Jackson M.J. Micro and Nanomanufacturing
Подождите немного. Документ загружается.


Manufacturing High Aspect Ratio Microstructures 101
process can achieve. As demand grew for the production of smaller
and smaller feature sizes, the requirements for etching substrates
moved from isotropic to anisotropic; thus ion beam etching (IBE)
was developed. IBE, however, had its own problems such as poor
selectivity between mask and substrate leading to the production of
components that did not meet specifications. Hence, chemical as-
sisted ion beam etching (CAIBE) was developed to counteract this
particular problem. The latest technique in the evolution of this
process is called ion beam assisted radical etching (IBARE). This
process has both high selectivity and high anisotropy, making it an
ideal way of creating high aspect ratio trenches with parallel sides
and square faces.
3.3.1 Ion Beam Assisted Radical Etching
In this process, the plasma is ignited by applying a radio fre-
quency voltage between two electrodes in a dielectric gas. Electrons
have enough energy to jump from their ground state and escape the
atomic bond, making the atom ionized. This results in a large num-
ber of charged particles in the chamber, namely, free electrons and
ions.
The electrodes are positive (anode) and negative (cathode), so
the electrons (negatively charged) migrate rapidly towards the anode
and ions (positively charged) move towards the cathode. During
these motions collisions with the gas occur generating neutrals, radi-
cals,
electrons, ions, and photons. Ions generated from the plasma
are strongly attracted toward the cathode. They can travel with
enough energy so that on impact with the cathode electrons from the
surface of the electrode they are released; this is a secondary genera-
tion of free electrons. Subsequently, newly created free electrons are
attracted toward the anode, as they pass through the chamber they
collide with neutrals and other particles in the gas creating further
ionization; thus secondary generation of free electrons and subse-
quent collisions sustain the plasma. Electrons usually acquire ener-
gies between leV and lOeV before colliding with other particles,
which are dependent on the gas pressure of the system.

102 Micro-and Nanomanufacturing
Electrons can be involved in collisions where they carry insuffi-
cient energy to cause ionization. The energy they have acquired is
used up in collisions within the gas, specifically by making orbiting
electrons jump energy shells. Because this energy is not enough to
cause ionization the electron decays back to its original energy level
prior to impact. Decay occurs because the electron cannot exist at
its new orbit for long, and the extra energy is released in the form of
a photon. Release of these photons is the source of the glow of the
plasma; the color of the plasma depends on the type of atom and the
magnitude of the decaying process.
3.4 Characteristics Of The Plasma
3.4.1 The Sheath Region
Above the cathode, the region is dense with electrons that have
just been liberated. They have low energy and there are few gas par-
ticles available for collision. Hence, there is little opportunity for
photon release. Therefore no light is being emitted from this region
and it is known as dark space. Similarly the anode has many elec-
trons around it and there are few chances for collision to cause light
emission; and this region is also known as dark space. The width of
the dark space depends on the chamber pressure; at low pressures
the mean free path of the electrons increases and the width of the
dark space increases. By controlling gas pressure within the cham-
ber, the energy with which the ions strike the surface can be con-
trolled.
Substrates are placed on the cathode to take advantage of the in-
cident ions. These substrates can be insulating, ion bombardment
ejects electrons and a charge builds up on the insulator to the point at
which the plasma can no longer be sustained. Hence an alternating
current at a radio frequency is used to drive and sustain the process.
The reversal of current maintains a charge balance, but if one elec-
trode is to be used then it must be energetically favored in some
way. The potential difference between electrodes is important be-

Manufacturing High Aspect Ratio Microstructures 103
cause this field accelerates ions providing them with more impact
energy. If the electrodes have equal area then they have the same po-
tential, increasing the area of one electrode changes the potential of
that electrode and overall the potential difference is increased. If the
area of the anode is increased, which is usually achieved by connect-
ing the chamber walls to the anode, the potential is at a maximum
thus favoring ion bombardment of the cathode.
3.4.2 Boundary Region
When moving from the plasma glow to the sheath there is a dis-
tinct dividing line called the boundary region, or boundary layer. It
can be considered as a surface emitting particles from the plasma.
