Jones D.S.J., Pujado P.R. Handbook of Petroleum Processing
Подождите немного. Документ загружается.

CATALYTIC REFORMING 231
The hydrogen-to-hydrocarbon (H
2
/HC) mol ratio is the ratio of mols of hydrogen in
the recycle gas to mols of naphtha charged to the unit. The recycle gas is a mixture of
hydrogen and light gases, typically 75–92 mol% hydrogen. The ratio of total recycle
gas to hydrocarbon is sometimes called the gas-to-oil ratio. Recycle hydrogen is
necessary to maintain catalyst-life stability by sweeping coke precursors from the
catalyst metals. The exact mechanism is proposed to be hydrogenation and inhibition
of polymerization. The rate of coke formation on the catalyst is a function of the
hydrogen partial pressure present. Increasing the H
2
/HC ratio increases the hydrogen
partial pressure and removes coke precursors from the metal sites, thereby increasing
stability with little effect on product quality or yields.
Except for units designed for continuous regeneration through the circulation of the
catalyst between the reactors and the regenerator, catalytic reforming units normally
will require a shutdown for regeneration every 6–12 months. This relatively long
cycle can be obtained by operating under milder conditions of high partial pressure of
hydrogen, lower reactor temperatures, and lower octane products. Continuous units
operate under severe conditions to yield high octane, high aromatics production, at
low hydrogen partial pressures, and higher reactor temperatures. Different catalysts
are used depending on the application.
Semi regenerative reformers make use of catalysts the contain platinum or plat-
inum modified by rhenium or, to a lesser extent, iridium. The support is most of-
ten gamma alumina, although there have been uses of eta alumina (4). Rhenium
or iridium is used to enhance the life of the catalyst over that observed for Pt-only
catalysts. All these catalysts are typically sulfided to minimize metal-catalyzed hy-
drogenolysis reactions that produce light gases and reduce gasoline yield. Additional
components were used on catalysts commercialized in the 1990’s. The use of two
catalysts in a SR unit; one catalyst in the front reactors and another catalyst in the
back reactors to provide maximum yield, activity, and stability was commercialized in
1994 (8).
There are two main shapes of catalysts, cylindrical and spherical. The cylindrical
catalysts are usually extruded alumina. The spherical catalysts may be formed through
a dropping method or by rolling wet, soft alumina dough. In some instances, factors
such as the resistance to flow or flow distribution concerns may cause one form to
be chosen over the other. The density of the catalysts may vary from approximately
0.5–0.8 g/cm
3
. The variability in density allows the refiner to load more pounds of
catalyst in a unit, should additional catalyst activity or stability be desired.
The process of moving catalyst from the reactors to the regenerator and back re-
quires the use of spherical catalysts, rather than extrudate, to avoid catalyst dusting
and breakage. Continuous regeneration units are operated at high severity and low
pressures to produce the greatest amount of aromatics and hydrogen possible. The
232 CHAPTER 5
catalyst is circulated at a rate such that it corresponds to about one regeneration per
week or even at a greater frequency if needed due to the rapid deactivation under these
conditions.
Typical catalysts used in these units had a composition of platinum and tin on gamma
alumina. The tin was used to reduce the hydrogenolysis activity of the platinum and
to improve yields. The reduction in metal-catalyzed cracking is also considered to
stabilize the catalyst relative to platinum only. Currently, new proprietary catalysts
are used to increase yields, lower coke make, or allow higher throughput (9).
Process flow schemes
Fixed bed semiregenerative reforming
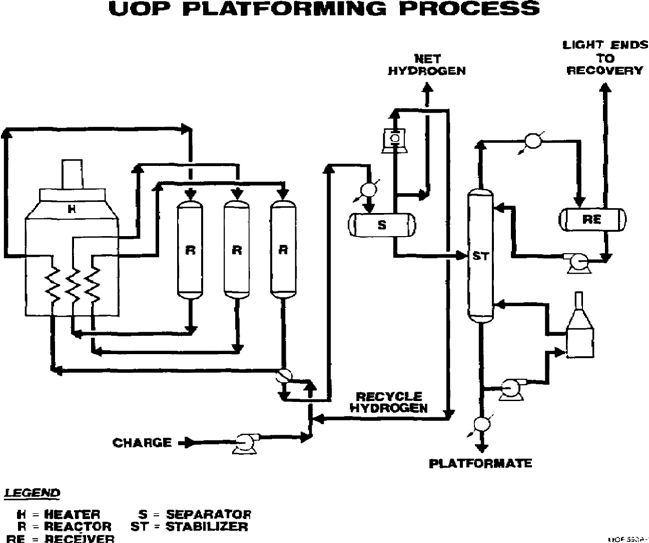
A typical SR Platforming flow diagram is presented in Figure 5.6. Feed to the unit
is mixed with recycled hydrogen gas, raised to the reaction temperature first by a
feed-effluent combined feed exchanger and then by a fired heater, and then charged to
Figure 5.6. Semiregenerative reforming process (reprinted with permission from UOP LLC).
