Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.

1.3 Covalent Bonds and Lewis Structures 19
WORKED PROBLEM 1.7 Draw Lewis structures for the following neutral species.
Use lines to indicate electrons in bonds,and dots to indicate nonbonding electrons.
*(a) CH
3
*(b) CH
2
(c) Br (d) OH (e) NH
2
(f)
ANSWER The first step is to determine the number of bonding electrons in each atom.
Then make the possible bonds and see how many electrons are left over. Because each
compound is neutral, the number of bonding electrons is equal to the atomic number
less the 1s electrons. For neutral hydrogen, there is always only the single 1s electron.
(a) Carbon has four bonding electrons and forms covalent bonds with three
hydrogens, each of which contributes a single electron. There is one electron
left over on carbon, which is shown as a dot in the Lewis structure:
(b) In carbon can form only two covalent bonds because only two hydro-
gen atoms are available.Two nonbonding electrons are left over:
WORKED PROBLEM 1.8 In each of the following compounds, there is at least one
multiple bond. Draw a Lewis structure for each molecule. Use lines to indicate
electrons in bonds, and dots to indicate nonbonding electrons.
(a) F
2
CCF
2
*(b) H
3
CCN (c) H
2
CO
(d) H
2
CCO (e) H
2
CCHCHCH
2
(f) H
3
CNO
(g)
ANSWER (b) If you did Problems 1.6 and 1.7, the methyl (CH
3
) group should be
familiar by now. Its three covalent bonds are constructed by sharing three of car-
bon’s four valence electrons with the single electrons of the three hydrogens:
The second carbon and the nitrogen bring four and five electrons, respective-
ly. Carbon–carbon and carbon–nitrogen covalent bonds can be formed, which
leaves two unshared electrons on carbon and four on nitrogen:
Formation of a triple bond between carbon and nitrogen completes the pic-
ture, and nitrogen is left with a nonbonding pair of electrons:
=
H
H
CH
C
.
.
N
.
.
..
H
H
CH
CN
..
H
H
CH
.
=
C
.
.
.
N
.
.
.
.
..
H
H
CH
C
.
.
N
.
.
..
C
..
..
..
.
H
H
CH
H
H
H
.
=
H
3
CO
O
‘
C
OH
H
H
=
..
..
C
H
H
C
..
..
CH
2
,
H
H
CH
..
.
.
..
..
H
H
CH
=
H
3
C
O
N

As we examine the nature of the bonding in the various molecules you will study
in organic chemistry, it is vital that you be able to draw Lewis structures quickly and
easily. It is also extremely important to be able to determine the charge on atoms,
sometimes referred to as a formal charge, in a given structure. For single atoms in
the second row of the periodic table, this process is easy as long as you remember
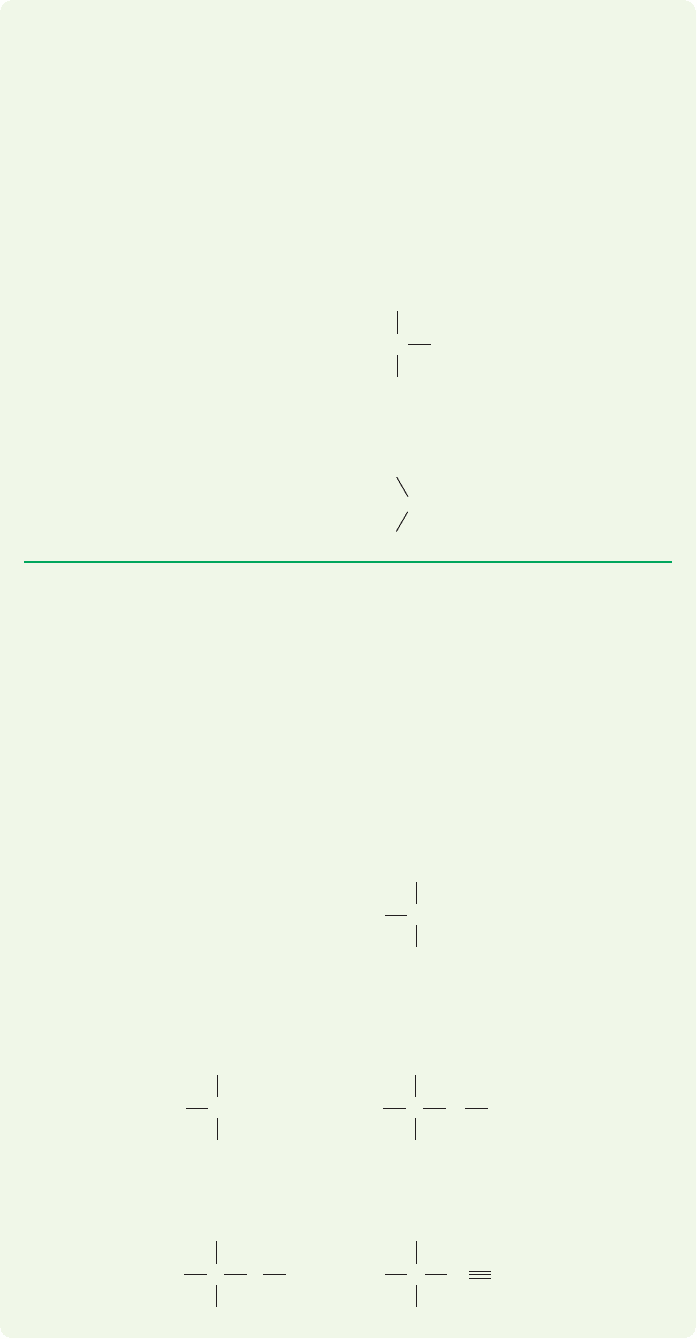
to count the two 1s electrons, which are never shown. Figure 1.22 gives Lewis struc-
tures and charge determinations for six species. Be sure you understand how the
charge is determined in each case.