Ions are directed by the field lines existing between the electrodes,
this causes particles to leave the boundary layer perpendicular to the
substrate. The ions can now be considered as a collimated stream of
particles. As ions leave the boundary layer they pass through the
sheath on their way to the substrate. The sheath region contains par-
ticles so that ion collisions are possible; if they occur it will cause
ions to deflect and they will no longer travel perpendicular to the
boundary layer or substrate. In addition, the collision has made ions
lose energy and therefore reduced the effectiveness of impact when
it reaches the cathode or substrate. These are two important effects
known as the ion angular distribution function (lADF), which is a
measure of how collimated the particles are before they reach the
substrate, and the ion energy distribution function (lEDF), which is a
measure of how much energy the ions have remaining. Radical colli-
sions in this region are unimportant because their initial direction is
isotropic so that further collisions producing random motions have
no effect on the overall direction.
3.5 Etching of Microstructures
Etching can occur if a chemical reaction producing a gaseous, or
high vapor liquid, or solid, is energetically favored. In other words.

104 Micro-and Nanomanufacturing
if a reaction can lower the overall energy of the system, then it is
possible for it to be energetically favorable to cause etching. In high-
pressure plasma reactors etching of Silicon, Si, is achieved using
Chlorine, C, and Fluorine F. After the reactions have taken place the
system must be at a lower energy; this is achieved because the radi-
cals (in this case F) are freely available and the bond energy between
Si and F is less than the bonding energy between Si and Si. In other
words it is energetically more favorable for the reaction to take
place. The reaction produces a SiF molecule resulting in an overall
net loss of Si; this is etching. A typical gas used would be CF4,
which will not etch silicon directly. F radicals are produced by ioni-
zation in the plasma. Other bonding groups in the system are Si2,
SiF2,
and SiF4. The Si substrate surface is coated with F, a bulk Si
atom is bonded to an Si surface atom which is itself bonded to two F
atoms, making SiF2. However, because it is bonded to the wafer it is
not free to move. An incoming F atom can replace the Si-Si bond
with SiF2, because Si already shares another surface F. The SiF2 then
leaves the surface and a small portion of Si has been removed. Al-
ternatively the incoming F atom could attach itself to SiF2, embed-
ded on the surface making SiFa, subsequently another F will join the
system producing SiF4, which will then leave the surface.
In the case of reactive ion etching a physical process for model-
ing the etching mechanism has been proposed. Chlorine gas in the
forms,
CI or CI2, can etch undoped silicon but only very slowly. N-
type doped silicon is etched spontaneously by CI, only. In the case
of undoped silicon, chlorine atoms migrate toward the surface and
chemisorbs. However, this does not break the Si-Si bond. Once a
layer of chlorine builds on the surface further absorption is pre-
vented by stearic hindrance. After a short time this surface becomes
negatively charged, in this case ionic bonding between the Si and CI
can occur. When this happens chemisorption sites are freed, thus in-
creasing the chances that CI atoms will penetrate the bulk and pro-
duce volatile SiCl. This process is dramatically enhanced by ion
bombardment, so areas subjected to ion bombardment etch much
faster than other regions and this produces anisotropic etch profiles.
However, the more heavily doped the substrate the more pronounced
the charging effect is. If reasonable etch rates are to be achieved then

Manufacturing High Aspect Ratio Microstructures 105
high doping means that undercut of the sidewalls is increased and
etching characteristics are no longer anisotropic.
3.5.1 Etching Phenomena
For IBARE techniques regardless of plasma chemistry, smaller
width trenches are etched more slowly than wider trenches, the con-
trolling factor being the aspect ratio of the trench rather than its
depth or width. One method of reducing this effect is to change the
geometry of the shape and control the aspect ratio.
3.5.2 Inhibitor Depletion in a Trench
In small trenches the supply of inhibitors is reduced partly due to
the narrow opening. The resulting depletion of inhibitors can lead to
a higher etch rate, and inverse reactive ion etching (RIE) lag should
be the result (i.e., the smaller trench is etched more quickly due to
radical ion etching lag).
3.5.3 Radical Depletion in a Trench
Radicals can etch a surface only if they are close by when it is ex-
posed to ions. Radicals can arrive at this site either by gas transport
or by surface flow. Radicals will attack any available site, in this
case the whole surface. Therefore, surface transport is not possible
because the path of each radical is blocked by its surrounding
neighbors. If this is the case the only way for radicals to enter the
trench is by transport in the bulk gas. This is called microloading;
the etch rate decreases and is inversely proportional to the amount of
substrate exposed to the plasma. Generally this effect is present
when radical density is exposed to an unusually high substrate area,
and gives rise to inverse RIE lag.
