Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.

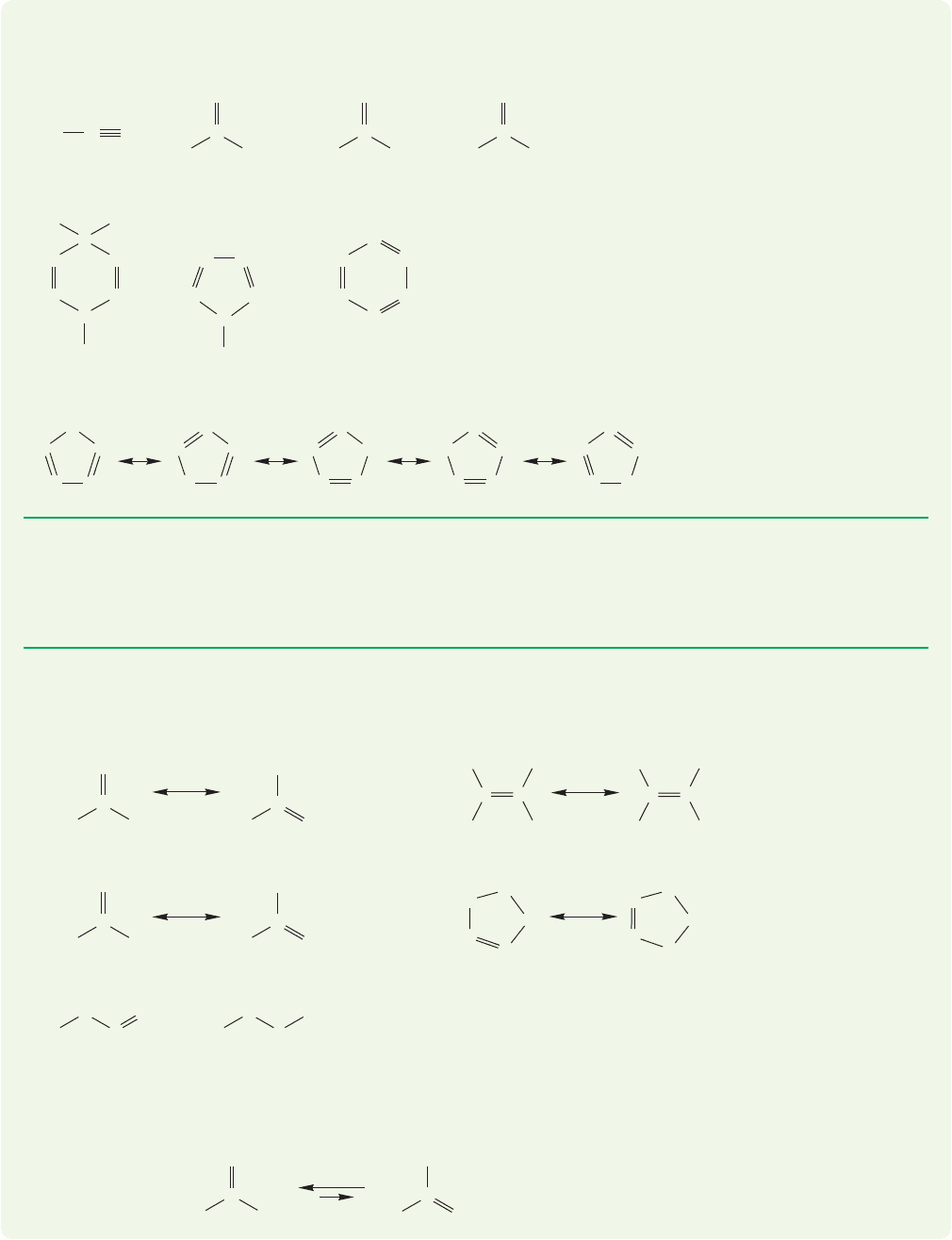
1.4 Resonance Forms 29
WORKED PROBLEM 1.18 Add dots for the electron pairs and write resonance
forms for the following structures:
(a)
*(b)
(c)
(d)
(e)
–
–
H
3
C
O
–
+
C
O
H
3
C
O
C
O
H
3
C
H
3
CC
H
H
2
C
CH
3
C
O
H
3
C
HC
HC
CH
2
CH
2
CH
2
C
CC CC
OH
H
3
CC
H
C
H
H
2
C
H
2
C
HC
HC
CH
2
C
H
H
2
C
CH
2
H
CH
3
H
3
C
H
H
3
C
CH
3
H
H
–
–
ANSWER (b) These two are not resonance forms because atoms have been moved,
not just electrons. These two molecules are in equilibrium. Molecules related
through the change of position of a single hydrogen are called tautomers.
O
C
CH
3
H
3
C
C
CH
2
H
3
C
OH
+
(a) (b) (c) (d)
(e) *(f) (g)
–
CH
2
H
2
C OCH
3
C
C
O
H
3
C OCH
3
C
O
–
OO
–
O
N
–
C
C
C
H
CH
CH
HC
CHHC
HC
CHHC
H
–
C
H
H
C
H
C
H
CHHC
CHHC
ANSWER (f)
PROBLEM 1.19 Write Lewis structures and resonance forms for the following
compounds. If you have problems visualizing the structure of some of these mol-
ecules, see the inside front cover of this book.
(a) HONO
2
(b)
OSO
2
OH (c) CH
3
COO
WORKED PROBLEM 1.20 Which of the following pairs of structures are not reso-
nance forms of each other? Why not? You may have to add dots to make good
Lewis structures first.
..