20 CHAPTER 1 Atoms and Molecules; Orbitals and Bonding
=
=
=
1
H
1 Proton
2 Electrons
Net 1
–
Li
.
H
..
=
=
1
H
1 Proton
1 Electron
Neutral
H
.
H
.
=
1
H
1 Proton
0 Electrons
Net 1
+
H
H
=
9
F
9 Protons
2 1s Electrons
8 Nonbonding
electrons
9 Positive charges
10 Negative charges
3 Positive charges
3 Negative charges
6 Positive charges
5 Negative charges
Net 1
–
F
..
..
..
..
F
..
..
..
..
=
3
Li
3 Protons
2 1s Electrons
1 Nonbonding
electron
Neutral
Li
.
C
+
.
.
.
=
6
C
6 Protons
2 1s Electrons
3 Nonbonding
electrons
Net 1
+
–
+
= 1 Negative charge
= No negative charge
= 2 Negative charges
= 1 Positive charge
= 1 Positive charge
= 1 Positive charge
–
H
..
–
–
+
C
.
.
.
+
FIGURE 1.22 A few examples of
charge determination.
=
..
..
..
H
H
C
..
..
..
H
H
C
1 Proton
1 Shared electron
1
H
HH
H
H
CC
HH
..
=
=
9 Protons
2 1s Electrons
6 Nonbonding
electrons
1 Shared electron
9
F
F
..
..
..
..
F
..
..
..
F
..
..
..
F
..
..
..
= 9 Positive charges
9 Negative charges
Neutral F
6 Protons
2 1s Electrons
4 Shared electrons
6
C
= 6 Positive charges
6 Negative charges
Neutral C
= 1 Positive charge
= 1 Negative charge
Neutral H
H
H
FIGURE 1.23 Electron counting
in some simple molecules.
In molecules, the determination of charge is a bit harder because you must take
care to account for shared electrons in the right way. There are two steps to this
process.First, we need to count the electrons that the atom “owns.”Remember that
every second-row atom has a pair of unshown 1s electrons. Count all nonbonding
valence electrons as owned by the atom and count one electron for each bond to
the atom. The second step is to consider the atomic number (positive charge of
the nucleus) for that particular atom and subtract the number of electrons that
the atom owns (negative charges). The resulting value is the formal charge on the
atom. In for example, each hydrogen has only a share in the pair of electrons
binding the two nuclei, and therefore each hydrogen is neutral (Fig. 1.23).
H
2
,
1.3 Covalent Bonds and Lewis Structures 21
=
The methyl anion
6 Protons
2 1s Electrons
2 Nonbonding
electrons
3 Shared electrons
6
C
= 6 Positive charges
7 Negative charges
Net 1
–
H
H
H
C
..
..
..
..
..
H
H
H
C
=
The ammonium ion
7 Protons
2 1s Electrons
4 Shared electrons
7
N
= 7 Positive charges
6 Negative charges
Net 1
+
HH
H
H
N
+
..
..
..
..
HH
H
H
N
+
–
–
FIGURE 1.24 Examples of the
calculation of the charge on an atom
in a molecule.
=
5 Protons
2 1s Electrons
4 Shared electrons
5
B
= 5 Positive charges
6 Negative charges
Net 1
–
HH
H
H
B
..
..
..
..
HH
H
H
B
6 Protons
2 1s Electrons
3 Shared electrons
6
C
= 6 Positive charges
5 Negative charges
Net 1
+
=
H
H
H
C
..
..
..
H
H
H
C
–
–++
FIGURE 1.25 More calculations of
charge in molecules.
PROBLEM 1.9 Draw Lewis structures for the following charged species. In each
case, the charge is shown closest to the charged atom.
(a) (b) (c) (d) (e) (f)
(g) (Hint: Nitrogen is the central atom.)
+
NO
2
+
OH
3
+
CH
3
-
Cl
+
NH
4
-
BH
4
-
OH
In F
2
each fluorine has two 1s electrons, six nonbonding electrons, and a share in
the single covalent bond between the two fluorine atoms. This count gives a total
of nine electrons, exactly balancing the nine positive nuclear charges (9 9 0).
In each carbon has a pair of 1s electrons and a share in four cova-
lent bonds for a total of six. Because each carbon has a nuclear charge of 6, both
are neutral.