CATALYTIC REFORMING 233
the reactor section. Because most of the reactions that occur in the Platforming process
are endothermic, the reactor section is separated into several stages, or reactors.
Interheaters are installed between these stages to maintain the desired temperature
range across the catalyst in the reactor section. Effluent from the last reactor is cooled
by the feed-effluent heat exchanger for maximum heat recovery. Air or water cooling
provides additional cooling to near-ambient temperature. The effluent is then charged
to the separation section, where the liquid and gas products are separated. A portion of
the gas from the separator is compressed and recycled back to the reactor section. The
net hydrogen produced is sent to hydrogen users in the refinery complex or for use as
fuel. The separator liquid is pumped to a product stabilizer, where the more-volatile
light hydrocarbons are fractionated from the high-octane liquid product.
Fixed bed cyclic reforming
Cyclic reforming is similar to SR reforming, but an additional reactor replaces one
of the primary reactors while that reactor is being regenerated. The frequency with
which a particular primary reactor is replaced and regenerated depends upon its rate
of deactivation. Large diameter valves and piping are used to vary the process flow
between reactors.
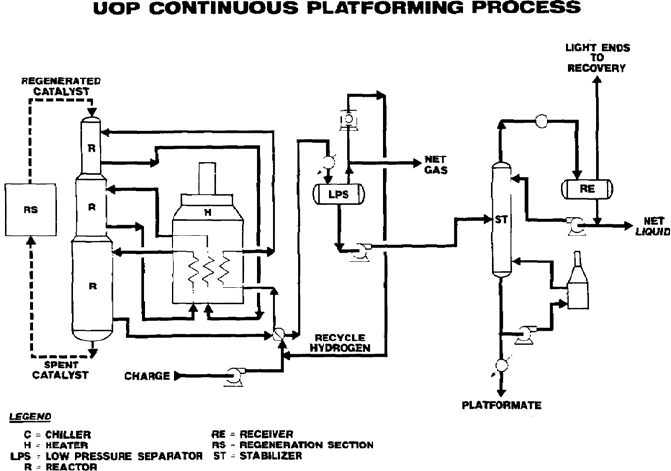
Platforming process with continuous catalyst regeneration
In parallel with bimetallic catalyst improvements and other process and regeneration
advances, UOP began to develop the CCR Platforming™ process (Figure 5.7). In the
CCR Platforming unit, partially aged catalyst in the reactors is continuously replaced
with catalyst that has been freshly regenerated in an external regenerator (CCR section)
to maintain a low average age for the reactor catalyst. Thus, the high selectivity
and high activity characteristics associated with new catalyst can be maintained at
significantly higher severities than with the SR Platforming process. For example, a
SR Platforming unit operates at a severity that steadily builds coke up on the catalyst
surface over the length of a cycle (6–18 months), at which point the unit is shut
down and the catalyst regenerated. Throughout the cycle, yields decline. Instead, in a
modern CCR Platforming unit, the catalyst is regenerated approximately every three
to seven days and the yield does not decline.
The ability to continuously regenerate a controlled quantity of catalyst is the significant
innovation of the CCR Platforming unit. The catalyst flows by gravity from the last
reactor into a catalyst collector vessel. The catalyst is lifted by either nitrogen or
hydrogen lifting gas to a catalyst hopper above the regeneration tower. Catalyst then
flows to the regeneration tower, where the catalyst is reconditioned. Regenerated
catalyst is returned to the top of the reactor stack by a transfer system similar to
that used in the reactor-to-regenerator transfer. Thus, the reactors are continuously
supplied with freshly regenerated catalyst, and product yields are maintained at fresh
234 CHAPTER 5
Figure 5.7. CCR Platforming process (reprinted with permission from UOP LLC).
catalyst levels. The regeneration and reactor sections of the unit are easily isolated to
permit a shutdown of the regeneration system for normal inspection or maintenance
without interrupting of the Platforming operation.
