Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.


1.10 Additional Problems 49
PROBLEM 1.65
Make the molecular orbitals for square H
4
by
allowing the molecular orbitals of H
2
to interact as shown below:
Order the new molecular orbitals by counting nodes, and add
the proper number of electrons. You might check your answer
by deriving the same orbitals. Do this by bending the molecular
orbitals for linear HHHH developed in Problem 1.64.
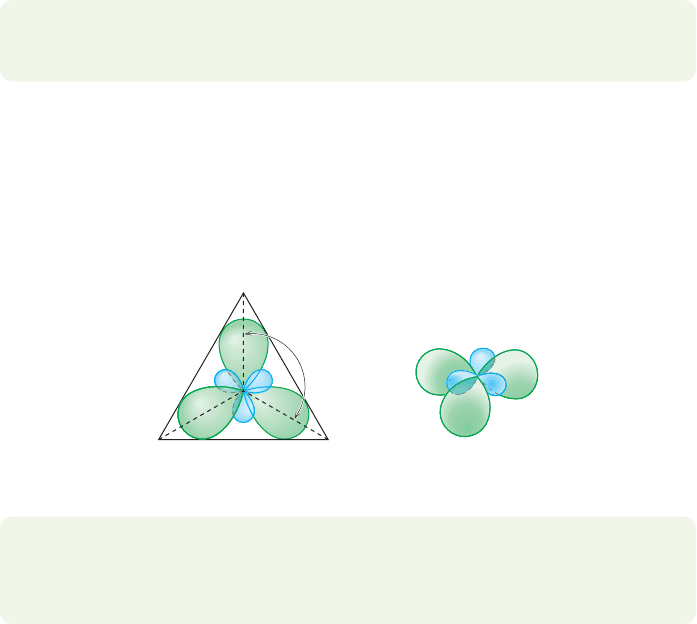
PROBLEM 1.66 Generate the molecular orbitals for planar
ammonia, NH
3
. Do this by taking combinations of the molecu-
lar orbitals for triangular H
3
(see Problem 1.62 for these
orbitals) and the atomic orbitals of nitrogen.
(a) Show clear pictures of the molecular and atomic orbitals you
are using to make the molecular orbitals of planar ammonia.
(b) How many molecular orbitals will ammonia have?
(c) Draw pictures of the molecular orbitals for planar ammonia.
Show clearly how these are generated.
(d) Order the bonding and nonbonding molecular orbitals in
terms of energy. Place them on a scale relative to the energy
of a lone, nonbonding 2p orbital. You do not have to order
the antibonding orbitals.
(e) Place the appropriate number of electrons in the orbitals. Be
careful to indicate the spin quantum number for each elec-
tron (use an up or down arrow to show spin).
PROBLEM 1.67 Generate the molecular orbitals for linear
methylene, by combining the atomic orbitals of
carbon with the molecular orbitals of hydrogen, H
2
.
(a) Show clear pictures of the molecular and atomic orbitals
you are using.
(b) How many molecular orbitals will linear methylene have?
(c) Draw pictures of the molecular orbitals for linear methylene.
H
O
C
O
H,
HH
HH
+
–
HH
HH
(d) Order these molecular orbitals in terms of energy. Place
them on a scale relative to the energy of a lone, nonbonding
carbon 2p orbital.
(e) Place the appropriate number of electrons in the orbitals,
being careful to indicate the spin quantum number for each
electron (use an up or down arrow to show spin).
Use Organic Reaction Animations (ORA) to answer the
following questions:
PROBLEM 1.68 Choose the reaction titled “Unimolecular
nucleophilic substitution” and click on the Play button. Do you
suppose the first step of this reaction is a homolytic or a het-
erolytic cleavage? Observe the Highest Occupied Molecular
Orbital (HOMO) track by clicking on the HOMO button.
The location (orbital) of the most available electrons will be
shown throughout the reaction. Notice that the electron density
goes with the bromine as it comes off. That should help you
answer this question.
PROBLEM 1.69 Choose the “Introduction” on the
bottom left of the Table of Contents page. Read this short
document. Under the “Technical Issues” heading there is a
discussion of solvent effects. After reading this section, how
do you think using a polar solvent in the “Unimolecular
nucleophilic substitution” reaction might affect the answer
to the previous question? That is, would a polar solvent
have more impact on a homolytic or a heterolytic cleavage?
PROBLEM 1.70 Choose the reaction “Alkene hydrohalo-
genation” and observe the molecule that initially comes
to the screen. It has a carbon–carbon double bond.
Click on the HOMO button. Observe the calculated area
for the π bond that is shown to answer the following
questions. Is the electron density of a π bond constrained
to the space between the carbons? Do you suppose the π
bond electrons are held more or less tightly than σ bond
electrons?

Alkanes
50
2.1 Preview
2.2 Hybrid Orbitals: Making a
Model for Methane
2.3 The Methyl Group (CH
3
) and
Methyl Compounds (CH
3
X)
2.4 The Methyl Cation (
+
CH
3
),
Anion (
CH
3
), and Radical
(CH
3
)
2.5 Ethane (C
2
H
6
), Ethyl
Compounds (C
2
H
5
X), and
Newman Projections
2.6 Structure Drawings
2.7 Propane (C
3
H
8
) and Propyl
Compounds (C
3
H
7
X)
2.8 Butanes (C
4
H
10
), Butyl
Compounds (C
4
H
9
X), and
Conformational Analysis
2.9 Pentanes (C
5
H
12
) and Pentyl
Compounds (C
5
H
11
X)
2.10 The Naming Conventions for
Alkanes
2.11 Drawing Isomers
2.12 Rings
2.13 Physical Properties of Alkanes
and Cycloalkanes
2.14 Nuclear Magnetic Resonance
Spectroscopy
2.15 Acids and Bases Revisited:
More Chemical Reactions
2.16 Special Topic: Alkanes as
Biomolecules
2.17 Summary
2.18 Additional Problems
.