Let’s see why the carbon of the methyl anion is negatively charged
and why the nitrogen of the ammonium ion (
NH
4
) is positively charged
(Fig. 1.24). In the methyl anion, carbon has a pair of 1s electrons, two nonbond-
ing electrons, and a share in three covalent bonds, for a total of seven electrons.
The nuclear charge is only 6, and therefore the carbon must be negatively
charged (6 7 1).The nitrogen atom of the ammonium ion has two 1s elec-
trons plus one electron from each of the four covalent bonds, for a total of six.
The nuclear charge is 7, and therefore the nitrogen is positively charged
(7 6 1).
(
-
:
CH
3
)
H
2
C
P
CH
2
,
Figure 1.25 shows two more ions and works through the calculations of charge.

PROBLEM 1.10 Add charges to the following compounds wherever necessary:
(a) (b)
(c) (d)
(e) (f)
(g) (h)
PROBLEM 1.11 Add electrons to complete the following Lewis structures. In each
case, the charge is placed as close as possible to the charged atom.
(a) (b)
(c) (d)
OH
3
(e)
OH (f)
NH
2
(g)
NH
2
(h)
1.4 Resonance Forms
Often, there will be more than one possible Lewis structure for a molecule. This
statement is especially true for charged molecules, but it applies to many neutral
species as well. (You may already have encountered this phenomenon in comparing
your answers to Problems 1.9 and 1.10 with ours.) How do we decide which Lewis
structure is the correct one? The answer almost always is that neither Lewis struc-
ture is complete all by itself. Instead, the molecule is best described as a combina-
tion of all reasonable Lewis structures, that is, as a combination of resonance forms,
which are different electronic representations of the same molecule. Whether or
not a Lewis structure is reasonable, whether or not it is an important contributor,
will be addressed below. The word electronic is emphasized to remind you that the
only differences allowed in a set of resonance forms are differences in electron dis-
tribution. There can be no change in the position of the atoms in resonance forms.
Let’s use nitromethane, H
3
CNO
2
, as an example. The nitro group, NO
2
, is a
functional group, a collection of atoms that behaves more or less the same way wher-
ever it appears in a molecule. As we work through the various structural types of
molecules in organic chemistry, you will become familiar with many functional
groups, but right now, at this early stage, almost everyone needs to look up a struc-
ture now and then. The problem here is that the condensed formula NO
2
doesn’t
contain structural information about how the atoms are connected to one another.
For that matter, neither does CH
3
. Over time, you will become familiar with many
of the functional groups and will be able to write the appropriate structures with-
out even thinking about it. For now, you can consult the collection of functional
groups and their structures on the inside front cover of this book.
Even though nitromethane is a neutral molecule, there is no good way to draw
it without separated charges. Figure 1.26 shows a Lewis structure for nitromethane
that has the nitrogen positive and one of the oxygens negative. But this is not the
only Lewis structure possible! We can draw the molecule so the other oxygen bears
the negative charge. The two renderings of nitromethane in Figure 1.27 are reso-
nance forms of the molecule. Note that the arrangement of the atoms is identical
in the two forms. Resonance forms are different electronic representations of the
same molecule, not pictures of different molecules.The real molecule is the combi-
nation of all its resonance forms and is often called a resonance hybrid.
CH
3
O
C
q
N
+
O
H
HC
-
P
CH
2
-
CH
2
CH
3
+
CH
2
HN
.
.
P
C
P
N
.
.
H
:
N
.
.
H
2
H
2
C
P
O
.
.
::
OH
3
:
O
.
.
.
.
H
:
C
.
H
.
CH
3
:
CH
2
22 CHAPTER 1 Atoms and Molecules; Orbitals and Bonding

1.4 Resonance Forms 23
PROBLEM 1.12 Draw a Lewis structure for nitric acid ( ), and verify that
the nitrogen is positive and one of the oxygens is negative (see Fig. 1.26).
