Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.

2.2 Hybrid Orbitals: Making a Model for Methane 59
PROBLEM 2.8 Why not allow a 2p orbital to overlap sideways with an s orbital?
Why would this not solve the problem of waste of the rear lobe (see below and
Fig. 2.10)?
Summary
There are two severe problems with the too-simple model for methane shown in
Figure 2.9. The hybridization model solves them both!
1. The electrons in the bonds are far from being as remote from each other as pos-
sible. The hybridization model yields four hybrid orbitals directed toward the
corners of a tetrahedron, the best possible arrangement.
2. Bonds are formed through overlap between the carbon 2p atomic orbitals and
hydrogen 1s orbitals, thus wasting the back lobes of the 2p orbitals. In the
hybridization model, overlap is much improved in the bonds formed using the
fat lobes of sp
3
orbitals and hydrogen 1s orbitals.Table 2.1 reviews the properties
of sp, sp
2
, and sp
3
hybrid orbitals.
So why didn’t we just tell you that methane is tetrahedral and be done with it
rather than taking several pages to explore that experimental observation? First of all,
learning science is not about gathering a collection of facts. Rather it is learning a
way of thinking—a way of solving problems—of reaching an approximate idea of how
Nature works. In science, we look at experimental data (methane has the formula
CH
4
, for example) and then postulate a model to explain our observations. In the
beginning,that model is almost certain to be either flawed or at the very least severe-
ly incomplete. So we test it against new data—against what can be learned from new
experiments, and modify our hypothesis. In this case, that process looks like this: We
find out that methane has no 90° angles—how can we change our model to get rid
of those errors and come to a better model of Nature? Ultimately, we come to the
tetrahedral structure. We have learned much from looking at why our early, too-
simple models were inadequate and from our process of investigation. You will forget
most of the facts in this book,eventually.No matter, you can always look them up here
or elsewhere. What we hope you will not forget is the investigative method used in
organic chemistry and all other sciences—and many, many other disciplines as well.
But it is a utilitarian world these days,and we can hear cries of,“just gimme the facts,
buster; I’m not in this course to learn about science,I just want to get on with my career
goals.”No! No! No! We would argue with you forever that if you feel this way, you are
making a profound, lifetime error, but even if such a goal is granted, there is a fatal
flaw in just giving the facts. Put simply, there is too much material to be learned this
year to memorize your way through it. You can survive at first, but somewhere about
+
–
TABLE 2.1 Properties of Hybrid Orbitals
Hybridization Constructed from Angle between Bonds (°) Examples
sp 50% s, 50% p 180 BeH
2
, linear HCH
sp
2
33.3% s, 66.7% p 120 BH
3
,BF
3
sp
3