–
HC
HC
H
C
CH
CH
..
..
––
HC
HC
H
C
CH
CH
..
–
HC
HC
H
C
CH
CH
..
–
HC
HC
H
C
CH
CH
HC
HC
H
C
CH
CH
PROBLEM 1.21 In the following pairs of resonance forms, assign weighting factors.
In each case, indicate which form you think is more important,and therefore con-
tributes more to the structure. You may have to add dots to make good Lewis
structures first. Hint: Carbocations are stabilized by substitution.
30 CHAPTER 1 Atoms and Molecules; Orbitals and Bonding
(a)
(b)
(c)
(d)
H
CH
2
C
O
H
H
C
O
H
H
C
O
H
N
CH
2
H
3
C
C
O
C
CH
2
C
H
O
O
N
H
3
C
H
3
C
H
3
C
C
CH
2
C
H
H
3
C
H
3
C
O
O
–
–
–
+
–
+
+
–
+
+
Summary
1. Many molecules are incompletely represented by just one Lewis structure. For
these molecules, resonance structures will provide a more accurate description.
2. Resonance forms are different electronic representations of the same molecule.
3. Resonance forms are not species in equilibrium—molecules do not oscillate back
and forth between resonance forms. The more accurate structure of the
molecule—called the resonance hybrid—is the weighted average of all forms.
4. Some resonance forms contribute more than others to the resonance hybrid.
1.5 Hydrogen (H
2
): Molecular Orbitals
In the first part of this chapter, we examined atomic structure and atomic orbitals
and began a study of the collections of atoms called molecules. Now we will enlarge
the discussion to include molecular orbitals, the regions of space occupied by elec-
trons in molecules. Covalent bonding between atoms involves the sharing of
electrons.This sharing takes place through overlap of an atomic orbital with anoth-
er atomic orbital or with a molecular orbital.
Chemistry is largely the study of the structure and reactivity of molecules. A cen-
tury ago, when chemistry was a young science,there seemed to be little time to worry
too much about the “how and why” of the science; there was too much discovering
going on, too much information to be collected.
Here is what Friedrich Wöhler (1800–1882), a great early unifier of organic
chemistry, had to say on the subject: “Organic chemistry just now is enough to drive
one mad. It gives one the impression of a primeval, tropical forest full of the most
remarkable things, a monstrous and boundless thicket, with no way of escape, into
which one may well dread to enter.” Nowadays, no longer is some metaphoric vast
jungle being explored by brute force; chemists are now thoughtfully aiming more
and more at new discoveries. The more we understand, and the better our models
of Nature are, the more likely it is that the current transformation from trial-and-
error methods to intellectually driven efforts will be successful.
Directed progress in chemistry depends on understanding how molecules react
and on the application of that understanding in creative ways. These days, the idea
WEB 3D
0 0.50
1.00
Distance between two hydrogens ( )
Energy
1.50 2.502.00 3.00
Energy of
two separated
H atoms
(a)
0.25 Å
Hydrogens
too close
A
H H
(c)
1 Å
Too far apart
H H
(d)
2 Å
Much too
far apart
H
2
molecule
H H
(b)
0.74 Å
Optimum
distance in
the hydrogen
molecule
H H
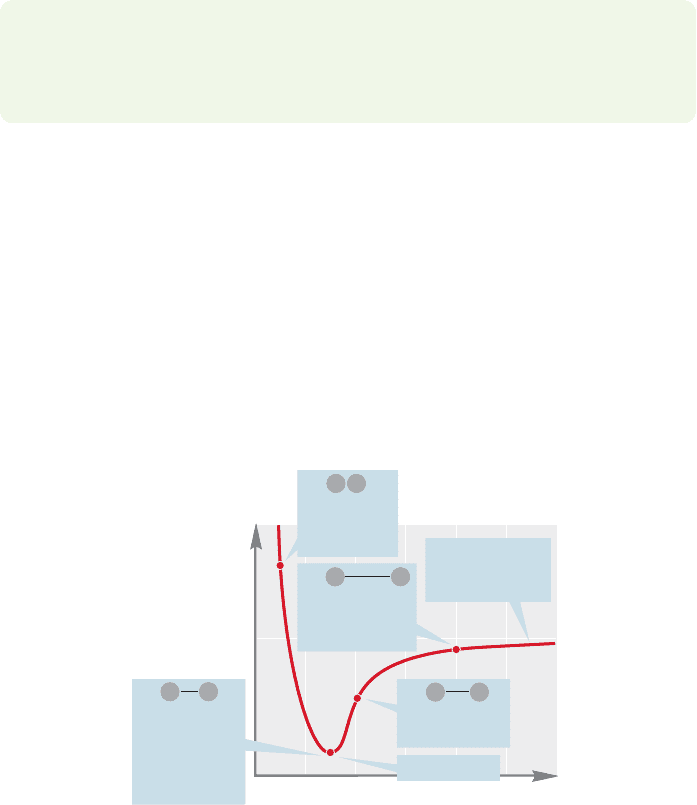
FIGURE 1.36 A plot of the energy for
the hydrogen molecule (H
2
) as a
function of the distance between the
two hydrogen nuclei.The point of
minimum energy corresponds to the
equilibrium internuclear separation,
the bond distance.
that both the structures of molecules and the reactions they undergo can be under-
stood by examining the shapes and interactions of atomic and molecular orbitals has
gained widespread acceptance in the chemical community, and an important branch
of theoretical chemistry embraces these ideas. Although the study of molecular
orbitals can be approached in a highly mathematical way, we will not follow that
path. One of the great benefits of our nonmathematical approach is that it can be
appreciated quite readily by nonmathematicians! We’ll find that even a very “low
level,” highly qualitative molecular orbital theory can provide striking insights into
structure and reactivity.