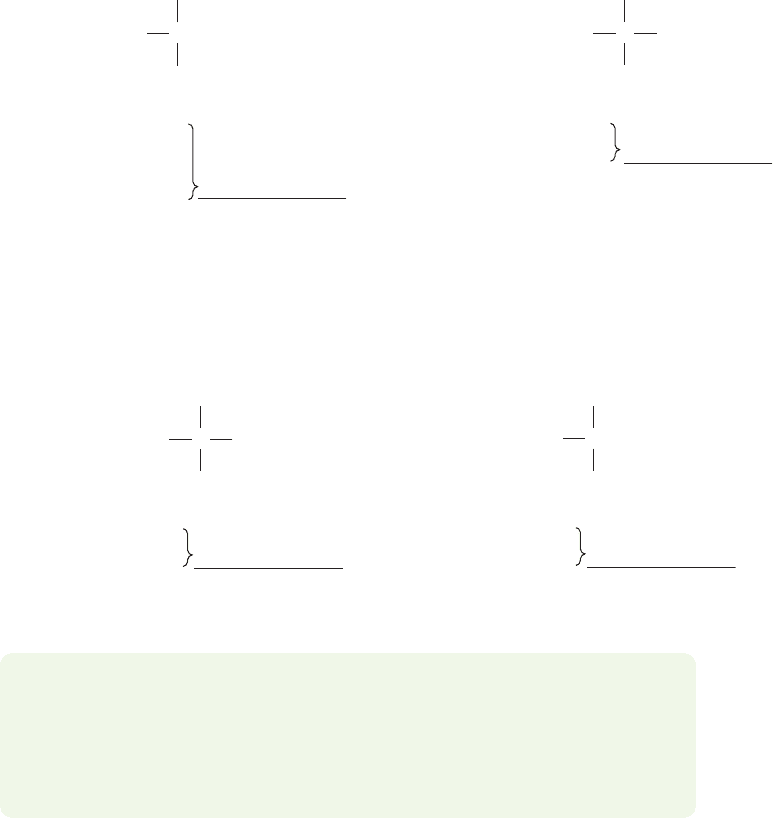
Neither Lewis structure in Figure 1.27 is completely accurate because in the
real nitromethane molecule, the two oxygens share the negative charge equally.
Thus, the two resonance forms in Figure 1.27 are equivalent electronic represen-
tations for nitromethane.Notice the special, double-headed arrow between the two
forms.This symbol is a resonance arrow, and in the notation of chemistry is used
exclusively to indicate resonance forms. Also notice the red, curved arrows in
Figure 1.27.These arrows are a notation used to show how we move a pair of elec-
trons from one point to another. In this case, such movement converts one reso-
nance form into the other. Using curved arrows to show how electrons move from
one point to another is called the curved arrow formalism. Other terms you might
encounter that are used to describe this type of analysis are arrow formalism or
electron pushing.
PROBLEM 1.13 Use the arrows to convert your Lewis structure for nitric acid
( Problem 1.12) into a resonance form.
WORKED PROBLEM 1.14 Draw another structure for nitromethane in which every
atom is neutral. Hint: There are only single bonds in this structure.
ANSWER You can arrive at the answer by using arrows to push electron pairs in
the following way:
+
–
H
H
H
C
O
O
N
..
..
..
..
..
..
H
H
H
C
..
..
..
..
O
O
N
HO
O
NO
2
,
HO
O
NO
2
H
N
H
H
C
O
O
..
..
..
..
..
–
8
O
8
O
7
N
+
8 Protons
2 1s Electrons
4 Nonbonding
electrons
2 Shared
electrons
= 8 Positive charges
8 Negative charges
Net neutral
8 Protons
2 1s Electrons
6 Nonbonding
electron
1 Shared
electrons
= 8 Positive charges
9 Negative charges
Net 1
–
6 Protons
2 1s Electrons
4 Shared
electrons
6
C
= 6 Positive charges
6 Negative charges
Neutral
7 Protons
2 1s Electrons
4 Shared
electrons
= 7 Positive charges
6 Negative charges
Net 1+
FIGURE 1.26 One electronic representation of nitromethane.
WEB 3D
H
3
C
N
O
Resonance arrow
Nitromethane
O
..
..
..
..
..
H
3
C
N
+
O
O
..
..
..
..
..
_
_
+
FIGURE 1.27 Two equivalent
resonance forms for nitromethane.
(continued)
Note that, as a new bond is formed between the two oxygen atoms, the two
charges originally on oxygen and nitrogen are canceled. Notice also the long
oxygen–oxygen bond. Remember: In drawing resonance forms, you move only
electrons, not atoms. The oxygen–oxygen distance must be the same in each
resonance form.
Is this cyclic form an important resonance form? Is it a good representation
of the molecule? The problem is the long bond between the oxygens. This bond
must be weak just because it is so long. In the language of organic chemistry, we
would say that the cyclic resonance form contributes little to the structure of
nitromethane.
Is the cyclic structure below a resonance form? Resonance involves different
electronic structures that do not differ in the positions of atoms.If atoms have been
moved, as in the figure below in which there is a normal oxygen–oxygen bond,the
two structures are in equilibrium and are not resonance forms.
Two things must be stressed here. First, although the curved arrows in Figure
1.27 have no physical significance, they do constitute an extraordinarily important
bookkeeping device. They map out the motions of electrons. But be careful—these
arrows, useful as they are, do not represent more than a bookkeeping process. We
will use curved arrows throughout this book, and all organic chemists use them to
keep track of electron movement, not only in drawing resonance forms but in writ-
ing chemical reactions as well. Being able to draw resonance forms quickly and
accurately is an essential skill for anyone wishing to master organic chemistry.
The second point to be stressed is that the bookkeeping represented by curved
arrows in resonance forms is accomplished by moving or “pushing”pairs of electrons.
Be careful when doing this sort of thing not to violate the rules of valence, not to
make more bonds than are possible, for this mistake is easy to make. Figure 1.28
gives some examples of this kind of electron-pair pushing in molecules best repre-
sented as combinations of resonance forms.
–
+
H
H
H
C
O
O
N
..
..
..
..
..
H
H
H
C
..