Now consider a masked wafer with a hole in the center. Surface
transport is now possible because there is no surface silicon to con-
sume the radicals. This is the so-called micro-loading effect, which

106 Micro-and Nanomanufacturing
occurs when the reactant density is depleted as a result of an exces-
sive substrate load. Etch rate will decrease inversely to the silicon
area which is exposed to the plasma glow, giving inverse RIE lag.
However, etch rate seems to be influenced by the geometry of the
mask shape. For a long, small silicon structure etching is faster than
the square of the same area. Hence, it is important to know the
transport mechanism so that RIE lag can be predicted. It is therefore
important to know how the radicals reach the trench because this de-
termines whether there will be any RIE lag.
3.5.4 Volume Transport
The gas flow within the system is one of three types: viscous, mo-
lecular, or a combination of both called transitional. The Knudsen
number is defined as the ratio of the mean free path of a molecule, X.,
to a characteristic dimension, d, of the channel through which the
gas is flowing, (a) Viscous flow - is characterized by a small Knud-
sen number (high pressure) because the high-pressure particle den-
sity is very high and particles are most likely to collide with each
other rather than the wall. Therefore, most collisions in this flow
type are intermolecular and characterize the gas as X/d < 0.01. (b)
Molecular flow - is characterized by large Knudsen numbers (low
pressure) because particles are more likely to collide with the walls
rather than each other because low pressure reduces the density.
Upon collision with a wall the particle or molecule is often ab-
sorbed. After a short time the molecule desorbs from the wall in a
random direction, these collisions characterize the nature of the gas.
Molecular flow restrictions result from the geometry being yd> 1.
(c) Transitional flow - characterized by median Knudsen numbers
(mid-pressures). Collisions with the wall and molecules are equally
as likely in this range. 0.01 < A./d < 1.
In a realistic situation where a substrate is covered by a mask it
is possible for radicals to move on the mask; therefore surface ge-
ometry determines radical supply to the bottom of the trench. Ions in
a trench can become depleted for a number of different reasons, the
most important is ion deflection. There are different forces that con-
tribute to the final force that is deflecting the ions. It is possible for

Manufacturing High Aspect Ratio Microstructures 107
the mask to become charged and the charge estabhshed on this sur-