25% s, 75% p 109.5 CH
4
,
NH
4
60 CHAPTER 2 Alkanes
the middle of the first semester you will run out of memory, and there is no way (yet)
to install any more. If you try to get through this course by memorizing a set of facts,
you will almost certainly not succeed. Success in this course—and in much of life—
depends on learning how to think, how to reason sensibly from new data. So that’s
what we will try to do throughout this book, and we have begun right here.
METHANE
There is a lot of methane in very surprising places. Some
anaerobic bacteria degrade organic matter to produce methane.
When that process occurs deep in the ocean, water can crys-
tallize in a cubic arrangement, encapsulating methane mol-
ecules to form hydrates called clathrates. And those clathrates
can hold a lot of methane. One cubic meter of this hydrate
could contain as much as 170 m
3
of methane! Nor are those
clathrates merely rare curiosities. Estimates vary, but the
Arctic might hold as much as 400 gigatons, and worldwide
estimates (guesses, really) run to as much as 10,000 gigatons.
That’s a good news–bad news story. If we ever solve the
daunting economic problems involved in finding these
clathrates and getting the methane out, our hydrocarbon
scarcity problems vanish for a long time. On the other hand, if
that methane is ever released in an uncontrolled fashion (as it
regularly is in science fiction disaster stories), watch out!
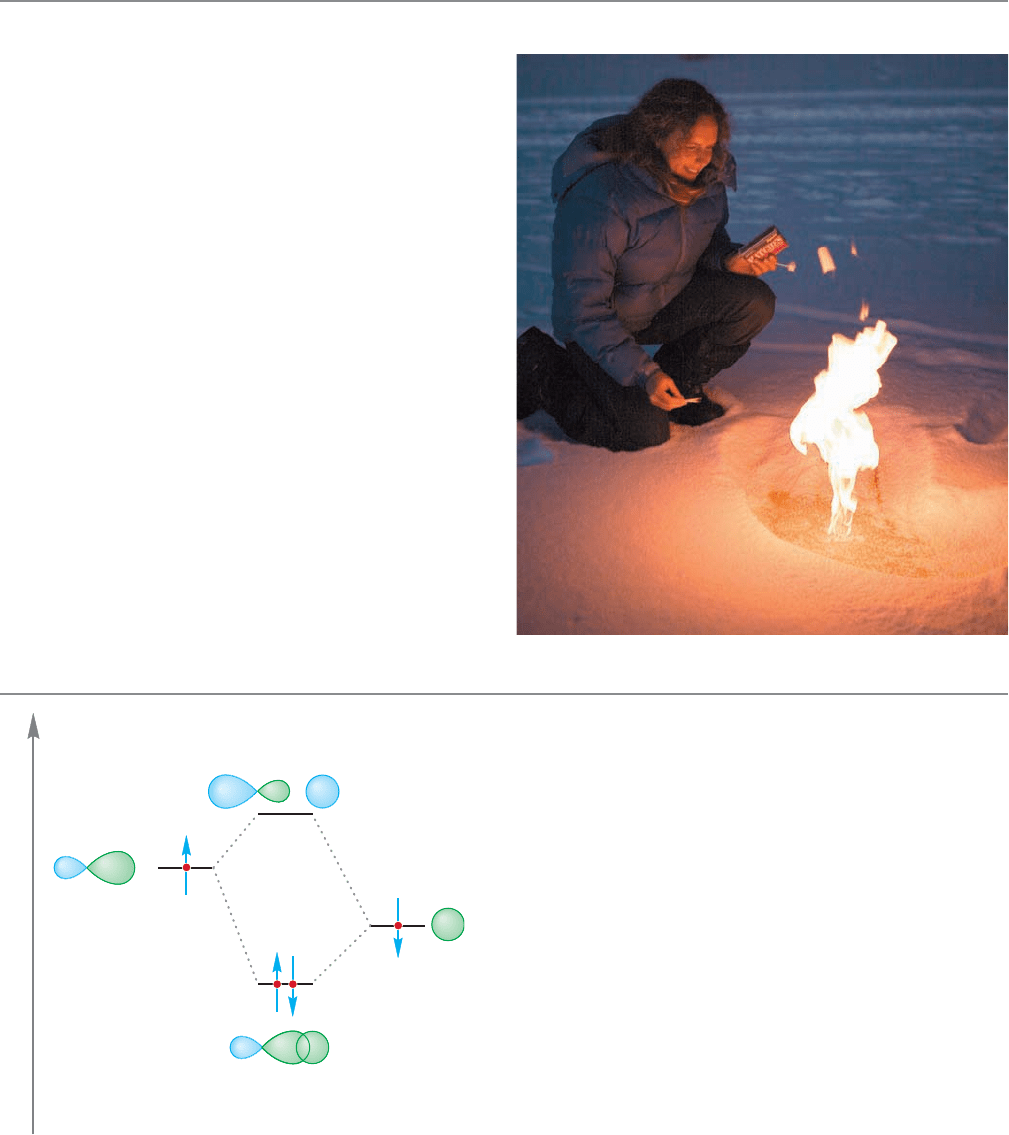
Lest you think that last scenario fanciful, as you read
this, there are Siberian and Alaskan lakes formed from melt-
ing permafrost that bubble with escaping methane. The
University of Alaska’s Katey Walter, shown here setting one
of those lakes afire, is investigating those burbling lakes. She
and her associates suggest that earlier atmospheric methane
spikes were at least partially the result of the escape of large
amounts of this greenhouse gas.
2.3 The Methyl Group (CH
3
) and
Methyl Compounds (CH
3
X)
As soon as one of the four hydrogens surrounding the cen-
tral carbon in methane is replaced with another atom, pure
tetrahedral symmetry is lost. We might well anticipate that
one of the four sp
3
hybrid orbitals of carbon could overlap
in a stabilizing way with any atom X offering an electron
in any atomic orbital (Fig. 2.11). For maximum stabiliza-
tion,we want to fill the new, bonding molecular orbital cre-
ated from this overlap, which requires two, and only two,
electrons.
All manner of derivatives of methane, called methyl
compounds ( ), are possible. The atom or group
replacing H is called a substituent,and one option for nam-
ing these substituted compounds is to drop the “ane” from
the name of the parent molecule,methane,and append “yl.”
CH
3
O
X
Energy
sp
3
* = sp
3
– X
= sp
3
+ X
X
FIGURE 2.11 Formation of a bond from overlap of an sp
3
hybrid orbital of C with an s atomic orbital of X.
C
O
X
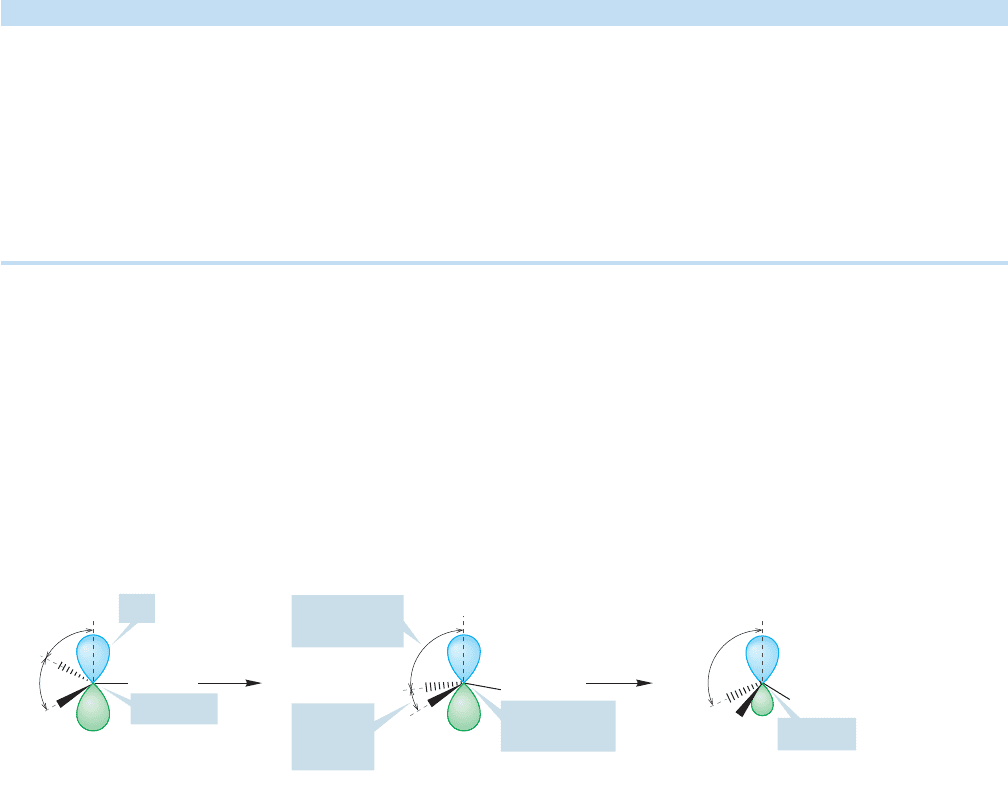
FIGURE 2.12 A thought experiment.The conversion of an sp
2
hybridized carbon into an sp
3
hybridized carbon.