:
2
A CELESTIAL VIEW Does it include molecular orbitals?
2.1 Preview 51
God never saw an orbital.
—WALTER KAUZMANN, FEBRUARY, 1964
1
2.1 Preview
The chemical reactions a given compound undergoes are a function of its structure.
No compound reacts exactly like another, no matter how similar their structures.Yet
there are gross generalizations that can be made, and some order can be carved out
of the chaos that would result if compounds of similar structure did not react in
somewhat the same fashion. To a first approximation, the reactivity of a molecule
depends on the functional groups (p. 22) it contains.
The inside front cover of this book shows some of the functional groups that
are important in organic chemistry. This material is not to be memorized, and it is
certainly not complete. You will want to work through it, however, to be certain you
can write correct Lewis structures for each group and to begin to become familiar
with the variety of functional groups important in organic chemistry. These differ-
ent kinds of molecules will all be discussed in detail in later chapters. We start in
this chapter with alkanes, the simplest members of the family of organic molecules
called hydrocarbons—compounds composed entirely of carbon and hydrogen.
Alkanes all have the molecular formula C
n
H
2n2
. The simplest alkane is
methane,CH
4
. Vast numbers of related molecules can be constructed from
methane by replacing one or more of its hydrogens with other carbons and their
attendant hydrogens. Linear arrays can be made,as well as branched structures and
even rings (ring compounds called cycloalkanes, have a slightly different formu-
la: C
n
H
2n
). Figure 2.1 shows a few schematic representations for these molecules.
1
Walter Kauzmann (p. 42) was chairman of the Princeton University chemistry department in 1964 when MJ
interviewed for a job. He made this comment in response to a typically convoluted molecular orbital answer
to some simple question.
H
H
H
H
C
The simplest
representations—not much
information here
Here, the connectivity is
shown, but there is no
three-dimensionalit
y
Three-dimensional structures.
Solid wedges come toward
y
ou, dashed wed
g
es retreat
The ultimate schematic
structures—neither carbons,
nor h
y
dro
g
ens are shown
CH
4
CH
3
CH
2
CH
3
Methane
Propane
Cyclopropane
H
H
H
H
H
H
CC
C
H
H
H
CH
HH
H
H
C
H
H
C
H
H
C
HH
HH
HH
C
C
C
H
2
C
CH
2
H
2
C
H
H
H
H
HH
C
C
H H
C
FIGURE 2.1 Several ways of drawing
alkane structures.
52 CHAPTER 2 Alkanes
Before we can go further, we need to make sense of these schematics, and as usual,
we must start with structure. The first task is to describe the shape of methane.
Once we have done this, we can go on to investigate other members of the al-
kane family.
PROBLEM 2.1 Draw Lewis structures for the linear alkanes butane (C
4
H
10
) and
pentane (C
5
H
12
).
ESSENTIAL SKILLS AND DETAILS
1. Hybridization. Above all, it is important to master the structural model introduced in
this chapter, hybridization.The hybridization model does a good job of allowing us to
predict the general structures of compounds and it is nicely suited for following
electron flow in chemical reactions.Thus, it fits in well with the curved arrow
formalism introduced in Chapter 1 (p. 23).
2. Structures. It is important to be able to use the various structural formulas, which range
from richly detailed three-dimensional representations to the ambiguous condensed
formulas that give no hint of the three-dimensional complexity often present in a
molecule.
3. Difference.The concept of difference is essential in organic chemistry. When are two
atoms the same (in exactly the same environment) and when are they different (not in
the same environment)? This question gets to the heart of structure and is much
tougher to answer than it seems. In this chapter, we will introduce this subject and we
will return to it in Chapter 4.
4. Names. In truth, it is not necessary to know every nuanced detail of the naming
convention for alkanes, but you do need to know some nomenclature.
5. The cis/trans convention. Cyclic molecules (rings) have sides—above the ring and
below the ring—and attached groups can be on the same or opposite sides of the
ring.
6. The Newman projection. Drawing and “seeing”Newman projections is a critical skill in
organic chemistry.
7. Bond strengths.You surely do not have to memorize all possible bond strengths, but by
the end of this chapter you should have a good idea of some of the important ones,
such as , , and .