face can deflect ions. Charged particles are attracted towards solid
bodies with a force inversely proportional to the square of their dis-
tance. In the case where a particle enters a trench the particle is sub-
jected to two forces, one from each wall. Any ions that are captured
by the walls due to image forces are unable to assist in etching at the
base of the trench. This effect is usually observed in small trenches
and can be reduced by increasing ion velocity; this is because the
ions have less time to become captured although that in turn can lead
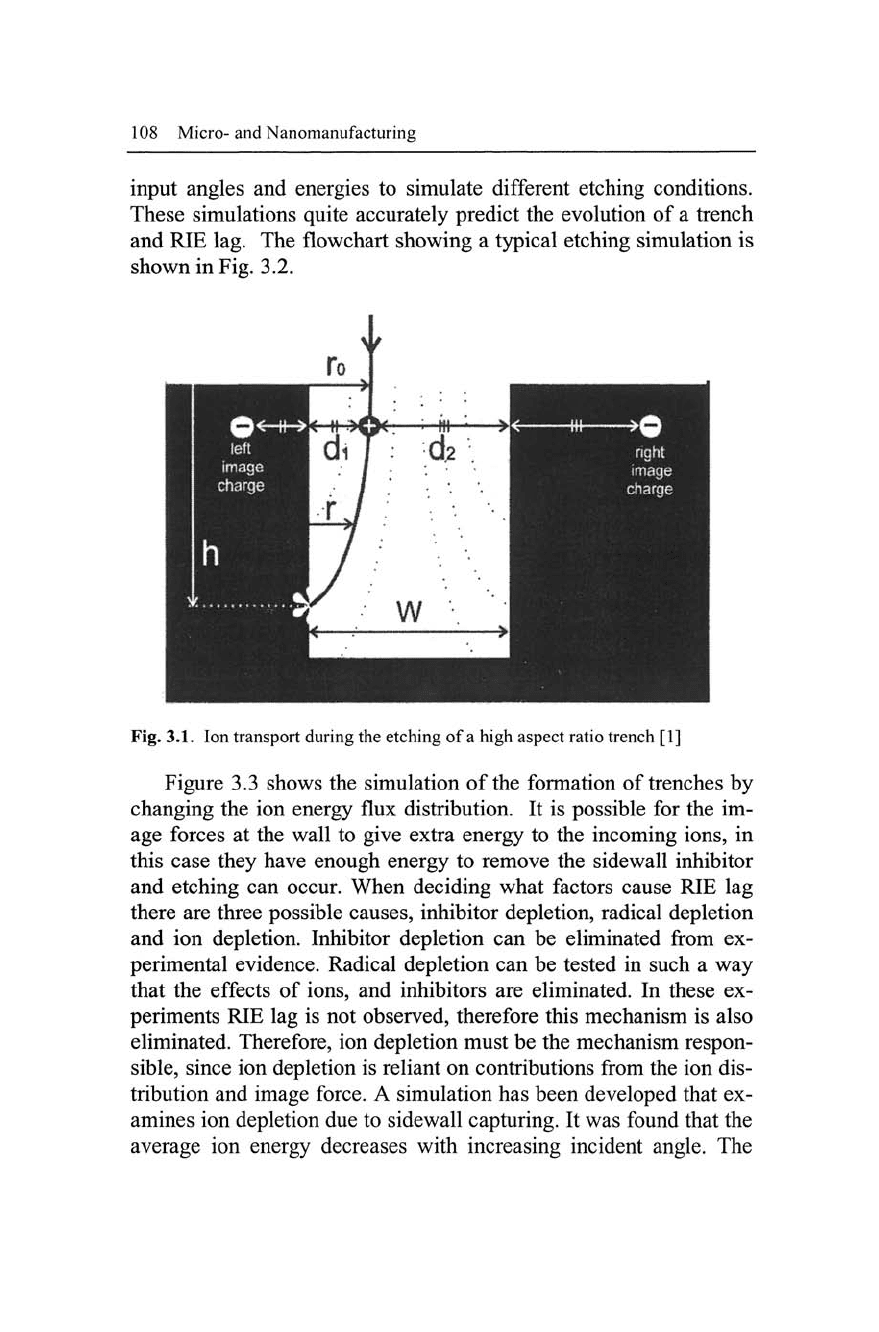
to increased sidewall etching (Fig. 3.1).
The forces between charged particles (known as Coulomb and
Lorentz forces) are not considered to be significant because the
chances of two ions existing in a trench together are small, however
the significance increases as the density increases. Initially when the
material is placed on the cathode the distance between the boundary
layer and substrate is constant and the associated electric field is
constant; therefore etching ions have equal properties along the sub-
strate length. However, when trenches form the distance from the
boundary layer to substrate changes locally, and in turn the electric
field also changes. It becomes distorted by an amount related to the
trench size. This is yet another cause of RJE lag, although insignifi-
cant at the microscale. In this case RIE lag is initiated by the distor-
tion that intensifies the electric field around it. Therefore, a concen-
trated number of ions build up to attack the inhibitor. After a short
time the charge builds up and incoming ions are repelled to the point
where they spread out and widen the trench producing inverse RIE
lag.
Not only is it possible for ions to be depleted by forces attracting
them to sidewalls, it is possible accidental collisions with other ions
in the plasma glow and plasma sheath region (ion dispersion) can
send ions to the sidewalls causing ion depletion. Of course these col-
lisions alter the ion energy as well. Finite element models have been
constructed to simulate the etching process, thus reducing time spent
on optimizing the process. The substrate is divided into elements and
these elements are subjected to incident energies simulating ion im-
pact. When the energy exceeds a certain level the element disap-
pears,
it is assumed that immediately after an ion has removed the
inhibitor a radical etches the substrate. It is possible to alter the ion
108 Micro-and Nanomanufacturing
input angles and energies to simulate different etching conditions.