..
..
..
..
O
O
N
24 CHAPTER 1 Atoms and Molecules; Orbitals and Bonding
C
O
O
..
..
..
..
..
..
..
..
..
–
–
–
–
H
3
CH
3
C
C
O
O
..
..
C
O
..
..
..
..
..
H
3
CH
3
CCH
2
C
O
C
H
2
.. ..
+
C
N
HH
H
2
NNH
2
..
..
+
C
N
HH
H
2
NNH
2
..
..
+
C
N
HH
H
2
NNH
2
FIGURE 1.28 Resonance forms.
CONVENTION ALERT
It is important to be extremely clear on the following point about resonance struc-
tures.Nitromethane does not spend half its time as one resonance form and half as the
other. There is no equilibration between the resonance forms. Nitromethane is best described
1.4 Resonance Forms 25
as the combination of the two electronic structures shown in Figure 1.27. In
nitromethane, the two resonance forms are equivalent because there is no differ-
ence between having the negative charge on one oxygen or the other, and thus
nitromethane can be reasonably described as a 50:50 combination (average) of the
two forms.
Here’s an analogy that might help: Frankenstein’s monster was always a mon-
ster. One might describe that poor constructed creature as part monster and part
human, but he was always that combination—he did not oscillate between the
two. In chemical terms, one would say that Frankenstein was a resonance hybrid
of monster and human. On the other hand, Dr. Jekyll and Mr. Hyde were in equi-
librium. When the good Dr. Jekyll drank the potion, he became the monstrous
Hyde. Later, when the potion wore off, he reverted to Jekyll. Part of the time he
was Jekyll, and part of the time he was Hyde. The two were in equilibrium, not
resonance.
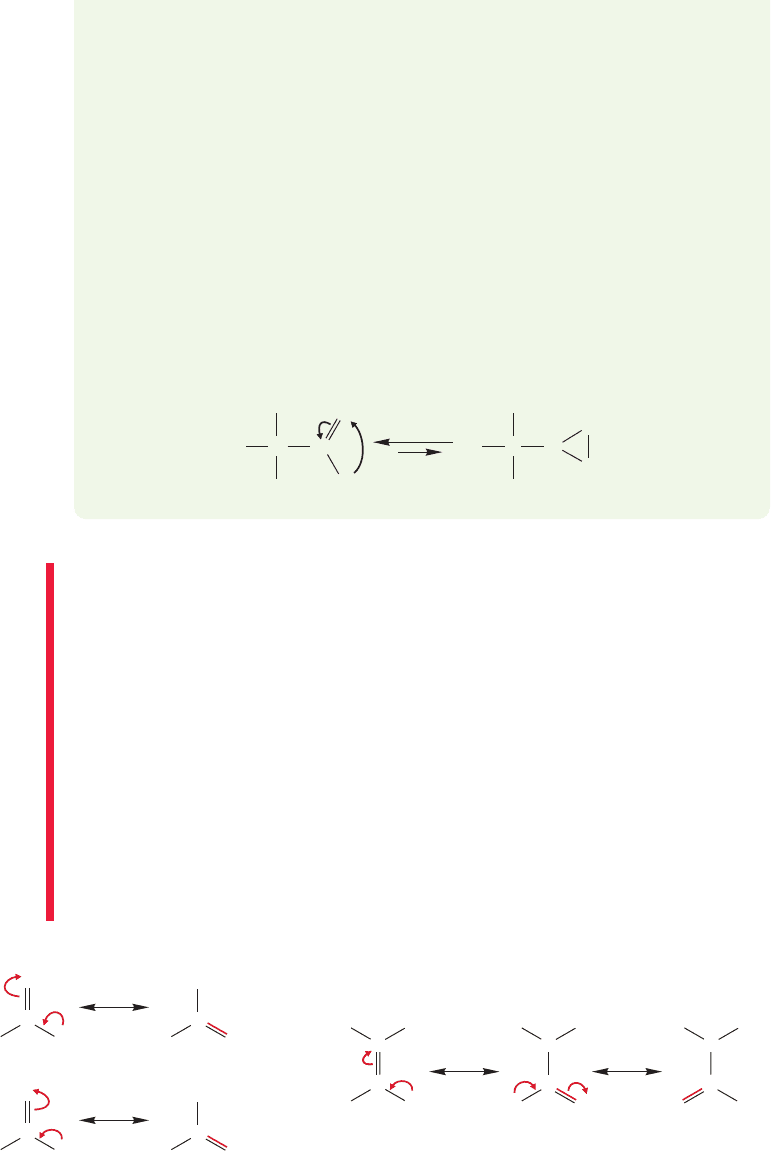
The special double-headed arrow ( ) used in Figures 1.27 and 1.28 is reserved
for resonance phenomena and is never used for anything but resonance. A pair of
arrows ( ) indicates equilibrium, the interconversion of two chemically distinct
species, and is never used for resonance (Fig. 1.29). This point is most important in
learning the language of organic chemistry. It is difficult because it is arbitrary.
There is no way to reason out the use of the different kinds of arrows; they simply
must be learned.