2.2 Hybrid Orbitals: Making a Model for Methane
2.2a Hybridization Our task is to devise a bonding model for the structure
of the simplest alkane, methane, CH
4
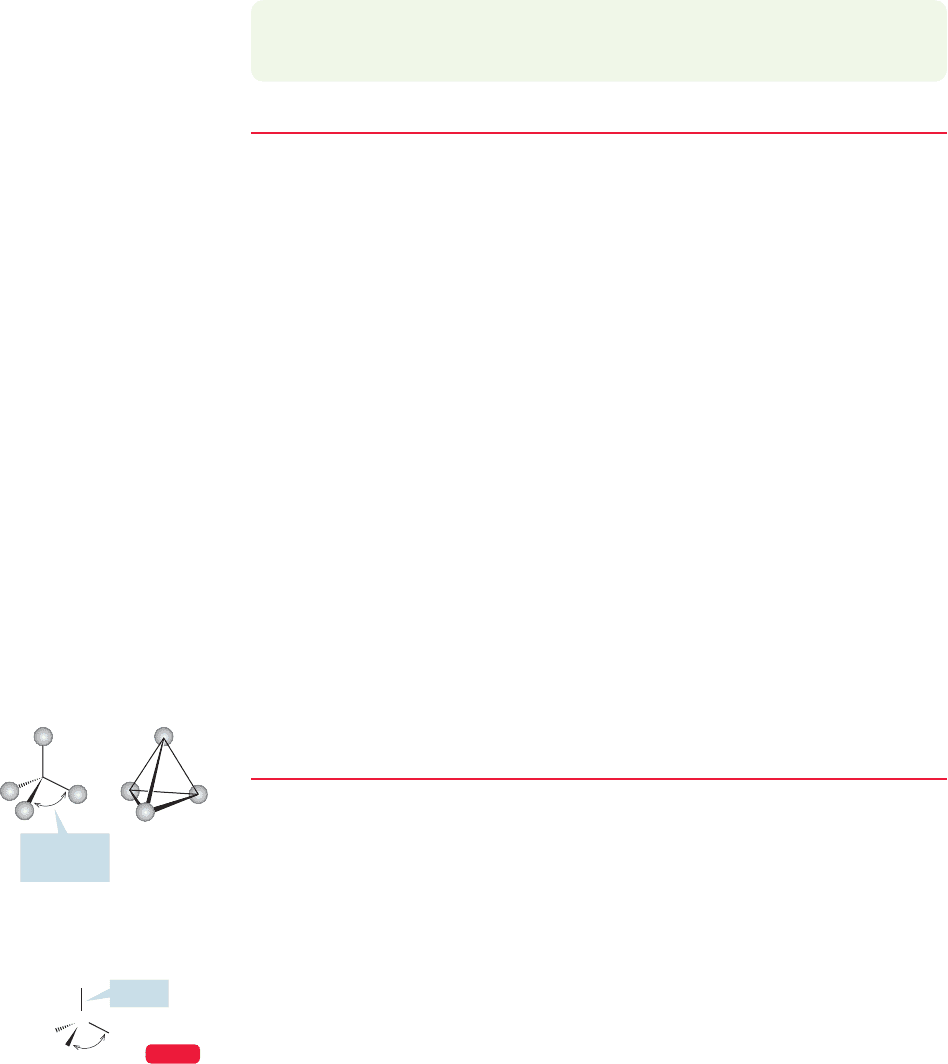
. Physical chemists tell us the structure:
methane is a tetrahedron, with all carbon–hydrogen bonds the same length
(Fig. 2.2). We had an earlier look at a tetrahedron in Problem 1.5 (p. 16), in
which the molecule carbon tetrachloride appeared. Our job now is to work out
a bonding scheme that leads to the experimentally determined tetrahedral struc-
ture of methane.
The method we use to generate our model of methane goes by the name
hybridization. Remember that we are working out a model, which means that no
matter how useful it is,what we produce here is bound to be flawed in some respects.
It is not the “ultimate truth”by any means! Our strategy will be to combine the four
H
O
HC
O
HC
O
C
(a)
(b)
A tetrahedron
H
H
H
H
C
=
109.5
Methane, a tetrahedral molecule
1.09
All angles
are 109.5
A
WEB 3D
FIGURE 2.2 (a) A tetrahedron.
(b) A tetrahedral molecule, methane,
showing the arrangement of the four
hydrogens about the central carbon.
2.2 Hybrid Orbitals: Making a Model for Methane 53
atomic orbitals of a carbon atom (2s,2p
x
,2p
y
,2p
z
) to produce four new orbitals for
the atom, called hybrid orbitals, that can overlap with the 1s orbitals of four hydro-
gens to produce methane.Despite the fancy name hybridization, we are really doing
something quite similar to the combining of atomic orbitals we saw often in Chapter
1. In Chapter 1 we combined orbitals from different atoms to make molecular
orbitals and here we will combine orbitals from the same atom to make hybrid atom-
ic orbitals. Remember that we already know that a combination of four atomic
orbitals must produce four new orbitals (p. 34).
It will greatly help our understanding of the hybridization process if we back
up just a bit and look at two simpler hybridization models before we return to
methane.