In Section 1.3, we constructed Lewis dot structures for molecules. Our next task
is to elaborate on this theme to produce better pictures of the bonds that hold atoms
together in molecules.We’ll need to consider how electrons act to bind nuclei togeth-
er, and we’ll take as our initial example hydrogen (H
2
),the second simplest molecule.
PROBLEM 1.22 What is the simplest molecule? If H
2
is the second simplest mol-
ecule, the answer to this question must be “H
2
minus something.” What might
the “something” be? The answer will appear farther along in the text, so think
about this question for a while now.
We start with two hydrogen atoms,each consisting of a single proton,the nucle-
us, surrounded by an electron in the spherically symmetrical 1s orbital. In principle,
the situation is this simple only as long as the two hydrogen atoms are infinitely far
apart. As soon as they come closer than this infinite distance, they begin to “feel”
each other. Remember: Wave functions do not vanish as the distance from the nucle-
us increases (Fig.1.6). In practice, we can ignore the influence of one hydrogen atom
on the other until they come quite close together, but as soon as they do, the ener-
gy of the system changes greatly. As one hydrogen atom approaches the other, the
energy of the two-atom system decreases until the two hydrogen atoms are 0.74
angstrom (Å, where 1 Å 10
8
cm) apart (Fig. 1.36). From this point the energy
of the system rises sharply, asymptotically approaching infinity as the distance
between the atoms approaches zero.
1.5 Hydrogen (H
2
): Molecular Orbitals 31
In the hydrogen molecule, two 1s electrons serve to bind the two nuclei togeth-
er. The system is more stable than two separated hydrogen atoms. In the mol-
ecule, the two negatively charged electrons attract both nuclei and hold them
together. When the atoms approach too closely, the positively charged nuclei begin
to repel each other, and the energy goes up sharply.
H
O
H
We begin our mathematical description of bonding by combining the two 1s
atomic orbitals (symbol ψ) of the two hydrogen atoms to produce two new molec-
ular orbitals.The first molecular orbital we will consider is called the bonding molec-
ular orbital. Its symbol is (this Greek letter is
pronounced “fy”). We will use as a shorter notation
for the bonding molecular orbital in this discussion.
Wave function mathematically represents the
bonding molecular orbital and results from a simple
addition of the two atomic orbitals. It can be written as
This bonding molecular orbital is
drawn in Figure 1.37, which shows that looks much like what one would expect
from a simple addition of two spherical 1s orbitals.
Many people use the Greek ψ for both atomic orbitals and molecular
orbitals, but we will use for molecular orbitals. Be careful in your other read-
ing, though, because you will encounter situations in which ψ is used for all
orbitals and even cases where is used for atomic orbitals and ψ for molecular
orbitals!
Because the bonding orbital is concentrated between the two nuclei (Fig.
1.37), two electrons in it can interact strongly with both nuclei. This attraction
explains why the molecular orbital in which the electron density is highest
between the nuclei, is strongly bonding.
Quantum mechanics tells us an important principle: The number of wave func-
tions (orbitals) resulting from the mixing process must equal the number of wave
functions (orbitals) going into the calculation. Because we started by mixing two
hydrogen atomic orbitals, there must be another molecular orbital that is formed.
It is called an antibonding molecular orbital, or (Fig. 1.38), and
results from a subtraction of one atomic orbital from the other to give
Electrons can occupy both bonding and antibonding molecular orbitals.An elec-
tron in a bonding orbital acts to hold the two nuclei together. As the name “anti-
bonding” suggests, an electron in an antibonding orbital does just the opposite—it
contributes to the dissociation of the two nuclei.
There is a third kind of molecular orbital; one in which an electron is neutral in
its effect on the two nuclei. Such an orbital is called a nonbonding orbital. We will
see some nonbonding orbitals when we come to molecules larger than H
2
.
By comparing Figures 1.37 and 1.38 you can see that in the antibonding molec-
ular orbital there is a nodal plane between the two hydrogen nuclei. Recall that
a node is a region in which the sign of the wave function is zero. As we pass from
a region where the sign of the wave function is positive to a region in which it is
negative, the sign of must go through zero. As noted earlier, the square of the
wave function corresponds to the electron density, and if is equal to zero, then£
£
£
A
ψ1H
a
, 1s2 - ψ1H
b
, 1s2 =£
antibonding
=£
A
.
£
A
£
antibonding
£
B
,
£
B
£
£
£
B
ψ1H
a
, 1s2 + ψ1H
b
, 1s2 =£
bonding
=£
B
.
£
B
£
B
£
bonding
32 CHAPTER 1 Atoms and Molecules; Orbitals and Bonding
ψ(H
a
) = ψ(H
a
, 1s) ψ(H
b
) = ψ(H
b
, 1s) Φ
B
(H
2
) = ψ(H
a
) + ψ(H
b
)
H
a
H
b
+
H
a
H
b
FIGURE 1.37 The bonding molecular
orbital ( ) of H
2
.£
B
ψ(H
a
) = ψ(H
a
, 1s) ψ(H
b
) = ψ(H
b
, 1s) Φ
A
(H
2
) = ψ(H
a
) – ψ(H
b
)
H
a
H
b
Nodal plane
–
H
b
H
a
FIGURE 1.38 The antibonding
molecular orbital of .1£
A
2H
O
H
CONVENTION ALERT
Molecular orbitals increase in energy
as the number of nodes increase.