The carbon–oxygen double bond in formaldehyde ( , Fig. 1.30), gives
us another opportunity to write resonance forms. In the resonance form on the left
in Figure 1.30, carbon has a pair of 1s electrons and shares in four covalent bonds;
therefore it is neutral (6 6 0). Oxygen has a pair of 1s electrons, four nonbond-
ing electrons,and a share in two covalent bonds,for a total of eight electrons
(8 8 0). Oxygen is also neutral. However, we can push electrons to gen-
erate the resonance form shown on the right in Figure 1.30, in which the
carbon is positive and the oxygen negative.The real formaldehyde molecule
is a combination, not a mixture, of these two resonance forms, a resonance
hybrid. Because charge separation is energetically unfavorable, these two resonance
forms do not contribute equally to the structure of the formaldehyde molecule. Still,
neither by itself is a perfect representation of the molecule, and in order to repre-
sent formaldehyde well, both electronic descriptions must be considered.
Formaldehyde is the weighted average of the two resonance forms in Figure 1.30.
How we determine a weighted average is what we look at next.
PROBLEM 1.15 There is a third resonance form for formaldehyde, but it con-
tributes very little to the structure and is usually ignored. Can you find it and
explain why it is relatively unimportant?
PROBLEM 1.16 Use the arrow formalism to convert each of the following Lewis
structures into another resonance form. Notice that part (e) of this question asks
you to do something new—to move electrons one at a time in writing Lewis forms.
H
2
C
P
O
Z
U
Z
U
CONVENTION ALERT
A B
Two chemically distinct species
One species, E, with two Lewis
descriptions, C and D
CDE
=
FIGURE 1.29 The difference between
equilibrium (two different species, A
and B; two arrows) and resonance
(different electronic representations,
C and D, for the same molecule, E;
double-headed arrow).
FIGURE 1.30 Formaldehyde.
WEB 3D
H
Resonance forms for formaldehyde
H
C
O
..
..
..
..
..
H
H
CO
+
–
(a)
(b)
(c)
(d)
(e)
..
C
H
3
C
H
3
C
CH
2
H
3
NBH
3
C
CH
3
CH
3
NB
+
–
C
H
H
CH
2
+
+
–
–
H
2
C
C
.
C
H
C
H
H
H
C
CH
2
.
–
+
H
3
C
H
3
C
O
..
..
..

WORKED PROBLEM 1.17 Use the arrow formalism to write resonance forms that
contribute to the structures of the following molecules:
ANSWER (c) As we have seen, it is not only electron pairs (nonbonding electrons)