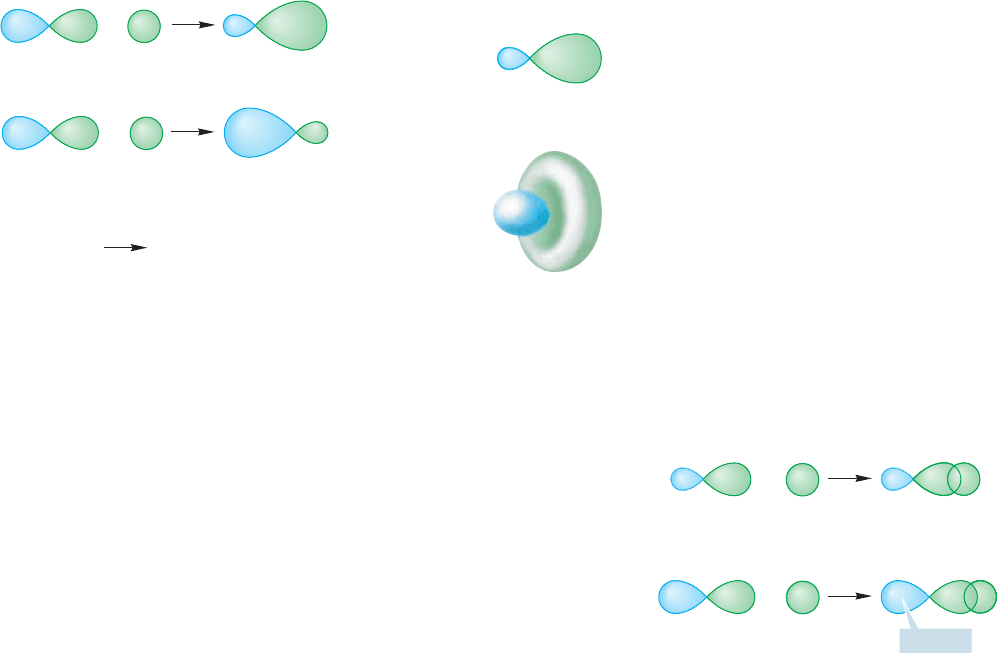
2.2b sp Hybridization Let’s first combine the 2s and 2p
x
atomic orbitals from
some arbitrary atom, as shown in Figure 2.3. The two can be combined in a con-
structive (2p 2s) or a destructive (2p 2s) way to create a pair of hybrid orbitals.
+
–
2p
x
2ssp Hybrid orbital
2p
x
2s Another sp hybrid
orbital
2s 2p
x
Two sp hybrid orbitals
One sp orbital in schematic form…
…and more correctly:
+
–
FIGURE 2.3 sp Hybridization.
FIGURE 2.4 A comparison between
overlap of a hydrogen 1s orbital with
(a) an sp hybrid orbital and (b) an
unhybridized 2p orbital. With the sp
hybrid orbital, overlap is maximized
because the non-overlapping back
lobe is small. With the unhybridized
2p orbital, all of the non-overlapping
back lobe is “wasted.”
Note in Figure 2.3 that the hybrid orbitals no longer have equal-sized lobes, as in a
2p orbital. In the 2p 2s combination, we get an expansion of one of the original
lobes (the one with the same sign as the 2s orbital) and a shrinking of the other (the
one with the opposite sign). The second combination, (2p 2s), is also lopsided,
but in the opposite sense.These two new atomic orbitals are called sp hybrid
orbitals. The designation sp means that the orbitals are composed of 50% s
orbital (they have 50% s character) and 50% p orbital (they have 50% p
character).
Figure 2.3 also shows the real shape of an sp hybrid orbital. It is tradi-
tional to use the schematic form in drawings, rather than attempt to repro-
duce the detailed structure of the orbital.
Overlap between an sp orbital of one atom and an orbital of some other
atom will be especially good if the fat lobe of the sp orbital is used (Fig. 2.4a).
Contrast this overlap with the overlap when a 1s orbital overlaps with one
lobe of a 2p orbital of another atom (Fig. 2.4b).The better the overlap, the greater
the stabilization and the stronger the bond. In a sense, overlap of a 1s orbital with
an unhybridized atomic 2p orbital “wastes” the back lobe of the 2p orbital.
Hybridization improves overlap!
Hybridization also minimizes electron–electron repulsion. The two new sp
hybrid atomic orbitals formed from 2p 2s and 2p 2s (Fig. 2.3) are aimed 180°
from each other. The electrons in the bonds are as remote from each other as
possible.
(b)
(a)
Wasted!