A few years after the introduction of the UOP CCR Platforming process, another con-
tinuously regenerable process design was offered by the Institut Fran¸cais du P´etrole.
Though similar to CCR Platforming, the continuous reforming units designed by the
Institut Fran¸cais du P´etrole differ most notably in that the reactors are located side-
by-side and the catalyst transfer is effected through transfer piping between reactors.
Advantages of CCR Platforming
From both an economic and technical standpoint, the CCR Platforming process is
superior to the SR and cyclic reforming processes. The CCR Platforming unit allows
for low-pressure operation, leading to higher yields. At these conditions, the SR
Platforming catalyst is completely deactivated after only a few days of operation.
Both the hydrogen and C
5
+yields are maximized with the CCR Platforming process.
Since the number of cyclic reformers is small relative to CCR Platforming process
units and SR process units, the following comparison will focus on contrasting CCR
Platforming units and SR units.

CATALYTIC REFORMING 235
Table 5.6. Relative severities of CCR versus SR
Platforming units
Operating mode SR CCR
Charge rate, barrels/day 20,000 20,000
LHSV, h
−1
Base Base × 1.8
H
2
/HC Base Base × 0.5
RONC 97 102
Reactor pressure, psig Base Base-50
Separator pressure, psig Base Base-145
Cycle life, months 12 Continuous
High yields and constant yields are important in the economics of reforming. As
the catalyst is deactivated by coke deposition in the SR Platforming process, the
yields begin to decline. With the CCR Platforming process, the reformate, aromatics,
and hydrogen yields remain consistent and constant. This is particularly important
for downstream users. The CCR section ensures proper redispersion of the metals
and chloride balance to maintain fresh catalyst activity. CCR Platforming units have
higher on-stream efficiency and are able to handle upset scenarios without long-term
shutdown or significant decline in performance.
Table 5.6 shows the relative operating severities for the SR and CCR Platforming
units. The CCR Platforming unit operates at higher severity and lower reactor catalyst
inventory. In addition, the CCR unit runs continuously compared to 12-month SR
Platforming cycle lengths.
Typical product yields for the SR and CCR Platforming units operating at the con-
ditions presented in Table 5.6 are shown in Table 5.7. Many of the benefits of
CCR Platforming are demonstrated in Table 5.7. More and higher-purity hydro-
gen is produced. The higher severity of the CCR Platforming unit results in simi-
lar liquid volume for the two units. However, the reformate produced by the CCR
Platforming is more valuable than that produced by the SR Platforming unit. Tak-
ing into account both the higher octane value and the increased on-stream efficiency
of the CCR Platforming unit, 80 million more octane-barrels, or 11.4 million more
Table 5.7. Yield comparison of CCR versus SR
Platforming units
SR CCR Delta
Hydrogen yield, SCF/bbl 1,085 1,709 +624
Hydrogen purity, mol% 80 92.6 +12.6
C
5
+ yield, LV% 79.3 79.4 +.1
C
5
+ yield, wt% 85.2 88.2 +3
Octane-barrel, 10
6
bbl/yr 513 583 +80

236 CHAPTER 5
Table 5.8. Economic summary
Description SR CCR
Gross key product value, $MM/yr 120 141
Raw materials less by-products, $MM/yr 98 103
Consumables, MM$/y 0.3 0.75
Utilities, $MM/yr 2.8 6.2
Total fixed costs, $MM/yr 5.5 6.5
Capital charges, $MM/yr 3.5 5.2
Net cost of production, $MM/yr 110 122
Pretax profit, $MM/yr 10 20
Pretax ROI, % 30 41
Payout period, (gross) years 1.5 1.3
metric octane-tons, are produced per year with the CCR Platforming unit than with
the SR Platforming unit. Octane-yield is defined as the product of the reformate yield,
octane, and operating days.
A summary of the operating revenues and costs expected for the SR and CCR Plat-
forming units in shown in Table 5.8. The nomenclature follows standard definitions.
The economics of the CCR Platforming process are superior as a direct result of the
differences in operating severity and flexibility of the two modes of operations. The
CCR Platforming unit produces more valuable reformate at 102 RONC versus the SR
Platforming reformate at 97 RONC. On-stream efficiency of the CCR Platforming
unit is 8,640 hr per year compared to about 8,000 hr per year for the SR Platforming
unit. Although the CCR Platforming utility costs are higher than those for the SR
Platforming unit, these costs are offset by the increase in both product quantity and
value as demonstrated by pretax profit and return on investment.