that can be redistributed (pushed) in writing resonance forms, but bonding electrons
as well. In this case, a pair of electrons in a carbon–carbon double bond is moved.
The positive charge on the right-hand carbon disappears, but reappears on the left-
hand carbon. In the real structure,the two carbons share the positive charge equal-
ly, each bearing one-half the charge. The structure is sometimes written with
dashed bonds to show this sharing:
It is important to be able to estimate the relative importance of resonance forms
in order to get an idea of the best way to represent a molecule. To do so, we can
assign a weighting factor,c, to each resonance form.The weighting factor is a num-
ber that indicates the percent contribution of each resonance form to the resonance
hybrid. Some guidelines for assigning weighting factors are listed below. Fortunately,
this is an area in which common sense does rather well.
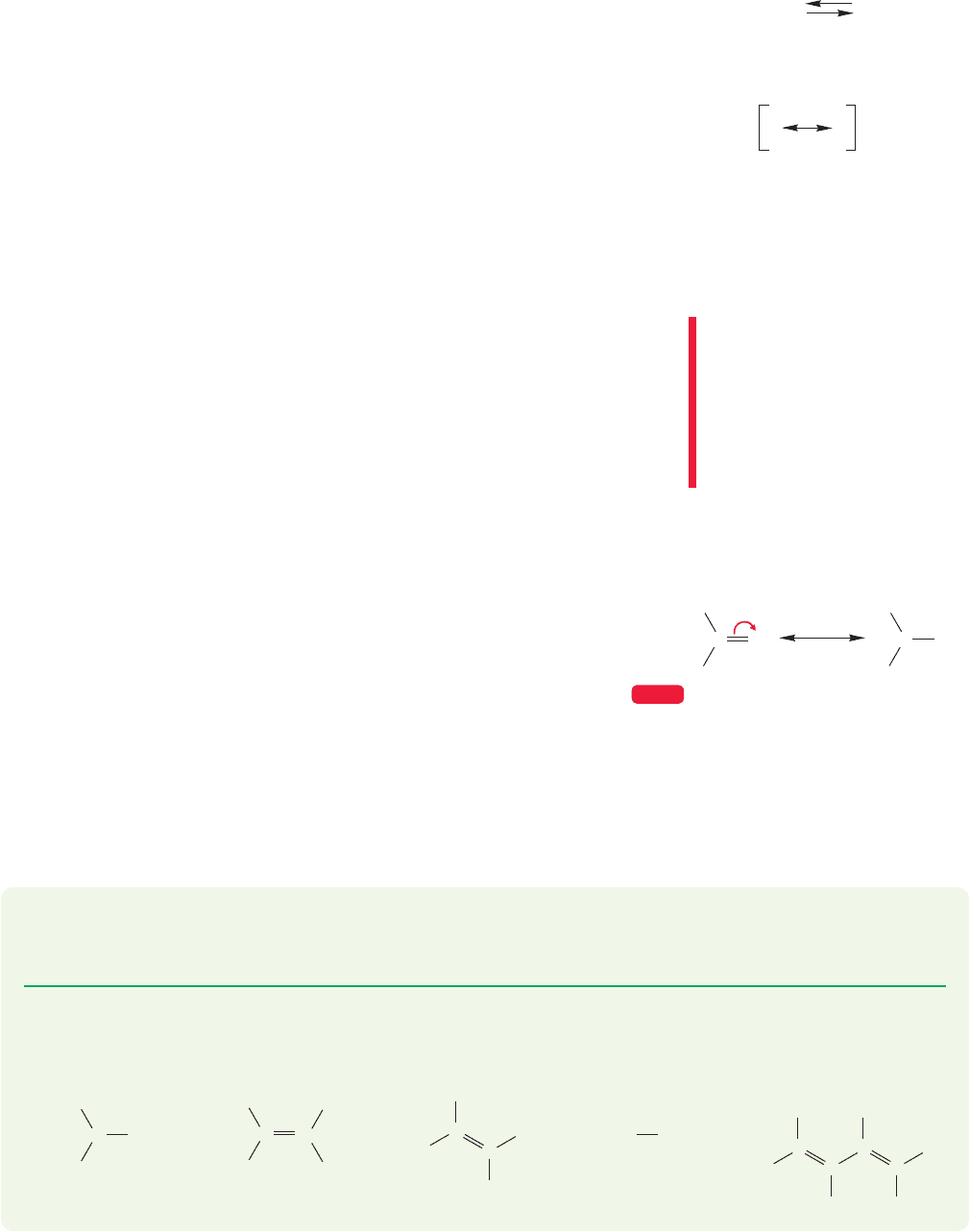
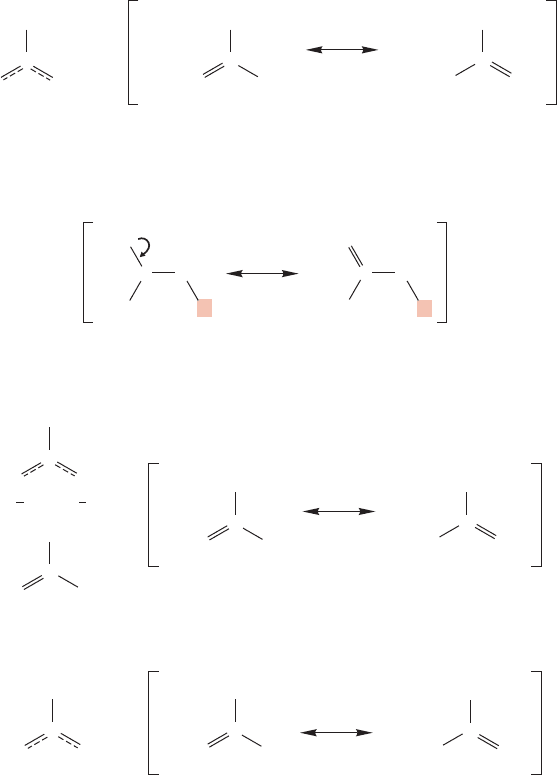
1. The more bonds in a resonance form, the more important a contributor the form
is. For example, 1,3-butadiene can be written as a resonance hybrid of four struc-
tures (Fig. 1.31).
Summary structure
C
C
H C
H
H
H
H
+
C
C
H C
H
H
H
H
+
C
C
H C
H
H
H
H
+
=
1
2
+
1
2
+
C
C
H C
H
H
H
H
+
C
C
H C
H
H
H
H
+
(c)
(a)
(b)
*(c)
(d)
(e)
H
H
C
NH
2
O
O
CH
3
C
–
C
C
H C
H
H
C
H
H
H
C
C
H C
H
H
H
H
+
+
..
..
..
..
..
OH
OH
CH
3
C
+
..
..
..
26 CHAPTER 1 Atoms and Molecules; Orbitals and Bonding
H
2
C
C
C
CH
2
+
–
A
H
2
C
C
C
CH
2
H
2
C
C
C
CH
2
BC
–
+
H
2
C
C
C
CH
2
C'
..
..
.
.
H
H
H
H
H
H
H
H
FIGURE 1.31 Four resonance forms contributing to 1,3-butadiene. Form A is by far the best.
1.4 Resonance Forms 27
It is reasonably well described by form A, which has a total of 11 bonds. Forms
B, C, and C each contain 10 bonds, and will contribute far less than A to the actu-
al 1,3-butadiene structure. Forms C and C are equivalent (this is why they are
labeled C and C rather than C and D) and contribute equally to the hybrid. Even
though A is clearly the major contributor, this does not mean that B, C, and C do
not contribute at all! It simply means that in our equation
Butadiene c
1
(A) c
2
(B) c
3
(C) c
3
(C)
the weighting factor c
1
is much larger than the other coefficients. Therefore,
1,3-butadiene looks much more like A than B, C,or C.