What is the hybridization at a point intermediate between the starting point (sp
2
) and end point (sp
3
)?
2.3 The Methyl Group (CH
3
) and Methyl Compounds (CH
3
X) 61
The methyl is followed by the name of the X group as a separate word. Table 2.2
shows a number of methyl derivatives with their melting points, boiling points, and
physical appearances.
TABLE 2.2 Some Simple Derivatives of Methane, Otherwise Known as Methyl Compounds
Common Name mp (°C) bp (°C) Physical Properties
Methane 182.5 164 Colorless gas
Methyl alcohol or methanol 93.9 65.0 Colorless liquid
Methylamine 93.5 6.3 Colorless gas
Methyl bromide 93.6 3.6 Colorless gas/liquid
Methyl chloride 97.7 24.2 Colorless gas
Methyl cyanide or acetonitrile 45.7 81.6 Colorless liquid
Methyl fluoride 141.8 78.4 Colorless gas
Methyl iodide 66.5 42.4 Colorless liquid
Methyl mercaptan or methanethiol 123 6.2 Colorless gas/liquidH
3
C
O
SH
H
3
C
O
I
H
3
C
O
F
H
3
C
O
CN
H
3
C
O
Cl
H
3
C
O
Br
H
3
C
O
NH
2
H
3
C
O
OH
H
3
C
O
H
H
3
C
O
X
The bonding in methyl compounds closely resembles that in methane, and it is
conventional to speak of the carbon atom in any as being sp
3
hybridized,
just as it is in the more symmetrical CH
4
. Strictly speaking, this is wrong because
the bond from C to X is not the same length as that from C to H and the
bond angle cannot be exactly the same as the angle.
Because sp
3
hybridization yields four exactly equivalent bonds directed toward the
corners of a tetrahedron, the bonds to H and X in cannot be exactly sp
3
hybrids, only approximately sp
3
(sp
2.8
, say). This point is very often troubling to stu-
dents, but it is really quite simple and, once one has seen it, even obvious. Consider
converting an sp
2
hybridized carbon into an sp
3
hybridized carbon, as in Figure 2.12.
H
3
C
O
X
X
O
C
O
HH
O
C
O
H
H
3
C
O
X
120
90
109.5
2p
C is sp
2
C is sp
3
Pure sp
3
Pure sp
2
Between sp
2
and sp
3
Between
120 and
109.5
Between 90
and 109.5
C is between
sp
2
and sp
3
We start with the carbon having three sp
2
hybrid orbitals and a pure, unhybridized
2p orbital ( angle 120°). As we bend the sp
2
hybrid orbitals, we will
come to a point at which the angle has contracted to 109.5°, which is
sp
3
hybridization. Our orbitals, originally one-third s and two-thirds p (sp
2
: 33.3% s,
66.7% p character), have become one-fourth s and three-fourths p (sp
3
: 25% s, 75%
p character). They have gained p character in the transformation from sp
2
to sp
3
.Now look
at the 2p atomic orbital in Figure 2.12. As we bend, this orbital goes from pure p to
one-fourth s, three-fourths p. It has gained s character in the transformation.There is a
smooth transformation as we pyramidalize the orbitals from sp
2
to sp
3
, and between
these two extremes, the hybridization of carbon is intermediate between sp
2
and sp
3
,
something like sp
2.8
.The hybridizations sp
2
and sp
3
are limiting cases, applicable only
in completely symmetrical situations.The hybridization of a carbon (or other) atom
is intimately related to the bond angles! If you know one, you know the other.
H
O
C
O
H
H
O
C
O
H
62 CHAPTER 2 Alkanes
In general,methyl compounds ( ) will closely resemble methane in their
approximately tetrahedral geometry; sp
3
is a good approximation, but methyl com-
pounds cannot be perfect tetrahedra.
In addition to CH
3
X, other substituted molecules are possible in which more
than one hydrogen is replaced by another atom or group of atoms. Thus, even for
one-carbon molecules, there are many possible substituted structures.
H
3
C
O
X
4
The name for carbocations was the focus of a long and too intense argument in the chemical community. A car-
bocation is sometimes called a carbonium ion by traditionalists or a carbenium ion by others. The compromise
carbocation is both aptly descriptive and avoids the emotional reactions of the staunch defenders of the other terms.
FIGURE 2.13 The formation of (a) the methyl cation (
CH
3
) and (b) the methyl anion (
CH
3
) by two
different heterolytic cleavages of a carbon–hydrogen bond in CH
4
.
:
..
..
..
(a) Carbocation formation
The methyl
cation
Hydride
(b) Carbanion formation
..
+
+
HH
H
H
C
HH
H
H
H
H
C
..
..
..
H
H
H
C
+
H
H
H
C
+
..
..
..
..
+
+
H
H
H
C
H
H
H
C
HH
+
H
+
H
H
H
H
H
..
..
..
..
C
..
C
–
–
The methyl
anion
A proton
–
H
..
H
–
..
PROBLEM 2.9 Use the halogens (X F, Cl, Br, or I) to draw all possible molecules
CH
2
X
2
. For example, CH
2
BrCl is one answer.
PROBLEM 2.10 Draw all possible molecules of the formula CH
2
X
2
, CHX
3
, and
CX
4
when X is F or Cl.
2.4 The Methyl Cation (
+
CH
3
), Anion (
ⴚ
CH
3
), and
Radical ( CH
3
)
Table 2.2 could be augmented to include
CH
3
,
CH
3
,and CH
3
,which are mem-
bers of a class of compounds called reactive intermediates.This name implies that
these molecules are too unstable to be isolable under normal conditions and must
usually be studied by indirect means, often by looking at what they did during their
brief lifetimes, or sometimes by isolating them at very low temperature.These three
species are especially important because they are prototypes—the simplest examples
of whole classes of molecules to be encountered later when the study of chemical
reactions takes over our attention.