A good analogy is the energy required
to increase the number of nodes in a
jump rope.This photo shows a rope
with zero nodes in the bottom
picture. A rope with one node
requires more energy to generate, and
a rope with two nodes even more.
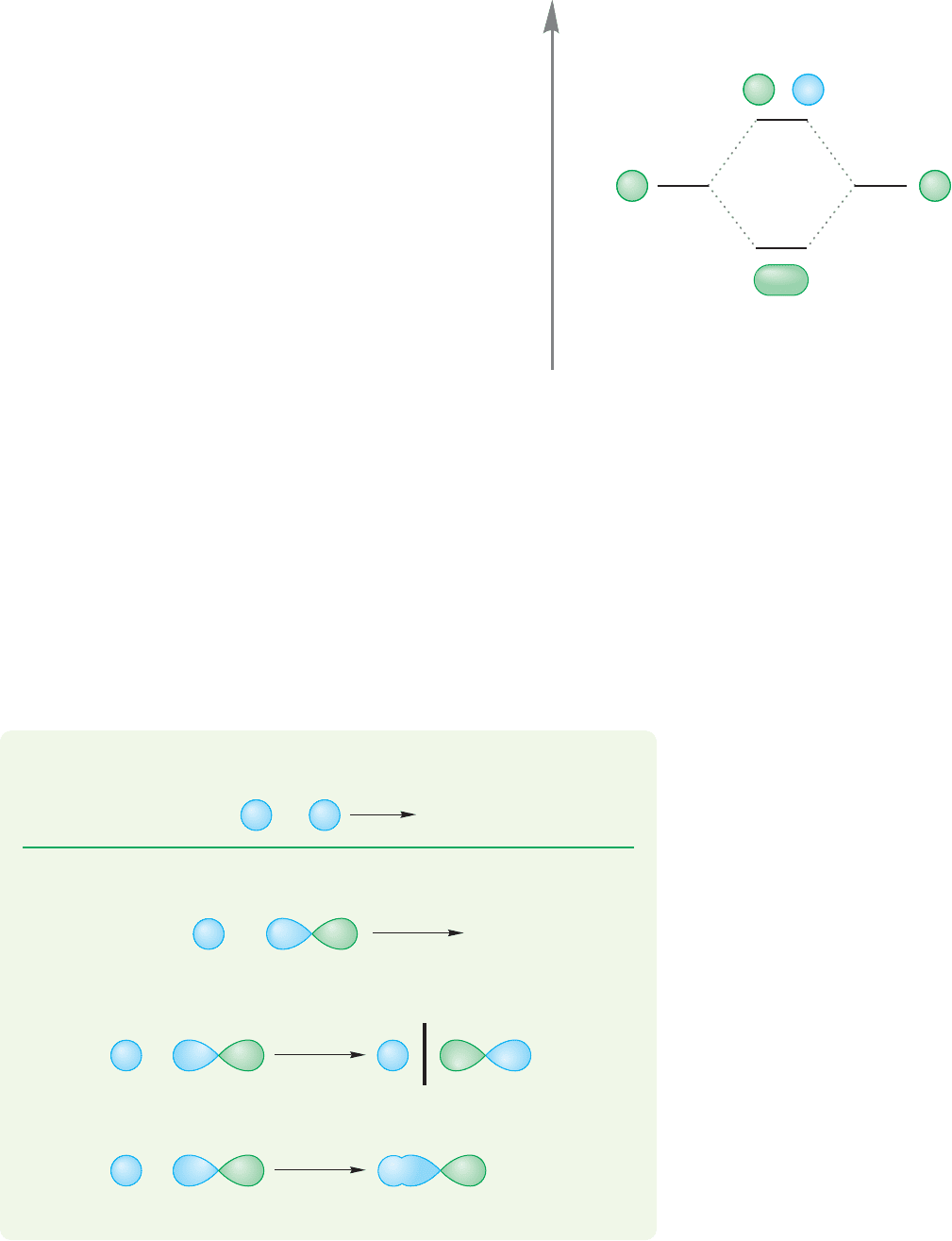
1.5 Hydrogen (H
2
): Molecular Orbitals 33
FIGURE 1.39 An orbital interaction
diagram—a graphical representation
of the combination of two atomic 1s
orbitals to form a new bonding and
antibonding pair of molecular
orbitals, and £
A
.£
B
is also zero. Therefore the probability of finding an electron
at a node must be zero as well. Accordingly, when a pair of elec-
trons occupies the antibonding molecular orbital of Figure 1.38,
the two nuclei are poorly shielded from each other, and electro-
static repulsion forces the two positively charged nuclei apart.
In summary, the combination of two hydrogen 1s atomic
orbitals yields two new, molecular orbitals, and This
idea seems intuitively reasonable. There are, after all, only two
ways in which two 1s atomic orbitals can be combined to con-
struct two new molecular orbitals. The signs of the wave func-
tions can be either the same, as in the bonding orbital or
opposite, as in the antibonding orbital One common anal-
ogy for this phenomenon is the jump rope, which has wavelike
properties. If we start with a jump rope that has an amplitude
of 3 meters and we add an in-phase wave of the same ampli-
tude, then the result will be a jump rope that has an amplitude
of 6 meters. If,however, we start with the original amplitude and we subtract a wave
(or add an out-of-phase wave) of the same amplitude, then we get a zero amplitude
wave. Subtracting the waves cancels them out.