sp 1s
2p 1s
54 CHAPTER 2 Alkanes
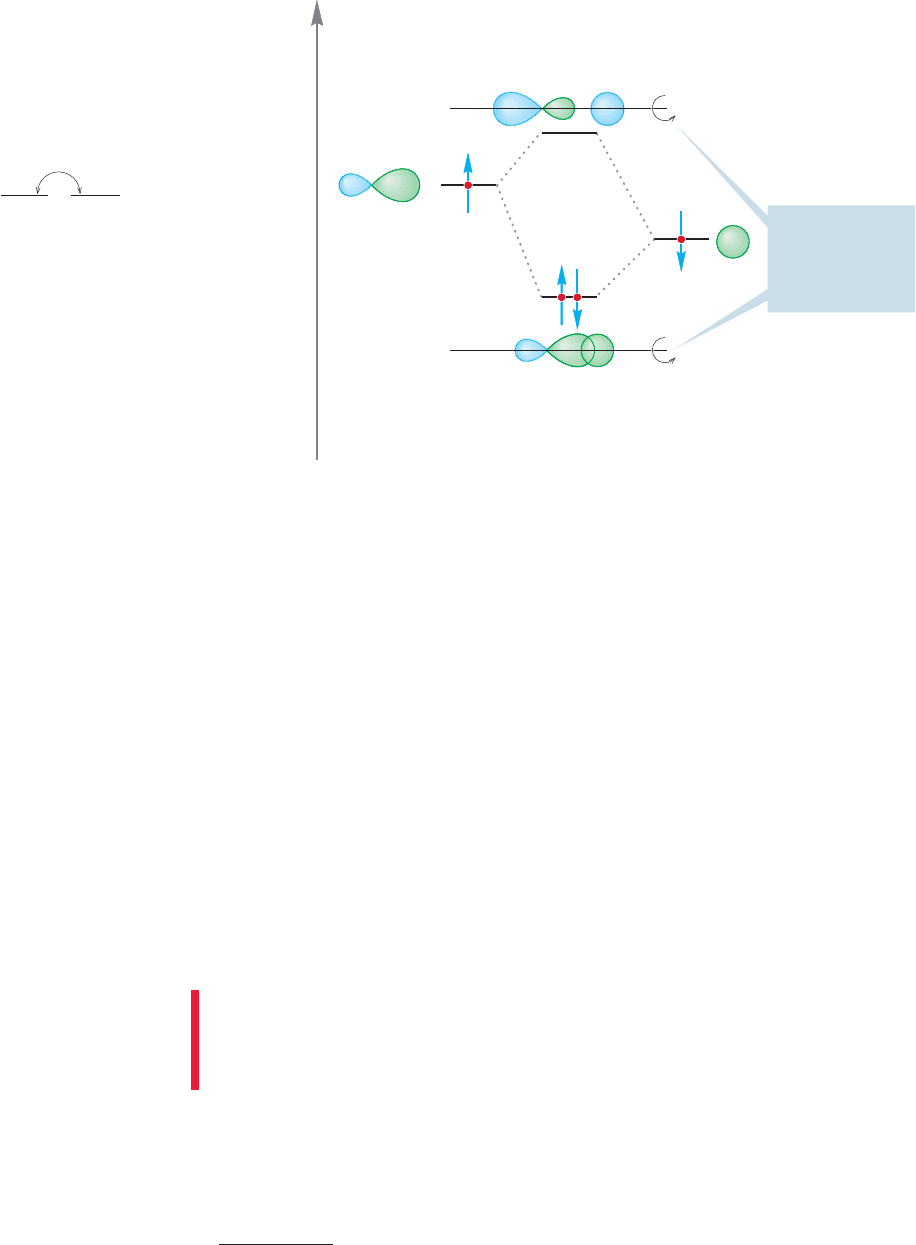
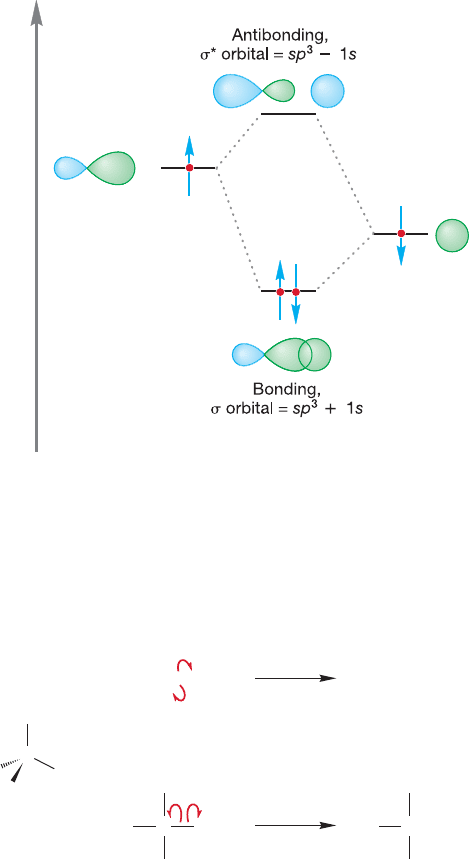
There are many compounds in which the bonding can be nicely described by sp
hybridization. The classic example is , a linear hydride of beryllium
(Fig. 2.5). Beryllium (
4
Be) has the electronic configuration 1s
2
2s
2
and thus brings
only two electrons to the bonding scheme (Remember: The filled shell 1s electrons
are not used for bonding).We construct our picture of beryllium hydride (BeH
2
) by
making two sp hybrid atomic orbitals from the Be 2s atomic orbital and one of the
three equivalent Be 2p orbitals, arbitrarily taking the 2p
x
orbital (which exists even
though the configuration 1s
2
2s
2
tells us there are no electrons in this atomic orbital).
The overlap of the 2p
x
2s hybrid orbital with a singly occupied hydrogen 1s orbital
and of the 2p
x
2s hybrid orbital with a second hydrogen 1s orbital produces two
bonds directed at an angle of 180° with respect to each other. As a result
of the linear shape of this compound we say that the beryllium is sp-hybridized.
Bonds of this type, with cylindrical symmetry, are called sigma bonds (σ bonds).
The orbital is unchanged through rotation around the axis between the Be and H.
The combination of a Be sp orbital and a H 1s orbital produces the sigma bonding
orbital (sp 1s) and must also yield an antibonding orbital (sp 1s), which in this
case is empty, as shown in Figure 2.5. This antibonding orbital also has cylindrical
symmetry and is therefore properly called a sigma antibonding orbital.
In Chapter 1, we used subscripts A and B to indicate antibonding and bonding
orbitals (Φ
A
, Φ
B
), but from now on we can use the simpler notation of an asterisk (*)
to indicate an antibonding orbital. Thus, in Figure 2.5, σ is the bonding molecular
orbital sp 1s and σ* is the antibonding molecular orbital sp 1s.