We call
CH
3
a methyl cation. To make this molecule, one could imagine
removing a hydride (
H) from methane to produce
CH
3
.It is the simplest exam-
ple of a carbocation,a molecule containing a positively charged carbon
4
(Fig.2.13a).
Now imagine forming the methyl anion (
CH
3
) by removing a proton (H
)
from methane (Fig. 2.13b), leaving behind a pair of nonbonding or lone-pair elec-
trons in the negatively charged methyl anion.The resulting
CH
3
is a simple exam-
ple of a carbon-based anion, or carbanion.
:
:
:
.
:
.
:
2.4 The Methyl Cation (
+
CH
3
), Anion (
ⴚ
CH
3
), and Radical ( CH
3
) 63
.
:
Both of these ways of producing
CH
3
and
CH
3
involve the concept of breaking
a carbon–hydrogen bond in unsymmetrical fashion, a process known as heterolytic
bond cleavage (p. 37 and Fig. 2.13). Remember the curved arrow formalism—the
red arrows of Figure 2.13 move the pair of electrons in the carbon–hydrogen bond
to the hydrogen or to the carbon.
Recall from p. 37 that there is another way of breaking a two-electron bond, and
that is to allow one electron to go with each atom involved in the breaking bond
(Fig. 2.14). This homolytic bond cleavage in methane gives a hydrogen atom (H )
and leaves behind the neutral methyl radical (CH
3
). Note the single-barbed “fish-
hook” curved arrow convention is used to represent movement of one electron.
.
.
:
+
HH
H
H
C
H
H
H
H
.
.
C
A hydrogen
atom
The methyl
radical
FIGURE 2.14 The homolytic
cleavage of a carbon–hydrogen bond
in methane to give a hydrogen atom
and the methyl radical.
sp
2
/1s Bond
120⬚
+
Empty p orbital
H
C
H
H
+
H
C
H
H
FIGURE 2.15 The sp
2
hybridized
methyl cation,
CH
3
.The three bonds
shown are the result of overlap
between carbon’s sp
2
hybrid orbitals
and the 1s atomic orbital of each
hydrogen. The four atoms all lie in the
same plane, which is perpendicular to
the plane of the page.
–
1.10
H
C
21.4
107.5
..
H
H
A
FIGURE 2.16 The structure of the
methyl anion,
CH
3
.The
hybridization of the carbon in this
carbanion is approximately sp
3
.The
molecular shape is pyramidal.
:
sp
2
Two inverting shallow pyramids
H
H
H
or
C
120
.
H
H
H
C
.
H
H
H
C
.
FIGURE 2.17 The methyl radical
(CH
3
) is either planar or a rapidly
inverting shallow pyramid.The
carbon is close to sp
2
hybridized.
.
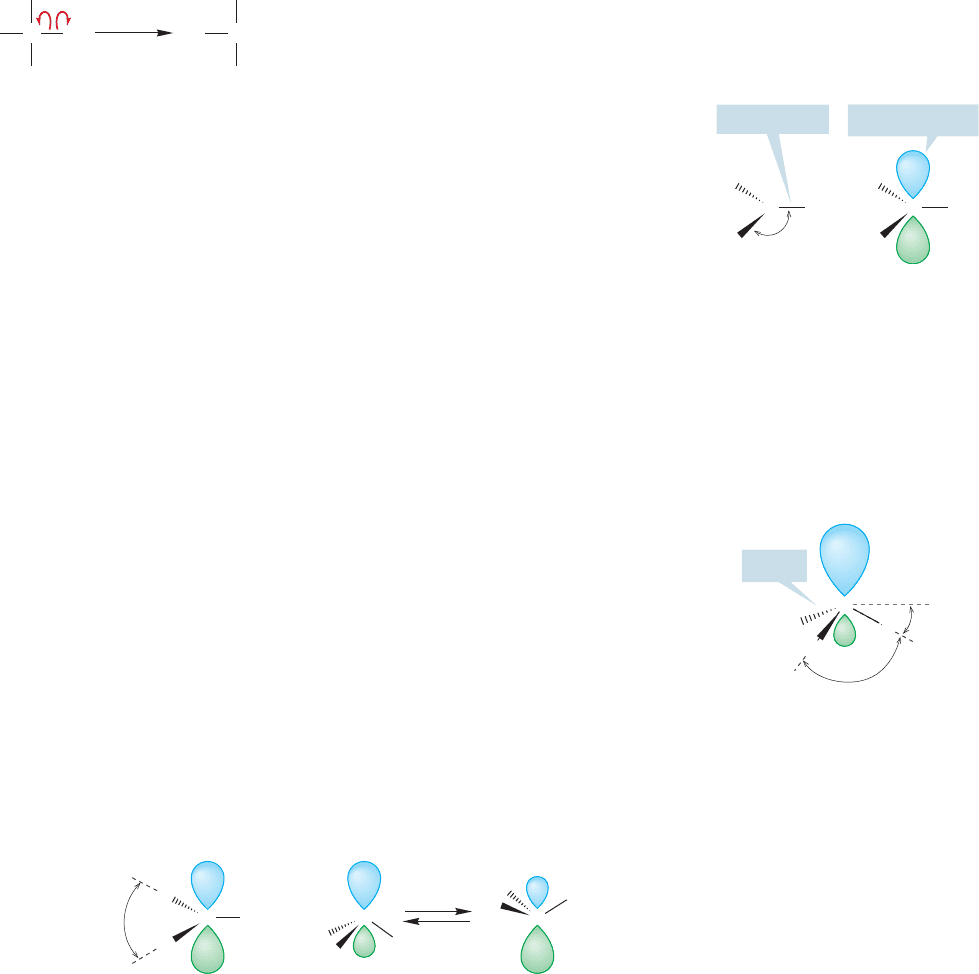
The methyl cation, anion, and radical have all been observed, although each
is extremely reactive, and thus, short-lived. They exist, though, and we can make
some predictions of structure for at least two of them. In the methyl cation
(
CH
3
), carbon is attached to three hydrogens, suggesting the need for three
hybrid atomic orbitals (recall BH
3
, p. 55), and therefore sp
2
hybridization
(Fig. 2.15).