Figure 1.39 shows a very simple and most useful graphic device for summing up
the energy comparison between atomic orbitals and the molecular orbitals formed
from mixing the orbitals. The graph is called an orbital interaction diagram, or an
interaction diagram for short.The atomic orbitals (ψ) going into the calculation are
shown at the left and right sides of the figure.For molecular hydrogen,these orbitals
are the two equivalent hydrogen 1s atomic orbitals, which must be of the same
energy. They combine in a constructive, bonding way (1s 1s) to give the lower-
energy and in a destructive, antibonding way (1s 1s) to give the higher-
energy Constructive combination for wave functions means that they are
in-phase with each other. Destructive combination means that they are out-of-phase.
PROBLEM 1.23 Sketch the molecular orbitals produced through the interaction of
two carbon 2s atomic orbitals.
WORKED PROBLEM 1.24 Sketch the orbitals produced through the interaction of
a carbon 2s atomic orbital overlapping end-on with a carbon 2p atomic orbital.
ANSWER The two atomic orbitals can interact in a bonding way (2s 2p) or in
an antibonding way (2s 2p):
2s 2p
Antibonding orbital—note
the new node (bar)
Bonding orbital
–
+
–
2s 2p
+
+
–
+
–
£
A
.
£
B
£
A
.
£
B
,
£
A
.£
B
£
2
Φ
A
= ψ(H
a
, 1s) – ψ(H
b
, 1s)
Φ
B
= ψ(H
a
, 1s) + ψ(H
b
, 1s)
ψ(H
a
, 1s) ψ(H
b
, 1s)
Energy

Energy
Φ
A
= ψ(H
a
, 1s) – ψ(H
b
, 1s)
Φ
B
= ψ(H
a
, 1s) + ψ(H
b
, 1s)
ψ(H
b
, 1s
1
) ψ(H
a
, 1s
1
)
Stabilization
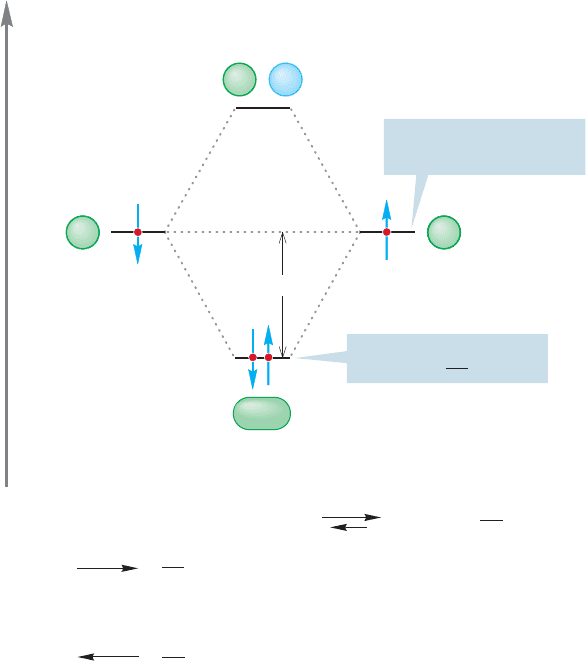
34 CHAPTER 1 Atoms and Molecules; Orbitals and Bonding
FIGURE 1.40 The electronic
occupancy for the molecule H
2
.
FIGURE 1.41 Two examples of
noninteracting (orthogonal) orbitals.
Note from Figure 1.39 that the orbital interaction diagram is con-
structed without reference to electrons. Only after the diagram has been
constructed do we have to worry about electrons. But now let’s count
them up and put them in the available molecular orbitals. In the con-
struction of H
2
, each hydrogen atom brings one electron. In
Figure 1.40, these electrons are placed in the appropriate 1s
orbitals ψ and ψ The spin direction of the elec-
trons is shown as paired, but we could also show them as
parallel. In the H
2
molecule, we put the two electrons into
the lower-energy The electrons must be paired,because
they are in the same orbital and their spin quantum numbers
must be different.The Pauli principle made this point earlier (p. 8).
The antibonding molecular orbital is empty because we are
dealing with only two electrons and they are both accommodated in the
bonding molecular orbital.