It is no accident that we use two of beryllium’s atomic orbitals (2s and 2p
x
) in
our description of the bonding in BeH
2
. Two hybrid orbitals are needed for bond-
ing to two hydrogens, which means we must combine two unhybridized atomic
orbitals to get them.
2
Be
O
H
H
O
Be
O
H
Energy
HH
180
Be
sp Hybrid
orbital of Be
H 1s
sp –
1s
The antibonding
* orbital
Axis of
symmetry:
Rotation around
this axis changes
nothing
sp +
1s
The bonding
orbital
Axis
Axis
FIGURE 2.5 The formation of one
bonding (σ) molecular orbital and
one antibonding (σ*) molecular
orbital from the overlap of a Be sp
hybrid orbital with a hydrogen 1s
atomic orbital. Note that the
interaction diagram shows the 1s
atomic orbital is at a lower energy
than the sp hybrid orbital.The figure
shows formation of only one of the
two beryllium–hydrogen bonds in
BeH
2
.The other bond is
made in identical fashion through
overlap of the second Be sp hybrid
orbital with the 1s atomic orbital of a
second H atom.
Be
O
H
2
Nothing in this discussion so far is specific to the element Be. Even though we are using this element as our
example, we have been constructing hybrid orbitals for any second row linear HXH molecule.
CONVENTION ALERT

2.2 Hybrid Orbitals: Making a Model for Methane 55
PROBLEM 2.2 What happens to the 2p
y
and 2p
z
atomic orbitals of Be in BeH
2
?
Elaborate the picture in Figure 2.5 to show the unused, empty 2p atomic orbitals
on Be.
3
WORKED PROBLEM 2.3 Use the hybridization model to build a picture of the
bonding in the linear molecule . (Recall that a molecular orbital
picture of this linear molecule was constructed in Problem 1.67, p. 49). If you did-
n’t do that problem, you might go back, try it now, and contrast your molecular
orbital answer with your hybridization answer.
ANSWER As with BeH
2
, the central atom, here carbon, is hybridized sp because
the CH
2
carbon only has two bonds. The 2s and 2p
x
atomic orbitals of carbon
are combined to form two sp hybrid orbitals (2s 2p
x
and 2s 2p
x
). One of
the bonds in CH
2
is made through overlap of the 2s 2p
x
sp hybrid orbital
and the 1s atomic orbital of one hydrogen. The other bond is made through
overlap of the 2s 2p
x
hybrid orbital and the 1s atomic orbital of the other
hydrogen.
The 2p
y
and 2p
z
atomic orbitals in the carbon remain unchanged. The atom
uses two of its four bonding electrons in the carbon–hydrogen bonds, leaving two
for the remaining 2p orbitals. Each contains a single electron.
H
O
C
O
H
3
This kind of thing generally drives physical chemists crazy. As an orbital is nothing more than the
electron probability region, how can there be an “empty orbital”? The problem is at least partially
avoided if the empty orbital is thought of as “the place the next electron would go” as mentioned in
Chapter 1.
2.2c sp
2
Hybridization In BH
3
, we must make three boron–hydrogen bonds
to form the molecule. The electronic configuration of boron (
5
B) is 1s
2
2s
2
2p
x
, and
thus there are three electrons available to form bonds with the three hydrogen 1s
Energy
HHC
sp Hybrid
orbital of C
constructed
from 2s
2p
x
H 1s
sp – 1s
The antibonding
σ∗ orbital
1s/sp Bonds
sp + 1s
Bonding
σ orbital
The 2p
y
and 2p
z
orbitals
H
H
C
.
.
+
–
56 CHAPTER 2 Alkanes
atomic orbitals.This time we combine the boron 2s,2p
x
, and 2p
y
orbitals to produce
the three orbitals we need in our model. These are called, naturally, sp
2
hybrid
orbitals (constructed from one s orbital and two p orbitals) and have 33% s charac-
ter and 67% p character. They resemble the sp hybrid orbitals, shown in Figure 2.3.
As with the sp orbital, the fat lobe of the sp
2
orbital allows for efficient overlap with
a hydrogen 1s or with any other orbital.
2s 2p
x
2p
y
Three sp
2
hybrid orbitals
U
Top view Side view
120
FIGURE 2.6 Top and side views of the
arrangement in space of the three sp
2
hybrid orbitals formed from the 2s,
2p
x
, and 2p
y
atomic orbitals of a
boron atom.
PROBLEM 2.4 Boron also forms a compound BF
3
. Create a bonding scheme and
draw a Lewis structure for this molecule.
How are the three sp
2
hybrid orbitals needed to form BH
3
arranged in space?
Your intuition may well give you the answer. They are directed so that electrons in
them will be as far as possible from one another. The angle between them is 120°,
and they are aimed toward the corners of a triangle (Fig. 2.6). An sp
2
carbon and
the three atoms surrounding it lie in the same plane.
PROBLEM 2.5 The 2p
z
orbital of boron was not used in our construction of the
three sp
2
hybrid atomic orbitals for the atom. Sketch it in, using the drawing of
Figure 2.6.