2. Separation of charge is bad. In Figure 1.31, the neutral resonance forms A and B
contribute more to the resonance hybrid than C or C, both of which require a
destabilizing separation of charge. (Form B contributes less than form A, as noted
in guideline 1, because it has fewer bonds.)
3. In ions,
delocalization of electrons (distributing them over as many atoms as pos-
sible) is especially important. Delocalization of electrons is a process that allows
more than one atom to share electrons and it is almost always stabilizing. Electrons
are like water, if allowed to spread out, they will. The allyl cation is a good exam-
ple—the two end carbons each bear one-half of the positive charge (Fig. 1.32).
=
H
H
2
CCH
2
C
H
+
H
2
CCH
2
C
H
+
AA'
c
1
c
2
H
2
C
CH
2
C
+
FIGURE 1.32 The allyl cation is an
equal combination of A and A.
H
H
CCH
2
Cl
H
H
CCH
2
Cl
+
+
..
..
..
..
..
c
2
c
1
FIGURE 1.33 In this carbocation, the
charge is shared by carbon and
chlorine.
H
2
C
CH
2
=
C
H
H
2
CCH
2
C
H
–
H
2
CCH
2
C
H
–
H
2
CCH
2
C
H
–
....
(–)
H
2
C
O
=
C
H
–
H
2
C
C
H
–
H
2
C
C
H
–
..
..
..
..
..
..
c
2
c
1
AB
OO
In this case, c
1
> c
2
c
2
c
1
In this case, c
1
= c
2
1
2
–
1
2
–
(a)
(b)
FIGURE 1.34 (a) The allyl anion.
(b) The enolate anion.
The electrons of the double bond have spread into the empty p orbital.The ion
shown in Figure 1.33 is another good example.Here the charge is shared by the car-
bon and the chlorine, as the two resonance forms show.
4. Electronegativity is important. In the allyl anion (Fig. 1.34a), for example, there
are two equivalent resonance forms. As in the allyl cation, the charge resides
equally on the two end carbons. Figure 1.34a shows two summary structures
for the allyl anion. In one, each of the two end carbons is shown with half a
negative charge; in the other, one resonance form is drawn, and the positions
sharing the charge are indicated by drawing the charge in parentheses ().
The related enolate anion, in which one CH
2
of allyl is replaced with an oxygen
(Fig. 1.34b), also has two resonance forms, but they do not contribute equally
because they are not equivalent. Each has the same number of bonds, and so
we cannot choose the better representation that way. However, form A has
the charge on the relatively electronegative oxygen while form B has it on
carbon, which is considerably less electronegative than oxygen. Form A is the
better representation for the enolate anion, although both forms contribute.
Mathematically, we would say that the weighting factor for A,c
1
, is larger than
that for B,c
2
.
5. Resonance forms that are equivalent contribute equally to a resonance hybrid. The
two forms of nitromethane in Figure 1.27 and the two forms of the allyl cation
(A and A) in Figure 1.32 are good examples. In both cases the resonance forms are
completely equal to each other (indistinguishable, same number of bonds, same
number of charges, charges on same atoms).
In cases such as these, the weighting factors for the two forms, c
1
and c
2
, must
be equal. An equation to represent the mathematics involved in our allyl cation
example is
Allyl c
1
(form A) c
2
(form A)
where c
1
is the weighting factor for resonance form A,c
2
is the weighting factor for
form A, and c
1
c
2
.
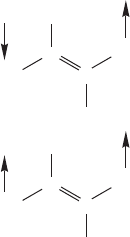
6. All resonance forms for a given species must have the same number of paired and
unpaired electrons. As we saw in Figure 1.5, two electrons occupying the same
orbital must have opposite spin quantum numbers. Most organic molecules,
including our example molecule 1,3-butadiene, have all paired electrons.
Therefore, resonance forms for 1,3-butadiene must also have all paired electrons.
Consider form B in Figure 1.31. It is often the convention to emphasize that all
electrons are paired in this molecule by appending electron-spin arrows alongside
each single electron in this form (Fig. 1.35). Opposed arrows ( and ) indicate
paired spins, arrows in the same direction ( and or and ) indicate parallel
(same-direction) spins.
[[\\
\[
28 CHAPTER 1 Atoms and Molecules; Orbitals and Bonding
C
C
CH
2
H
2
C
H
2
C
.
.
C
C
CH
2
.
.
The opposing arrows indicate
that the spin quantum numbers
of the two electrons are different;
this is a resonance form of
1,3-butadiene
The identical arrows indicate
that the spins of the two electrons
are the same; this is not a
resonance form of 1,3-butadiene
H
H
H
H
FIGURE 1.35 In 1,3-butadiene, all
electrons are paired, and all resonance
forms contributing to the structure
must also have all electrons paired.