8
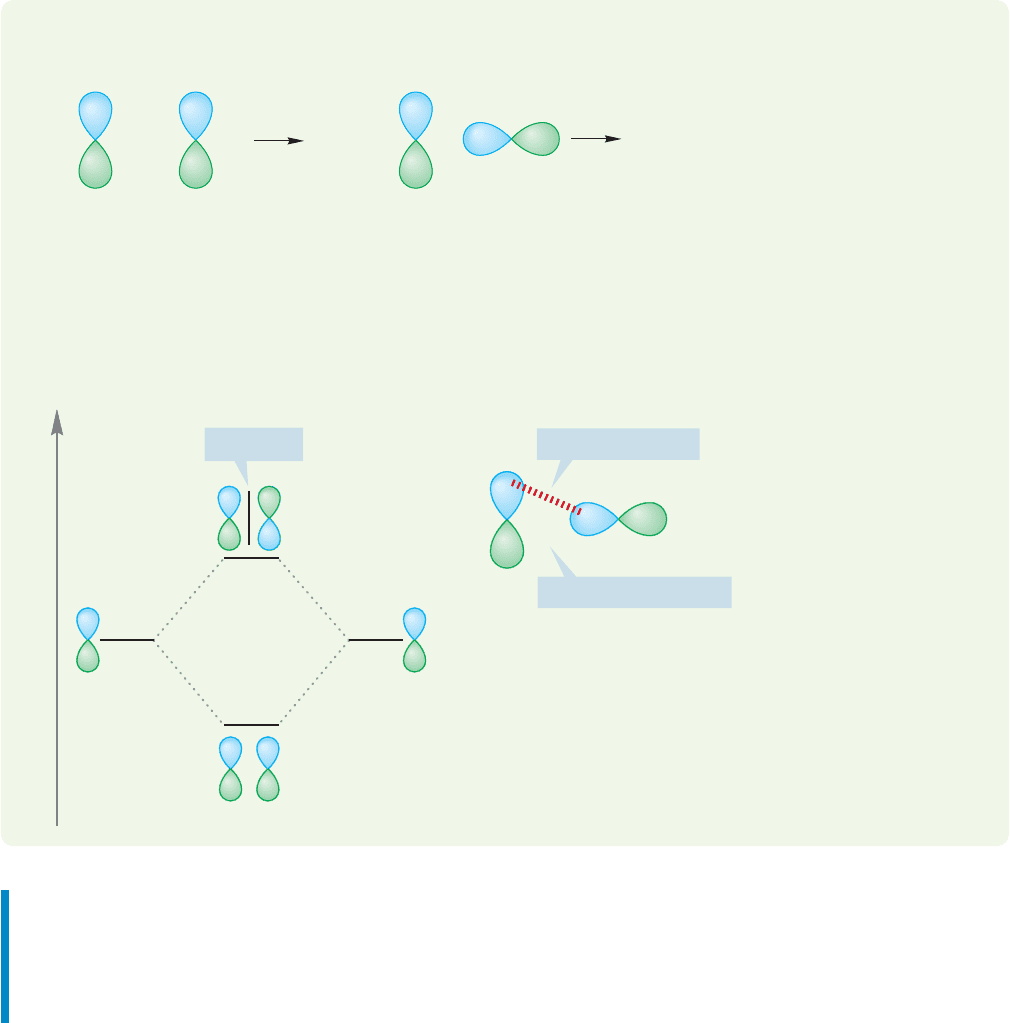
So far we have dealt only with mixing one atomic orbital with another atomic
orbital. But it is also possible to mix a molecular orbital with another molecular
orbital or an atomic orbital with a molecular orbital.Not all combinations of orbitals
are productive. If two orbitals approach each other in such a way that the new bond-
ing interactions are exactly balanced by antibonding interactions, there is no net inter-
action between the two and no bond would form.Such orbitals are called orthogonal
orbitals, orbitals that do not mix.Therefore,the way in which orbitals approach each
other in space—how the lobes overlap—is critically important. Figure 1.41 shows
two cases of atomic orbitals that are orthogonal to each other. As the orbitals
approach each other, the number of bonding overlaps (shown with red dashed line)
and antibonding overlaps are the same. The result is no net bonding. In each case,
as the orbitals are brought together the bonding interactions (blue–blue starting to
overlap) are exactly canceled by the antibonding interactions (blue–green starting
to overlap).
Here are some “rules” for orbital construction:
1. The number of orbitals produced must equal the number of orbitals you begin with.
If you start with n orbitals, you must produce n new orbitals. Here is a way to check
your work as you proceed.
2. Keep the process as simple as you can. Use what you know already, and combine
orbitals in as symmetrical a fashion as you can.
3. The closer in energy two orbitals are, the more strongly they interact. At this prim-
itive (but useful) level of theory, you need mix only the pairs of orbitals closest in
energy to each other.
4. When two orbitals interact in a bonding way (wave functions for the two orbitals
have same sign), the energy of the resulting orbital is lowered; when they interact
in an antibonding way (wave functions have different signs), the energy of the
resulting orbital is raised.
5. When two orbitals interact, the only options for mixing are adding (in-phase mixing)
or subtracting (out-of-phase mixing). When three orbitals interact we will have
1£
A
2
£
B
.
1H
b
2.1H
a
2
8
There can be no denying that the concept of an empty antibonding orbital is slippery! It makes physicists
very uneasy, for example. Chemists see the empty orbital of Figures 1.39 and 1.40 as “the place the next elec-
tron would go.”
Antibonding
Bonding
Antibonding
Bonding
1.5 Hydrogen (H
2
): Molecular Orbitals 35
three ways to mix the orbitals. With four orbitals there are four combinations
possible, and so on.
6. To put new orbitals in order according to their energy, count the nodes. For a given
molecule, the more nodes in an orbital, the higher it is in energy.
There is a connection between stability and energy. The lower the energy of an
orbital, the greater the stability of an electron in it. A consequence of this stability is
that the strongest bonds in molecules are formed by electrons occupying the lowest-
energy molecular orbitals.Throughout this chapter we will be taking note of what fac-
tors lead to low-energy molecular orbitals and thus to strong bonding between atoms.
WORKED PROBLEM 1.25 Contrast the interactions between two 2p orbitals
approaching in the two different ways shown below.
ANSWER (a) A pair of 2p atomic orbitals aligned parallel to each other interacting
side by side produces two new molecular orbitals, one bonding (2p 2p) and one
antibonding (2p 2p). (b) The orbitals aligned perpendicular to each other produce
no molecular orbitals because there is no net bonding or antibonding.The two exact-
ly cancel, producing no net interaction. In this case, the two orbitals are orthogonal.
Perpendicular
+
–
?
Parallel
(a) (b)
+
–
?
(a)
Energy
2p 2p
2p2p
2p 2p
Antibonding orbital
Bonding orbital
New node
–
+
Bonding interaction
There is no net interaction
here as the bonding and
antibonding interactions
(overlaps) exactly cancel
Antibonding interaction
(b)
Summary
Overlap of two atomic orbitals produces two new molecular orbitals, one bond-
ing and the other antibonding.Two electrons can be accommodated in the lower-
energy bonding molecular orbital. In this way, two atoms (or groups of atoms)
can be bound through the sharing of electrons in a covalent bond.