Catalysts and suppliers
For detailed updated lists of catalysts and suppliers consult the periodic reviews
published by the Oil and Gas Journal.
The main catalyst suppliers are:
Axens/IFP Group Technologies
Criterion Catalyst Co.
Exxon Research & Engineering Co. (ERECO)
Indian Petrochemicals Corp., Ltd.
Instituto Mexicano del Petr´oleo (IMP)
UOP LLC
Some of the catalyst suppliers may restrict availability to process licensees only.
CATALYTIC REFORMING 237
References
1. A. L. Huebner, “Tutorial: Fundamentals of Naphtha Reforming,” AIChE Spring Meeting
1999, Houston, TX, 14–18 March 1999.
2. American Petroleum Institute Research Project 45, Sixteenth Annual Report, 1954.
3. E. L. Marshall and K. Owen, eds., Motor Gasoline, The Royal Society of Chemistry,
London, 1995, p. 8.
4. G. A. Mills, H. Heinemann, T. H. Milliken, and A. G. Oblad, Ind. Eng. Chem., 1953:45;134–
137.
5. D. R. Stull, E. F. Westrum, and G. C. Sinke, The Chemical Thermodynamics of Organic
Compounds. John Wiley & Sons, New York, 1969.
6. S. M. Augustine, G. N. Alameddin, and W. M. H. Sachtler, J. Catal., 1989:115(1); 217–232.
U.S. Pat. 4,469,812 (September 4, 1984) C. M. Sorrentino, R. J. Pellet, R. J. Bertolacini
(to Standard Oil Company—Indiana).
7. J. A. Weiszmann, In Meyers, ed., Handbook of Petroleum Refining Processes, McGraw-
Hill, New York, 1986, p. 31.
8. M. D. Moser, D. H. Wei, R. S. Haizmann, CHEMTECH, October, 1996, pp. 37–41.
Chapter 6
Fluid catalytic cracking
Warren Letzsch
∗
Crude oil comprises hundreds of molecules that boil over a wide temperature range.
The lighter products can be separated directly by distillation into LPG, gasoline,
naphtha, kerosene, and diesel fuels. Heavier products (BP > 650
◦
F/344
◦
C) include
vacuum gas oils and resids. Thermal and catalytic cracking processes in petroleum
refining reduce the molecular weight of these heavier constituents and produce more
valuable lighter products such as LPG, gasoline and diesel fuels.
Catalytic cracking was first commercialized in 1936 by Eugene Houdry. This fixed bed
process was a major improvement over the thermal cracking processes it replaced due
to the improved yield distribution and superior product properties. Multiple vessels
were utilized that alternated between cracking, stripping, regeneration, and purge
cycles. This configuration was quickly replaced by a moving bed reactor and a separate
regenerator or kiln that first used a bucket lift to move the pelleted catalyst followed
later by a pneumatic air lift system. The last of these units was built around 1960.
Standard Oil of New Jersey developed their own cracking process rather than pay the
large royalty being asked at the time. They commercialized the fluid catalytic cracking
(FCC) process in three years, starting in 1939 and culminating in 1942 with the start-
up of PCLA#1 at their Baton Rouge, Louisiana refinery. The inherent superiority of
the fluid process to transfer both heat and catalyst ultimately made it the catalytic
cracking process of choice.
Many different designs of fluid catalytic crackers have been introduced over the years.
Table 6.1 is a list of the various FCCU configurations and the approximate year of
their commercial introduction.
Fluid catalytic cracking has evolved considerably over the more than 60 years since
its inception. As seen in Figure 6.1 these changes have encompassed all aspects of
*Senior Refining Consultant, Stone & Webster Inc—A Shaw Group Company.