Unlike the methyl cation, the carbon in the methyl anion is not only attached
to three hydrogens but also has a pair of nonbonding electrons. The cation has an
empty pure p orbital (zero s character) and therefore the species is as flat as a pan-
cake ( angle 120°). The methyl anion has two more electrons than
the cation and we have to consider them in arriving at a prediction of the anion’s
shape.Recall from Figure 1.7 that s orbitals have density at the nucleus. Because the
nucleus is positively charged and electrons are negatively charged, it is reasonable to
assume that an electron is more stable (lower in energy) in an orbital with a lot of
s character. A pyramidal structure seems appropriate for the methyl anion, although
of course it cannot be a perfect tetrahedron because this is a CH
3
X molecule (where
X is a lone pair of electrons).We can’t predict exactly how pyramidal the species will
be, and an anion’s shape is difficult to measure in any case, but recent calculations
predict the structure in Figure 2.16.
It is harder to predict the structure of the neutral methyl radical ( CH
3
),in which
there is only a single nonbonding electron. At present, it is not possible to choose
between a planar species and a rapidly inverting and very shallow pyramid,although
it is clear that the methyl radical is close to planar (Fig. 2.17). Do not be disturbed
by this! Chemists still do not know many seemingly simple things (such as the shape
and hybridization of the methyl radical). There is still lots to do!
.
H
O
C
O
H
64 CHAPTER 2 Alkanes
PROBLEM 2.11 Draw a structure for the methyl radical at the halfway point for the
inversion shown in Figure 2.17. What is the hybridization of the carbon atom in
the structure you drew?
Summary
Methane can be substituted in many ways through replacement of one or more
hydrogens with another atom or groups of atoms. In principle,removal of a hydro-
gen from methane can lead to the methyl anion (
CH
3
), the methyl radical
(CH
3
), or the methyl cation (
CH
3
) depending on the nature of the hydrogen
removed (
H, H or
H). In this chapter, we have discussed only the shapes of
these intermediates—reactions are coming later. It will be important to remem-
ber that carbocations are flat and sp
2
hybridized and that simple carbanions are
pyramidal and approximately sp
3
hybridized.
:
.
.
:
2.5 Ethane (C
2
H
6
), Ethyl Compounds (C
2
H
5
X), and
Newman Projections
There is a special substituent X that produces a most interesting . In this
case, we let X CH
3
, another methyl group.Now becomes
which can also be written as C
2
H
6
. This molecule is ethane (Fig. 2.18), the second
member of the alkane family. You might try to anticipate the following discussion
by building a model of ethane and examining its structure now.
H
3
C
O
CH
3
H
3
C
O
X
H
3
C
O
X
(a)
CC
H
H
H
H
X
H
3
CH
3
CCH
3
(b)
Ethane (C
2
H
6
)
H
H
H
CX H
H
H
C
H
H
CH
(c)
X = CH
3
X = CH
3
X = CH
3
H
X
H
H
C
H
H
WEB 3D
FIGURE 2.18 The construction of
ethane ( ) by the
replacement of X in with a
methyl group.
H
3
C
O
X
H
3
C
O
CH
3
Even in a molecule as simple as ethane there are a number of inter-
esting structural questions. From the point of view of one carbon, the
attached methyl group takes up more room than the much smaller
hydrogens. So we expect the angle to be slightly larger
than the angle. And so it is: 111.0° and
109.3° (Fig. 2.19).
How are the hydrogens of one end of the molecule arranged with
respect to the hydrogens at the other end? There are two important struc-
tures for ethane, which are related by rotation about the bond.
Molecules that differ in spatial orientation as a result of rotation around a
C
O
C
H
O
C
O
H
H
O
C
O
CH
3
H
O
C
O
H
H
3
C
O
C
O
H
111.0
109.3
1.53
1.11
CC
H
H
H
H
H
H
A
A
WEB 3D
FIGURE 2.19 The detailed structure of ethane.