36 CHAPTER 1 Atoms and Molecules; Orbitals and Bonding
104 kcal/mol
Energy of two separate
hydrogen atoms, 2 H
Energy of one hydrogen
molecule, H
H
Two separate hydrogen atoms molecular H H, H
2
H H
ΔH° = –104 kcal/mol
This reaction is exothermic by 104 kcal/mol
+
.
H
.
H
.
H H
ΔH° = +104 kcal/mol
This reaction is endothermic by 104 kcal/mol
+
.
H
.
H
Φ
A
Φ
B
ψ(H
b
, 1s
1
) ψ(H
a
, 1s
1
)
Energy
FIGURE 1.42 Molecular hydrogen
(H
2
) is more stable than two isolated
hydrogen atoms by 104 kcal/mol.
1.6 Bond Strength
In Figure 1.40, the energy of the electrons in the two atomic orbitals is greater than
their energy in the bonding molecular orbital As noted earlier, lower energy
means greater stability, but just how much energy is the stabilization apparent in
Figure 1.40 worth? The difference between the energies of the two electrons in
and the energies of the two separate electrons in atomic 1s orbitals is 104 kcal/mol.
This amount of energy is very large, and consequently the H
2
molecule is held
together (bound) by a substantial amount of energy; this amount of energy is
released into the environment if H
2
molecules are formed from separated hydrogen
atoms. It would require the application of exactly this amount of energy to gener-
ate two hydrogen atoms from a molecule of H
2
. The 104 kcal/mol represents the
stabilization of two electrons in (Fig. 1.42).
£
B
£
B
£
B
.
Actually, if we could measure it, the temperature would rise ever so slightly as
two hydrogen atoms combined to make a hydrogen molecule because 104 kcal/mol
would be released as heat energy into the vessel.The environment in the vessel would
warm up as the process proceeded.A reaction that liberates heat,one where the prod-
ucts are more stable than the starting materials, is called an exothermic reaction.
The opposite situation, in which the products are less stable than the starting ma-
terial, requires the application of heat and is called an endothermic reaction.
The enthalpy change of any chemical reaction is estimated as the differ-
ence between the total bond energy of the products and the total bond energy of
(≤H°)

1.6 Bond Strength 37
the reactants.The value of for a reaction between hydrogen atoms to give H
2
is 104 kcal/mol.
The sometimes troublesome sign convention shows for an exothermic
reaction as a negative quantity. For an endothermic reaction, is positive. For
example,
If A B C then the reaction is exothermic
If A B C then the reaction is endothermic
In Figure 1.42
is an exothermic reaction
From this we can also know that the reverse reaction
is an endothermic reaction
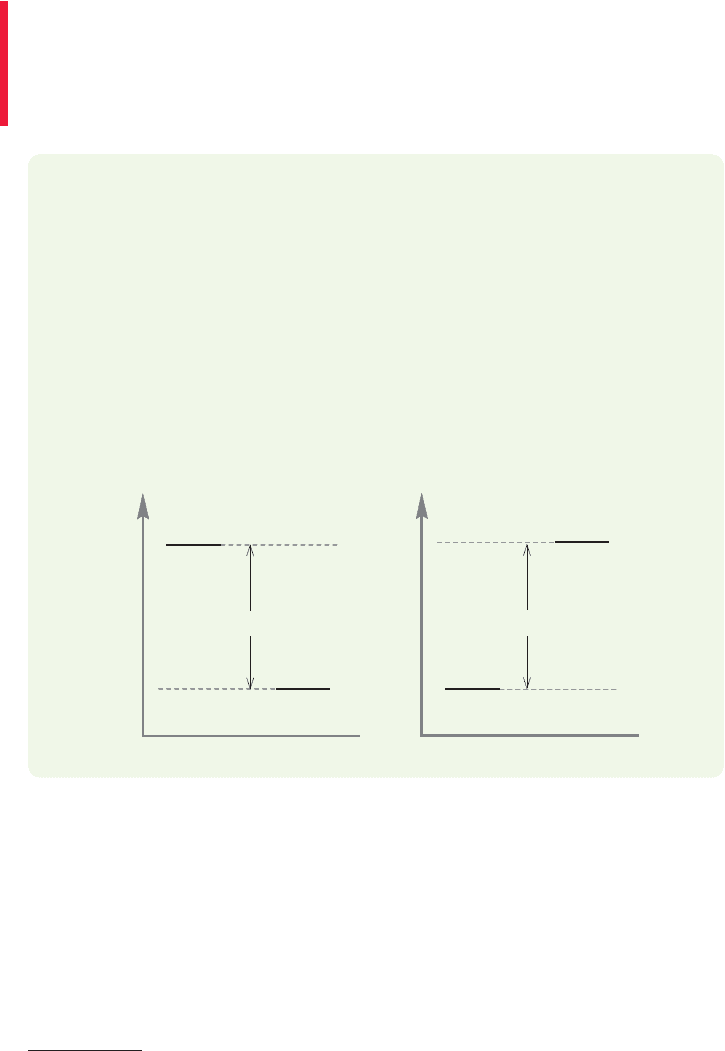
Figure 1.43 is a graph that plots the energy of reactants and products in a
chemical reaction against a variable known either as the reaction progress or the
reaction coordinate. A reaction coordinate monitors a change or changes taking
place as the reaction proceeds. These changes may be distance between atoms,
angles between bonds, or a combination of relationships that result from the
movement of atoms. For example, in the reaction a suitable
reaction coordinate might be the distance between the two hydrogen atoms. It is
a fair approximation at this point to take this “reaction coordinate” as indicative
of the progress of the overall reaction, and for that reason we will use the term
reaction progress from now on.