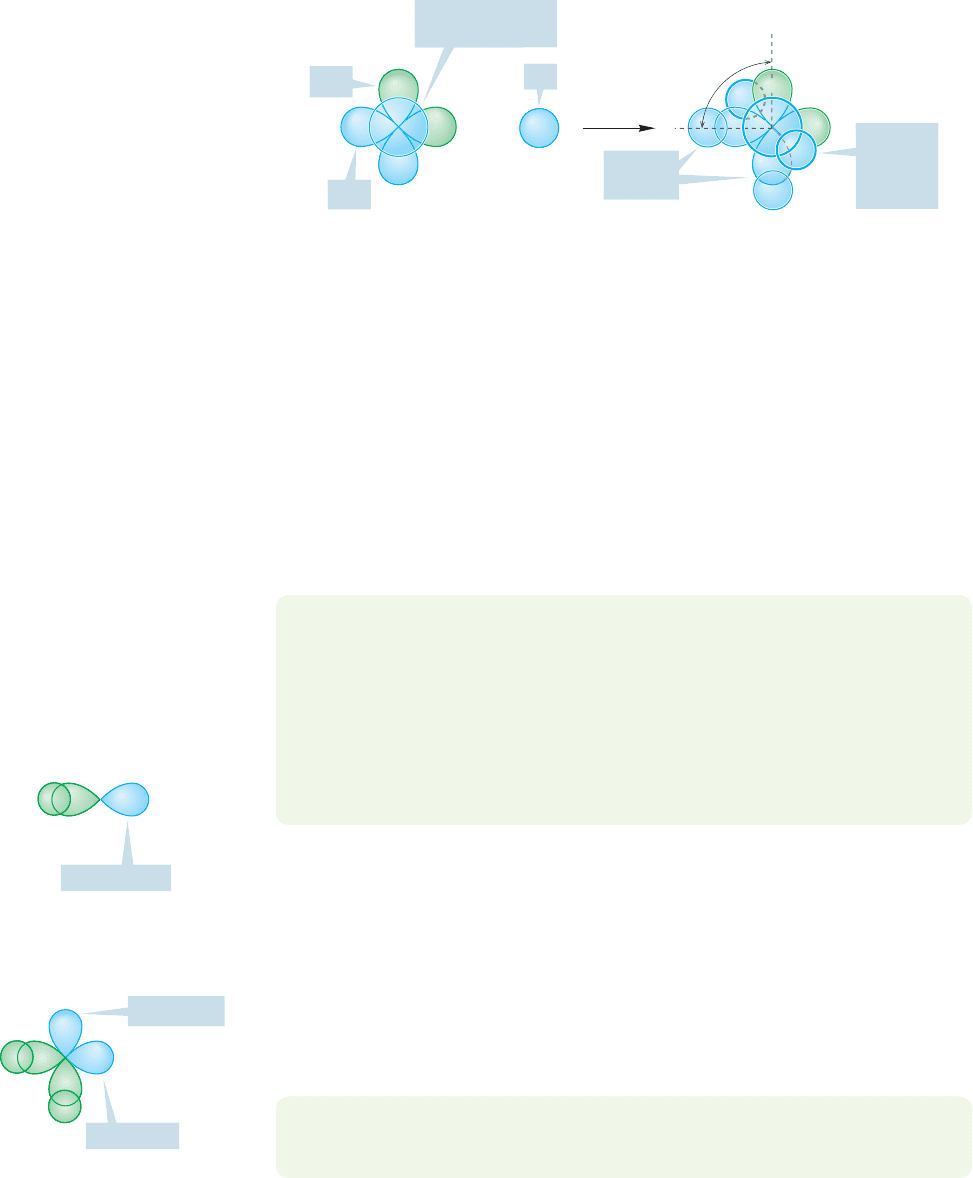
2.2d sp
3
Hybridization: Methane For CH
4
we need four hybrid atomic
orbitals because there are four hydrogens to be attached to carbon. Fortunately, we
have just what we need in the 2s,2p
x
,2p
y
, and 2p
z
atomic orbitals of carbon. In our
mathematical description, we combine these orbitals to produce four sp
3
hybrid
orbitals, which also look similar to sp hybrids (Fig. 2.3).
2s 2p
x
2p
y
2p
z
Four sp
3
hybrid orbitals
The familiar lopsided shape appears again, and thus we can expect strong bond-
ing to the four hydrogen 1s atomic orbitals. By now you will surely anticipate that
these orbitals will be aimed in space so as to keep them, and any electrons in them,
as far apart as possible. How are four objects arranged in space so as to maximize
the distance between them? The answer is,at the corners of a tetrahedron (Fig.2.2).
The carbon in methane is hybridized sp
3
(25% s character; 75% p character) and
the four hybrid atomic orbitals are directed toward the corners of a tetrahedron.
The 1s atomic orbital in each of four hydrogen atoms overlaps with one of the four
U
2.2 Hybrid Orbitals: Making a Model for Methane 57
sp
3
orbitals to form a bonding molecular orbital and an antibonding molecular
orbital (Fig. 2.7).The resulting angles are each 109.5°, and the length
of a carbon–hydrogen bond in methane is 1.09 Å (Fig. 2.2).
H
O
C
O
H
Energy
sp
3
1s
FIGURE 2.7 The formation of a
carbon–hydrogen bond through the
overlap of an sp
3
hybrid orbital with a
hydrogen 1s atomic orbital. Note the
formation of both the bonding (σ)
molecular orbital and the
antibonding (σ*) molecular orbital.
energy
energy
H
H
H
H
C
=
..
..
..
..
⌬H = +105 kcal/molHomolytic cleavage of one
carbon–hydrogen bond in methane
HH
H
..
