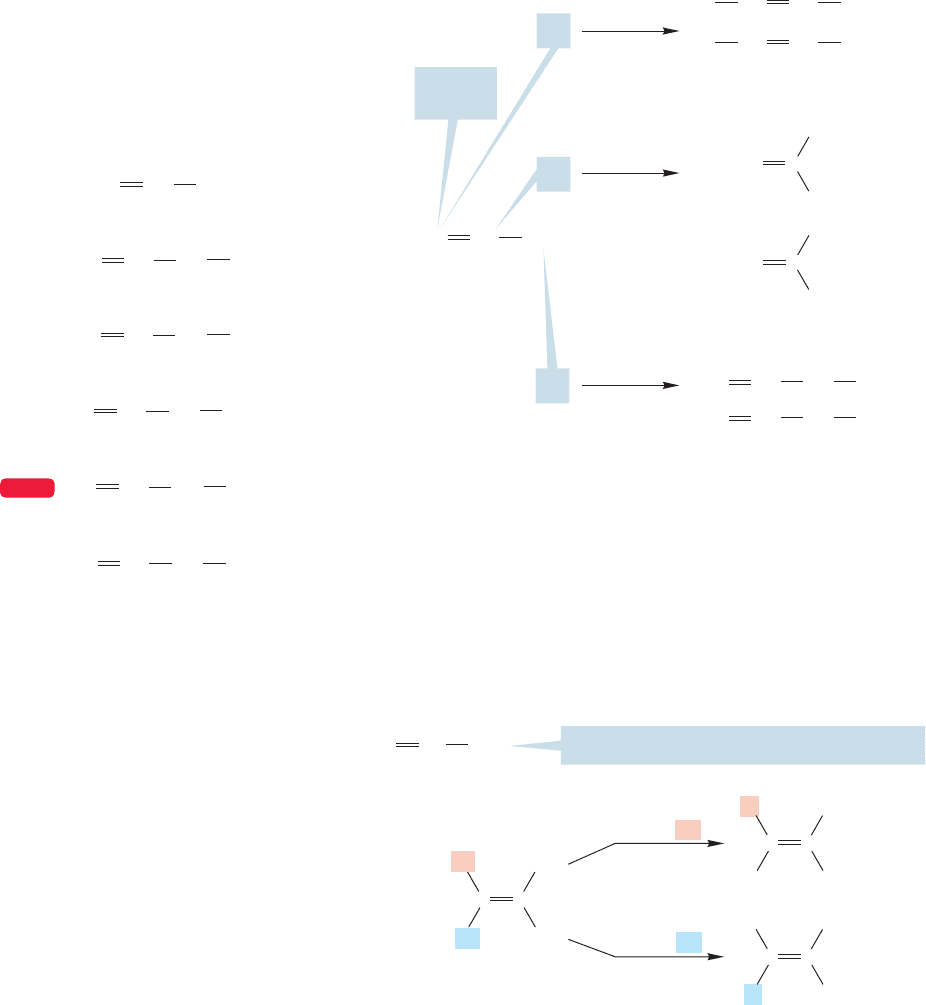
108 CHAPTER 3 Alkenes and Alkynes
replace H
a
1-Chloropropene
2-Chloropropene
3-Chloropropene
or allyl chloride
H
2
CCH
3
H
a
H
b
Terminal
position
H
c
CH
H
2
CC
HC CH
3
CHX
X
HC CH
3
CH
3
H
2
C
H
2
C
H
2
C
C
Cl
Propene
CH
3
CHCl
CH
2
CH X
CH
2
CH Cl
replace H
b
replace H
c
FIGURE 3.18 Substitution of three different hydrogens in propene
appears to give three different compounds.propenyl
O
X
We can now imagine replacing a hydrogen in propene with an “X”atom or group,
but the situation is not as simple as it was with ethylene. Although there is only one
kind of hydrogen in ethylene, there are several different hydrogens in propene that
might be replaced. A quick count of the different hydrogens in propene is mislead-
ing. Care must be taken from now on to draw out the three-dimensional structures
of the alkenes on which we are doing our mental replacements.In practice, this task
is quite simple, because alkenes are planar, and most structures are easily drawn. If we
just write a shorthand structure,we predict only three new kinds of compounds when
a hydrogen is replaced with an X atom or group. As shown in Figure 3.18, these
compounds are all known.Note the use of the frequently encountered common name
allyl for the group. Figure 3.19 shows some allyl compounds.
There is one severe problem with the approach of Figure 3.18.In reality, there are not
three kinds of substituted propenes, but four.The more detailed drawing in Figure 3.20
shows why. The schematic structure for propene used in Figure 3.18 is inadequate
H
2
C
P
CH
O
CH
2
The allyl group
H
2
CCH
2
CH
H
2
CCH
2
ClCH
Allyl chloride
H
2
C
CH
2
Br
CH
Allyl bromide
CH
2
NH
2
H
2
C
CH
Allylamine
CH
2
OH
H
2
C
CH
Allyl alcohol
CH
2
CN
H
2
C
CH
Allyl cyanide
WEB 3D
FIGURE 3.19 Some allyl compounds.
H
2
CCH
3
CH
replace H
a
The two H
a
atoms are really
different! H
a
is on the same
side as the methyl group,
but H
a
'
is on the opposite side
This schematic drawing hides too much detail
replace H
a
'
H
a
'
These compounds
are not the same!
X
H
a
'
H
b
CC
CH
3
H
a
X
H
b
CC
CH
3
H
a
H
b
CC
CH
3
FIGURE 3.20 A detailed drawing of
propene reveals four different
potential substitution sites.