A reaction progress graph can be read either left to right or right to left.Reading
Figure 1.43 left to right tells us that the energy of the product is lower than the ener-
gy of the reactants, and so the graph shows the release of energy, in this case,
104 kcal/mol, as H
2
is formed in an exothermic reaction. If we read the graph from
right to left,we see the endothermic formation of two hydrogen atoms from the H
2
molecule.
This amount of energy that must be added to break the H
2
bond to produce two
hydrogen atoms, 104 kcal/mol, is called the bond dissociation energy (BDE). It is
the amount of energy that must be applied for homolytic bond cleavage.The high-
er the BDE, the more difficult it is to break the bond. A bond can break in either
of two ways (Fig. 1.44). In the homolytic cleavage, one electron from the bond goes
with one atom and the other electron of the bond goes with the other atom. This
kind of bond cleavage gives neutral species.The other option for bond breaking has
both electrons from the bond go to the same atom creating an anion–cation pair.
This process is called heterolytic bond cleavage. Normally, heterolytic cleavage is
not the path followed because it usually costs more energy to develop separated
charges than to make neutral species.
H
.
+ H
.
U
H
2
,
¢H ° =+104 kcal>mol,H
O
H
U
H
.
+ H
.
¢H ° =-104 kcal>mol,H
.
+ H
.
U
H
O
H
¢H ° =+x kcal>mol,
U
¢H ° =-x kcal>mol,
U
¢H °
¢H °
¢H °
H
104 kcal/mol
H H
Reaction progress
(reaction coordinate)
.
H
.
Energy
FIGURE 1.43 Another schematic
picture of the formation of molecular
hydrogen from two hydrogen atoms.
CONVENTION ALERT
Two versions of heterolytic bond
cleavage for the molecule
X
Y;
ions are formed
Y
X
+
Y
X
Homolytic bond cleavage
for the molecule X
Y;
neutral species are formed
..
Y
+
X
Y
X
–
..
..
.
Y
X
Y
X
.
–
FIGURE 1.44 In one kind of bond
cleavage, the pair of binding electrons
is split evenly between the atoms,
giving a pair of neutral species.This
lower-energy process is called
homolytic cleavage. Heterolytic
cleavage produces a pair of ions.
38 CHAPTER 1 Atoms and Molecules; Orbitals and Bonding
Notice the difference in the red curved arrows in the two parts of Figure 1.44.The
curved arrow showing electron movement in the heterolytic cleavage has the stan-
dard double-barbed arrows, representing the movement of two electrons to the same
atom. The homolytic cleavage pathway uses single-barbed or “fishhook” arrows rep-
resenting the movement of one electron to each atom.
WORKED PROBLEM 1.26 Sketch the profile of an endothermic reaction. See Figure
1.43 for the sketch of an exothermic reaction.
ANSWER Aha! A trick question. If you have written an exothermic reaction, you
have already written an endothermic reaction as well. You need only read your
answer backward. We are psychological prisoners of our tendency to read from
left to right. Nature has no such hangups! The formation of molecular hydrogen
from two hydrogen atoms (Fig. 1.43, left to right) is exothermic (104 kcal/mol of
energy is given off as heat), and the formation of two hydrogen atoms from a sin-
gle hydrogen molecule is endothermic by the same amount (104 kcal/mol of heat
energy must be applied).
In practice,a reaction that requires about 15–20 kcal/mol of thermal energy pro-
ceeds quite reasonably at room temperature, about 25 °C. It is important to begin to
develop a feeling for which bonds are strong and which are weak, to start to build
up a knowledge of approximate bond strengths.
9
The bond in the hydrogen mol-
ecule is a strong one. As we continue our discussion of structure, we’ll make a point
of noting bond energies as we go along. Unfortunately, there is no way to acquire
this knowledge except by learning some bond strengths. Fortunately, there are not
too many numbers to remember. Table 1.9 gives a few important bond dissociation
Energy
Reaction progress
Reaction progress
Exothermic formation
of H
2
from 2 H
Endothermic formation
of 2 H from H
2
2 H
H
2
.
.
.
Energy
2 H
H
2
.
104 kcal/mol
104 kcal/mol
9
To describe a bond as strong or weak is an arbitrary function of human experience. A bond that requires
100 kcal/mol to break is “strong”in a world where room temperature supplies much less thermal energy.It would
not be strong on the sunlit side of Mercury (where it’s hot: the average temperature is about 377 °C,or 710 °F).
Similarly, if you were a life form that evolved on Pluto (where it’s cold: the average temperature is about 220 °C,
or 361 °F), you would regard as strong all sorts of “weak” (on Earth) interactions, and your study of chem-
istry would be very different indeed. One area of research in organic chemistry focuses on extremely unstable
molecules held together by weak bonds. The idea is that by understanding extreme forms of weak bonding
we can learn more about the forces that hold together more conventional molecules. Chemists who work in
this area deliberately devise conditions under which species that are normally most unstable can be isolated.
They create very low temperature “worlds” in which other reactive (predatory) molecules are absent. In such
a world, exotic species may be stable, as they are insulated from both the ravages of heat (thermodynamic sta-
bility) and the predations of other molecules (kinetic stability).
CONVENTION ALERT