..
..
HH
H
H
C
H
C
H
H
H
C
.
.
H
H
H
H
C
.
.
H
+
+
FIGURE 2.8 Two ways of showing
homolytic cleavage of one
carbon–hydrogen bond in methane.
Each single-barbed arrow represents
movement of one electron.
The carbon–hydrogen bond in methane is very strong; its bond dissociation ener-
gy is 105 kcal/mol.This means that 105 kcal/mol must be applied in order to break
one of the carbon–hydrogen bonds in methane homolytically. This reaction is
endothermic by 105 kcal/mol (Fig. 2.8). Compare this value to the 104 kcal/mol
bond strength of the very strong bond in H
2
(p. 36). The bond in
methane and the bond in H
2
have very similar strengths.H
O
H
C
O
HH
O
H
2.2e Why Hybridization? Why do we need to develop this new structural model
called hybridization? If we want to form methane, why don’t we just overlap the 1s
atomic orbitals of four hydrogens with the 2s and 2p atomic orbitals of carbon? Well,
we certainly could have done just that, but the results are not very satisfying—this
approach doesn’t give us a decent approximation of the tetrahedral structure of methane.
However, let’s follow this flawed procedure. We can learn a lot from examining what is
wrong with models that are too simple. Further, what we do here approximates what
really happens in science in that we make a series of successive structural approxima-
tions in which we come increasingly close to the real methane. In Section 2.2d,we went
right to a satisfactory model for methane, thus shortcutting the intellectual process.
58 CHAPTER 2 Alkanes
Figure 2.9 shows the overlap of the occupied carbon 2s,2p
x
,and 2p
y
atomic orbitals
with four hydrogen 1s orbitals. Two predictions are clear: First, we should form one
bond from the overlap of the carbon 2p
x
orbital and one hydrogen 1s orbital
and one bond from the overlap of the carbon 2p
y
orbital and another hydro-
gen 1s orbital; second, these bonds must be at 90° to each other because of the 90°
angle between the carbon 2p
x
and 2p
y
orbitals. The third and fourth bonds are diffi-
cult to locate exactly, as they are formed from the overlap of a carbon 2s orbital with
two hydrogen 1s orbitals. Both the 2s and 1s orbitals are spherically symmetric, and
thus no directionality can be induced by the shape of the orbitals. We might guess
that these two new carbon–hydrogen bonds would be aimed so as to keep the elec-
trons in the bonds as far from each other as possible, thus minimizing repulsions.
C
O
H
C
O
H
PROBLEM 2.6 In a quick analysis there appear to be too many electrons in the region
of Figure 2.9 where the carbon 2s orbital and the two hydrogen 1s orbitals overlap.
You might think that an atomic orbital with two electrons can’t make a bond with two
other atoms, each bringing an electron. It is true that no orbital can have more than
two electrons (the Pauli principle). You are used to thinking of two-electron bonds,
and our new system is clearly more complicated than that.But there is no violation of
the Pauli principle in the unhybridized model illustrated in Figure 2.9. Explain.
Hint: Remember that overlap of n orbitals produces n new orbitals. See Problem 1.60.
Using unhybridized carbon atomic orbitals to form methane, a model that yields
the structure shown in Figure 2.9,has a fatal flaw.Modern spectroscopic methods show
that there are no 90° angle in methane and that all carbon–hydrogen
bonds in the molecule are of equal length! Knowing that the Figure 2.9 structure is
wrong, we can begin to construct a model that does a better job of describing Nature.
Don’t be offended by this—science works this way all the time.The goal is to use what
we learn from our wrong guesses to come closer next time. We can already anticipate
one problem in this model—the electrons in some of the bonds are only 90° apart.
They could be farther apart, and thus electron–electron repulsion could be reduced.
H
O
C
O
H
PROBLEM 2.7 Consider the ammonium ion (
NH
4
). Predict the hybridization of
the nitrogen and the shape of the molecule.
A second problem with the model used in Figure 2.9 might be labeled “ineffi-
cient overlap” or “wasted orbitals.” Recall from Figure 2.4 that a 2p atomic orbital
is poorly designed for efficient end-on overlap because overlap can take place only
with one lobe.The back lobes of 2p orbitals are unused and thus wasted (Fig. 2.10).
Our unhybridized model for methane has two such overlapping pairs of orbitals.
This partial picture of methane
contains two such 1s/2p end-on
overla
p
s; two rear lobes are wasted
Waste not!
Waste not!
FIGURE 2.10 End-on overlap of a 2p
atomic orbital with a 1s atomic
orbital.
1s/2p End-on overlap;
notice the wasted lobe
not involved in bonding
Wasted lobe
2p
y
2p
x
..
.
.
1s
2s
(1s is not shown)
Methane
(CH
4
)
1s/2p
Overlap
4
+
1
H
1s
1s/2s/1s
Overlap
outlined
in bold
..
90
.
6
C
(1s
2
)2s
2
2p
x
2p
y
.
.
..
..
FIGURE 2.9 A structure for methane,
CH
4
. Bonds are formed by the
overlap of four hydrogen 1s orbitals
with the 2p and 2s atomic orbitals of
one carbon atom.This model requires
some 90° bond angles and requires
the bonds to be of different lengths.
Carbon’s 1s electrons are shown in
parentheses because they are not used
in bonding.