2.5 Ethane (C
2
H
6
), Ethyl Compounds (C
2
H
5
X), and Newman Projections 65
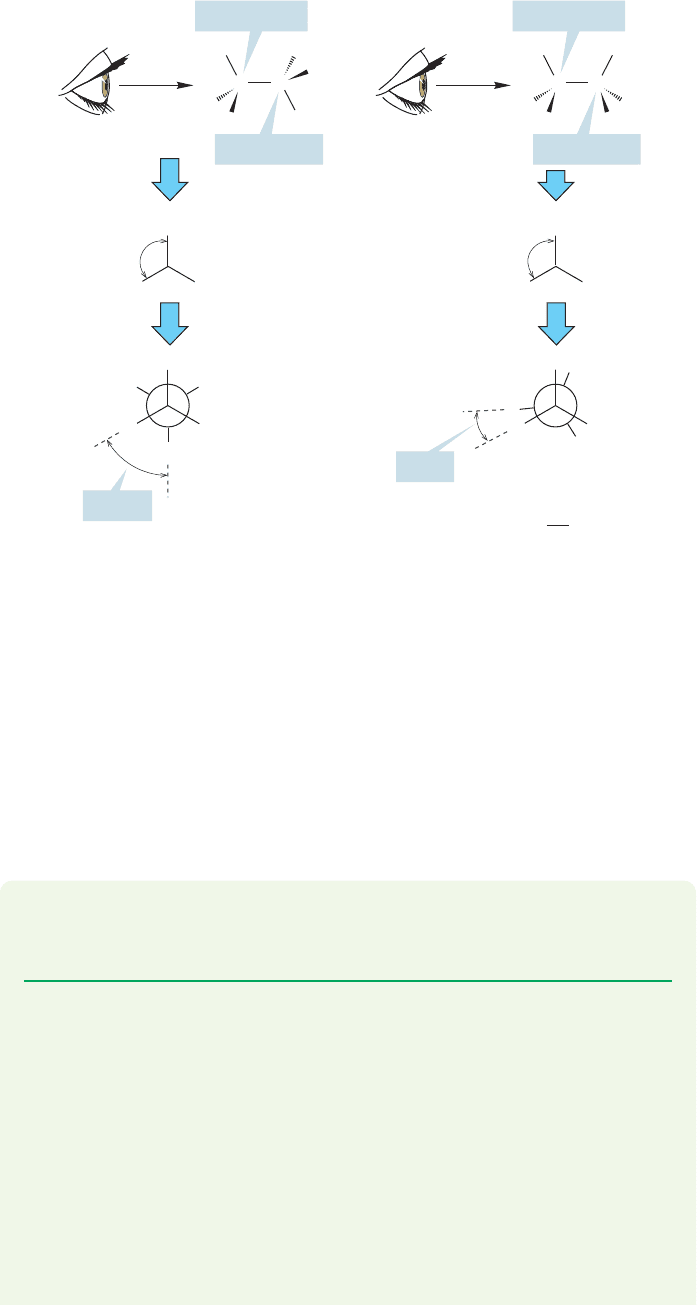
sigma bond are called conformations.The two important conformations for ethane
are eclipsed ethane and staggered ethane,each shown in a side view in Figure 2.20.
If one looks down the bond (Fig. 2.21) one can see that in the eclipsed con-
formation each of the three hydrogens on the front methyl eclipses a hydrogen on the
back methyl. In the staggered conformation, the view down the shows the
hydrogens in front arranged so that each hydrogen in back can be seen.When we look
down the bond,the angle between a hydrogen in front and a hydrogen in back
is called the dihedral angle.The dihedral angle is measured in degrees θ (pronounced
tha¯-ta). The dihedral angle between the hydrogens in the eclipsed ethane is 0°. The
dihedral angle in the staggered conformation is 60°. Ethane can exist in an infinite
number of conformations,depending on the value of the dihedral angle.We focus here
on the two extreme structures,eclipsed and staggered ethane. Be sure you see how these
two forms are interconverted by rotation about the carbon–carbon bond. Use a model!
A particularly effective way of viewing different conformations of a molecule is
called a Newman projection, after its inventor, Melvin S. Newman (1908–1993) of
Ohio State University. Like all devices, the Newman projection contains arbitrary
conventions that can only be learned, not reasoned out. Its utility will repay you for
the effort many times over, however, so do it! We first imagine looking down a par-
ticular bond, in this case the carbon–carbon bond in ethane. The three
carbon–hydrogen bonds attached to the front carbon are now drawn in as shown in
the middle row of Figure 2.21. Notice that from this end-on view the
angle on the front carbon is 120°.The rear carbon is next represented as a circle, and
the H atoms attached to it are put in as shown in the bottom row of Figure 2.21.
Constructing a Newman projection is easy for the staggered form (Fig. 2.21a)
but not so simple for the eclipsed molecule (Fig. 2.21b). If we were strictly accurate
in the drawing, we wouldn’t be able to see the hydrogens attached to the rear car-
bon of the eclipsed conformation because each is directly behind an H on the front
carbon (the dihedral angle is 0°). So we cheat a little and offset these eclipsed hydro-
gens just enough so we can see them.
Newman projections are extraordinarily useful in making three-dimensional
structures clear on a two-dimensional surface. For example, Figure 2.21a makes it
obvious that all six hydrogens in staggered ethane are the same—they are equiva-
lent in the language of organic chemistry.
H
O
C
O
H
C
O
C
C
O
C
C
O
C
rotation
Eclipsed ethane
The dihedral angle,
θ, is the angle between the red C H bonds
CC
H
H
H
H
H
H
Staggered ethane
CC
H
H
H
H
H
H
θ = 0
CC
H
H
H
H
H
H
θ = 60
H
CC
H
H
H
H
H
FIGURE 2.20 Two conformations of
ethane: the eclipsed and staggered
forms.
CONVENTION ALERT
PROBLEM 2.12 Write Newman projections for the staggered conformations of ethyl
chloride ( ) and 1,2-dichloroethane ( ).
In the second case, there are two staggered conformations of different energy. Can
you estimate which is more stable?