239

240 CHAPTER 6
Table 6.1. Evolution of fluid catalytic crackers
Commercial fluid catalytic crackers
1942 Model I upflow
1943 Model II downflow
1945 Sinclair design
1947 Model II side by side
1951 Kellogg orthoflow A
1952 Exxon Model IV
1953 Kellogg orthoflow B
1955 Shell two stage reactor
1956 UOP straight riser (SBS)
1958 Exxon riser cracker
1961 HOC cracker (Phillips)
1962 Kellogg orthoflow C
1967 Texaco design
1971 Gulf FCC process
1972 Exxon flexicracker
1972 Amoco ultracracking
1973 UOP high efficiency design
1973 Kellogg orthoflow F
1981 Total petroleum resid cracker
1982 Ashland/UOP RCC unit
1985 IFP R2R
1990 Kellogg/Mobil HOC
1991 Deep catalytic cracking (RIPP and S&W)
1993 Exxon flexicracker III
1996 MSCC UOP/Coastal
2002 Catalytic pyrolysis process
the process as it has adapted to meet ever-changing demands and to accommodate
new technologies.
The initial units (1940s) were tall, had dense bed reactors and were made of carbon
steel. Dilute phase catalyst coolers and regenerator steam coils were employed to limit
the regenerator temperatures. Recycle rates of 100–150% were needed to achieve the
desired conversions. Later designs (late 1940s to early 1950s) were undertaken to
reduce the height of the crackers, make them more compact and cater to the many
small refiners around the United States.
In the late 1950s side by side designs with straight feed risers were introduced to im-
prove gasoline selectivity. Residue cracking in fluid cracking units was first practiced
in the early 1960s. This unit was designed for 100% atmospheric bottoms and had
large amounts of heat removal.
Catalytic cracking was truly revolutionized in the early 1960s with the advent of zeo-
lite containing fluid cracking catalysts. Catalyst activities were raised by an order of
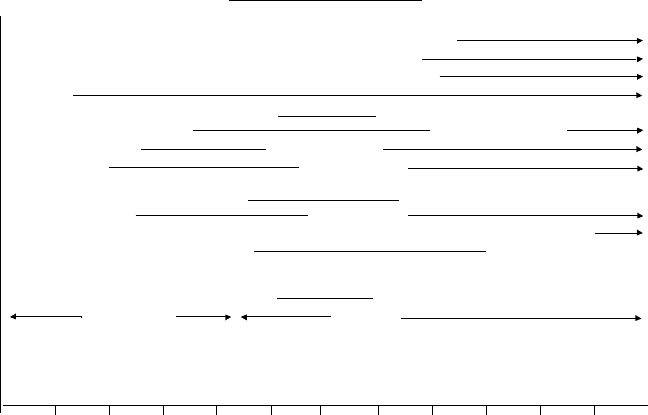
FLUID CATALYTIC CRACKING 241
Processing Objectives
Gasoline
Resid
Olefins
Octane
Environmental
CATALYSTS
EQUIPMENT
Carbon Steel Stainless Steel
Dilute Coolers & Bed Coils
Dense Bed Coolers
Dense Bed Reactor Riser Cracking
Decreasing Recycle
Advanced Feed Injection
Reduce Dilute
Phase Cracking
Partial CO Burn
Full CO Burn
Amorphous Zeolite
Low Alumina High Alumina
Spray DriedGround
Increasing Concentrations
Shape Selectivity
DeSoxCO Promotion
1942 1952 1972 1982 19921962
YEAR
OPERATING MODE
Controlled Recycle
Figure 6.1. Fluid catalytic cracking development.
magnitude and units needed to be redesigned to take full advantage of the new catalyst
technology. These design changes included the elimination of reactor dense beds and
the use of the feed riser as the sole conversion vessel. Recycle was greatly reduced
and replaced with more fresh feed. It was found that coke left on the regenerated
catalyst impaired the catalyst’s activity and selectivity and the average carbons on
regenerated catalyst were reduced from 0.3–0.5 wt% to 0.1 wt% or less. Complete
CO combustion in the regenerator was introduced to provide the needed regener-
ator burning conditions which necessitated higher regenerator temperatures. Alloy
internals replaced the carbon steel and chrome-moly hardware in the regenerator and
the regenerated catalyst standpipe. Catalyst inventories were minimized due to the
favorable coke burning kinetics and modifications in the regenerator design (1970s).
In the early 1980s several new resid cracking designs were introduced. The spent
catalyst regeneration was staged in these designs and dense bed catalyst coolers were
optional. These coolers have also been placed with single stage regenerators for
residual catalytic cracking units. The growth of this segment of the catalytic cracking
process is shown in Figure 6.2. Over two million barrels per day of on purpose
resid crackers have now been licensed and another million barrels of FCC capacity
processes some resid along with their normal gas oils. Typical yields from resid
cracking along with the feedstock properties are given in Table 6.2.