Cl
O
CH
2
O
CH
2
O
ClCH
3
O
CH
2
O
Cl
66 CHAPTER 2 Alkanes
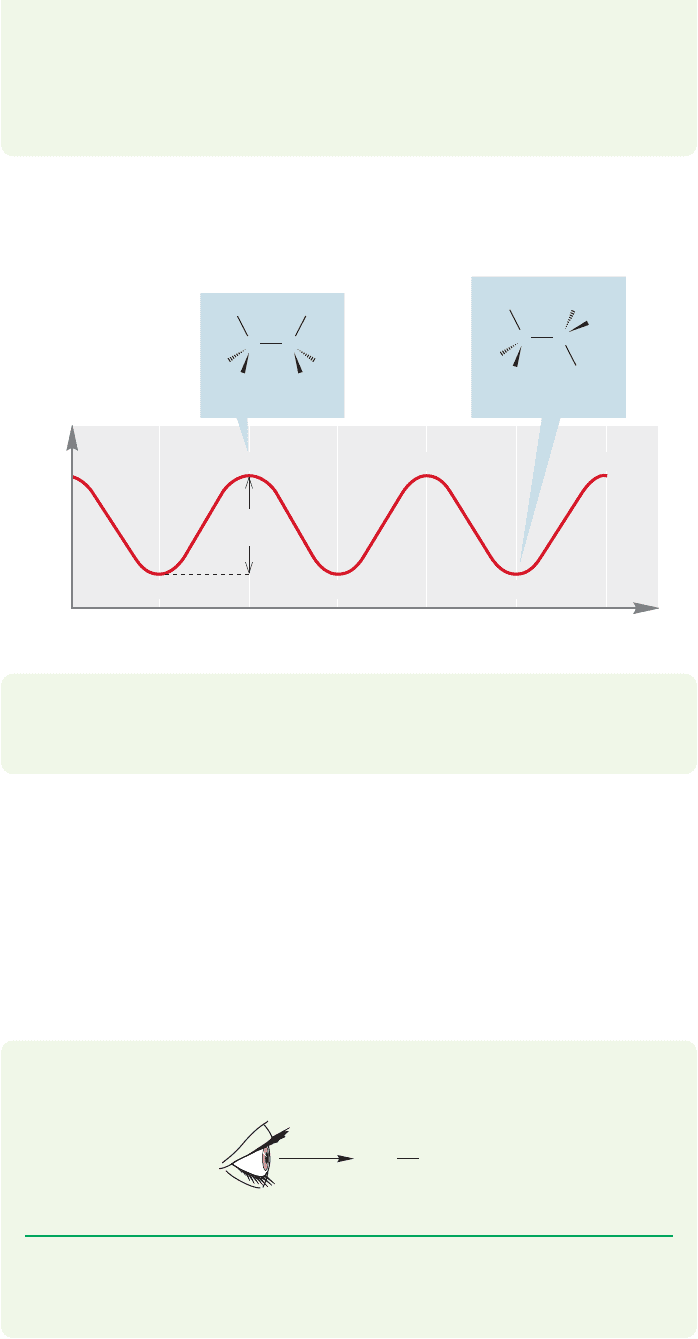
Which of the two limiting conformations of ethane represents the more stable
molecule? We would surely guess that it would be the staggered conformation, and
that guess would be exactly right. But the reason has little to do with the “obvious”
spatial requirement for the hydrogens.The hydrogens do not compete for the same
space in the eclipsed conformation. There may be some electrostatic repulsion
between the electrons in the bonds in the eclipsed bonds, but the eclipsed confor-
mation is mostly destabilized by the repulsive interaction of two filled orbitals in each
of the three pairs of eclipsed carbon–hydrogen bonds (Fig. 2.21b).
PROBLEM 2.13 Use an orbital interaction diagram like the one for He
2
in Figure
1.46, p. 40, to show the destabilization in eclipsed ethane. How many eclipsing
filled orbital–filled orbital interactions are present?
PROBLEM 2.14 There is a related orbital effect that stabilizes staggered ethane.Use an
orbital interaction diagram like the one for H
2
in Figure 1.47, p. 42, to show the sta-
bilization in staggered ethane. This problem is much harder than Problem 2.13, so
here is some help in the form of a set of tasks.The explanation that this problem leads
to was first pointed out to MJ by an undergraduate just like you about 25 years ago.
(a) Draw staggered ethane in a Newman projection.
(b) Draw the antibonding orbital for one of the bonds of the front carbon.
(c) Consider the bond that is directly behind the antibonding orbital
you have drawn. Can you see the overlap between the filled bonding orbital
in back and the empty antibonding orbital you drew?
C
O
H
C
O
H
C
H
H
H
H
H
H
(a) Staggered ethane
Rear carbon
Front carbon
(b) Eclipsed ethane
θ = 60⬚
θ = 0⬚
Notice how we must
cheat in this Newman
projection by offsetting
the rear C
H bonds
slightly; otherwise they
could not be seen
C
C
H
H
H
Rear carbon
Front carbon
C
H
H
H
HH
H
120⬚
HH
H
First draw the front carbon
with attached hydrogens; note
that the “C” is not drawn in
Now the rear carbon is
added as a circle; the
attached hydrogens are
drawn from the edge of
the circle
120⬚
H
H
H
HH
H
H
H
H
H
H
H
FIGURE 2.21 (a) A Newman
projection for staggered ethane.
The dihedral angle (θ) between two
carbon–hydrogen bonds is 60°.
(b) A Newman projection for
eclipsed ethane. The dihedral angle
(θ) between two carbon–hydrogen
bonds is 0°.
(continued)
2.5 Ethane (C
2
H
6
), Ethyl Compounds (C
2
H
5
X), and Newman Projections 67
(d) What factor stabilizes the staggered conformation of ethane over the
eclipsed conformation? How many interactions in the staggered conforma-
tion of ethane have overlap of bonding and antibonding orbitals?
If you can fight your way through this problem, you are in excellent shape in
terms of manipulating orbitals!
We can now make a plot of dihedral angle as a function of energy (Fig. 2.22).
Calculations show that the eclipsed form of ethane is an energy maximum, the top
of the energy barrier separating two staggered forms. Such a maximum-energy point
is called a transition state (TS).
PROBLEM 2.15 You recently saw a transition state, although the term wasn’t used.
Where? Hint: Where have we described two forms of a molecule that intercon-
vert by passing through another species? It is close by.
How big is the energy difference between the staggered and eclipsed conforma-
tions of ethane? This question asks how high the energy barrier is between two of the
minimum-energy staggered forms in Figure 2.22.The barrier turns out to be a small
number, 2.9 kcal/mol, an amount of energy easily available at room temperature. At
normal ambient temperatures,ethane is said to be freely rotating because there is ample
thermal energy to traverse the barrier separating any two staggered forms. On some
very cold planet, though, where there would be much less thermal energy available
than on Earth, alien organic chemists would have to worry more about this rotation-
al barrier. Thus, their lives would be even more complicated than ours.
Energy
Dihedral angle (θ)
0⬚ 60⬚ 120⬚ 180⬚ 240⬚ 300⬚ 360⬚
E
S S S
E E E
2.9
kcal/mol
H
H
H
C
C
H
H
H
Eclipsed ethane
H
H
H
C
H
H
H
C
Staggered ethane
FIGURE 2.22 A plot of the energy of
ethane as rotation around the
carbon–carbon bond occurs.
CH
2
OH
Eye
H
3
C
PROBLEM 2.16 Draw the low-energy Newman projection for the structure depicted
below by looking down the carbon–carbon bond.
PROBLEM 2.17 Imagine 1,2-dideuterioethane ( ), where D deu-
terium, on some cold planet such as Pluto. If there were not enough energy available
to overcome the barrier to rotation,how many 1,2-dideuterioethanes would there be?
DCH
2
O
CH
2
D
68 CHAPTER 2 Alkanes
The chemical and physical properties of ethane closely resemble those of
methane. Both are gases at room temperature, and both are quite unreactive under
most chemical conditions. But not all conditions! One need only light a match in a
room containing either ethane or methane and air to find that out.This reaction is
actually very interesting. If we examine the debris after the explosion, we find two
new molecules, water and carbon dioxide:
2 H
3
C CH
3
7 O
2
4 CO
2
+ 6 H
2
O + and
Ethane
match
Heat Ligh
t
A thermochemical analysis indicates that water and carbon dioxide are more sta-
ble than ethane and oxygen. Presumably, this is the reason ethane explodes when
the match is lit. Energy is all too obviously given off as heat and light. But why do
objects that are stable in air, such as ethane or wood, explode or burn continuously
when ignited? How are they protected until the match is lit?
We’ll approach these questions in Chapter 8, but
it’s worth some thought now in anticipation. This
matter is serious because the molecules in our bod-
ies are also less thermodynamically stable than their
various oxidized forms,and we live in an atmosphere
that contains about 20% oxygen. Why can humans
live in such an atmosphere without spontaneously
bursting into flame? This issue has been of some con-
cern, and not only for scientists.
5
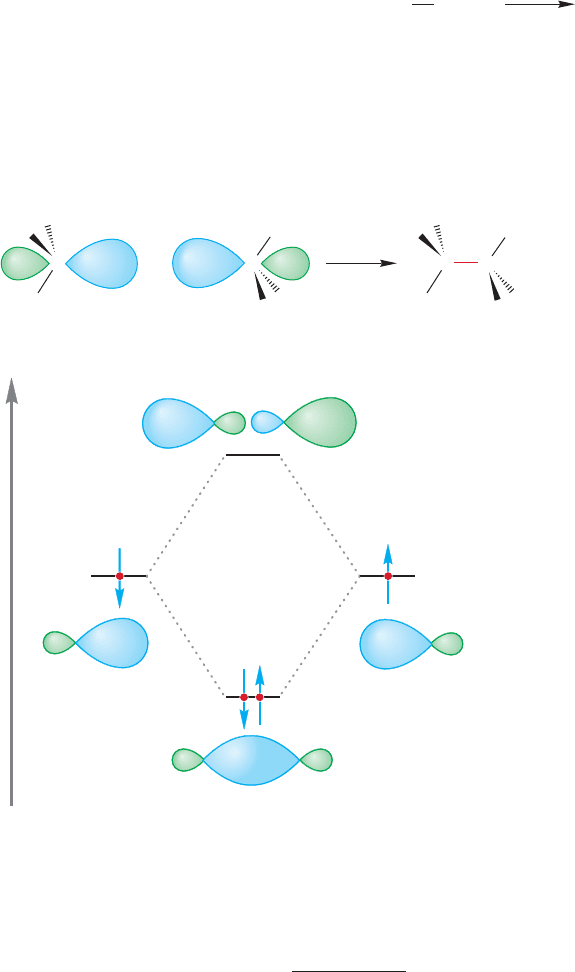
We can imagine making ethane by allowing two
methyl radicals to come together (Fig.2.23).The two
sp
3
hybrid orbitals, one on each carbon, overlap to
form a new carbon–carbon bond.This orbital overlap
produces a bonding molecular orbital, which is a σ
orbital because of its cylindrical symmetry. Of course,
the process simultaneously creates an antibonding
molecular orbital (σ*). The bonding orbital is filled
by the two electrons originally in the two methyl rad-
icals and the antibonding orbital is empty.We might
guess that the overlap of the two equal-energy sp
3
hybrid orbitals would be very favorable,and it is.The
carbon–carbon bond in ethane has a strength of
about 90 kcal/mol.This value is the amount of ener-
gy by which ethane is stabilized relative to a pair of
separated methyl radicals and is therefore the amount of energy required to break
this strong carbon–carbon bond.The formation of ethane from two methyl radicals
is exothermic by 90 kcal/mol:
ΔH ° 90 kcal/molCH
3
U
H
3
C
O
CH
3
.
+
.
H
3
C
5
Eliot ...claimed to be deeply touched by the idea of an inhabited planet with an atmosphere that was eager
to combine violently with almost everything the inhabitants held dear ...“When you think of it, boys,” he
said brokenly, “that’s what holds us together more than anything else, except maybe gravity. We few, we happy
few, we band of brothers—joined in the serious business of keeping our food, shelter, clothing and loved ones
from combining with oxygen.”
—Kurt Vonnegut,
God Bless You, Mr. Rosewater
H
H
H
H
H
H
C
H
H
H
C
C
H
H
H
C
Energy
+
.
.
.
.
Two methyl radicals Ethane
CH
3
.
H
3
C
.
The bonding
molecular orbital,
σ
sp
3
sp
3
sp
3
+ sp
3
sp
3
– sp
3
The antibonding
molecular orbital,
σ∗
FIGURE 2.23 An orbital interaction
diagram for the formation of ethane
through the combination of a pair of
methyl radicals.