These simulations quite accurately predict the evolution of a trench
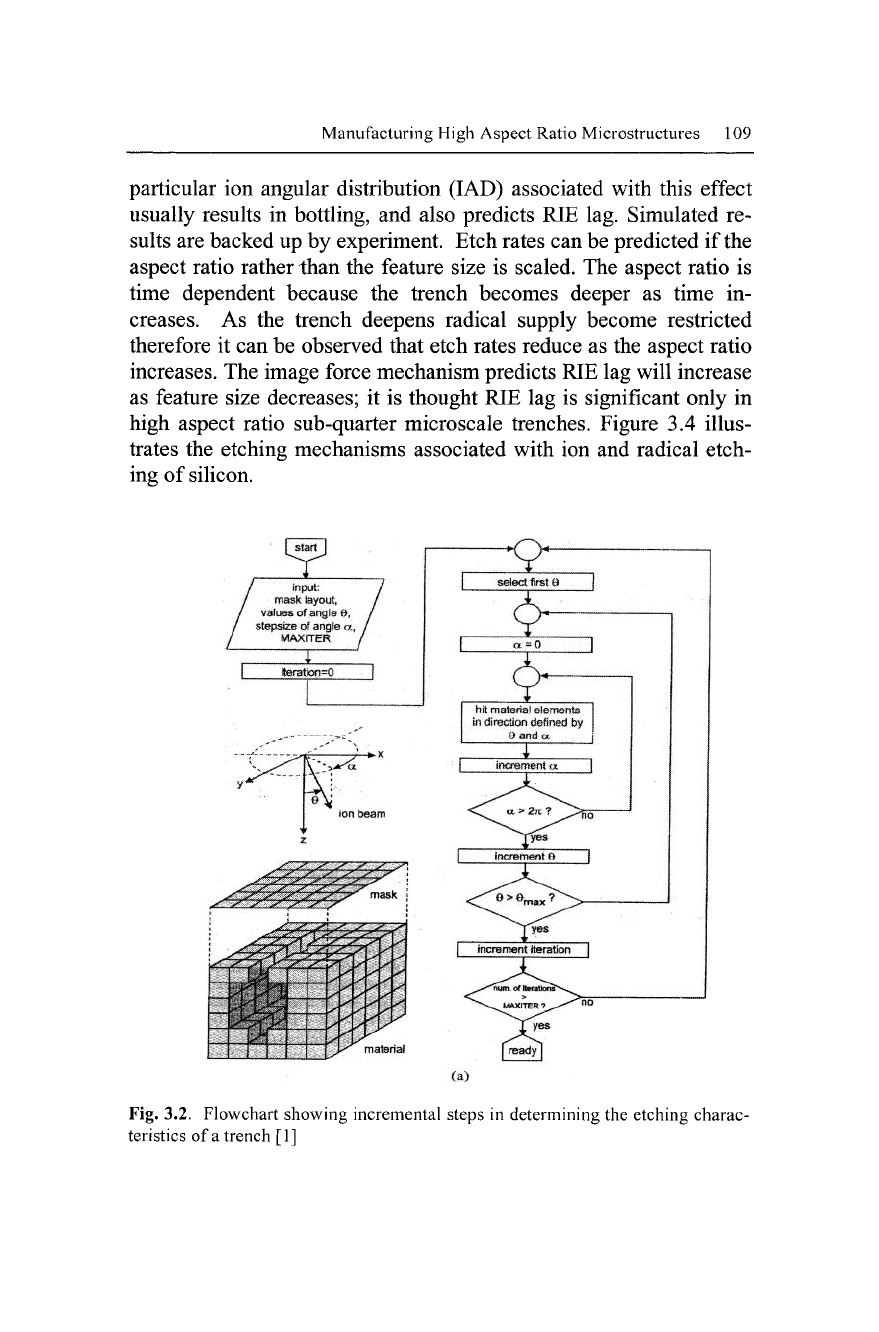
and RIE lag. The flowchart showing a typical etching simulation is
shown in Fig. 3.2.
Q<
H
>
>
ieft
image
charge
h
Y
To
I
IT
1 T
^ , ;
„„,
Ml '*^ \
^ • »"-fn •""——;^
^ w
4, %
^ ^
<
w >Q
nght
image
charge
Fig. 3.1. Ion transport during the etching of a high aspect ratio trench [1]
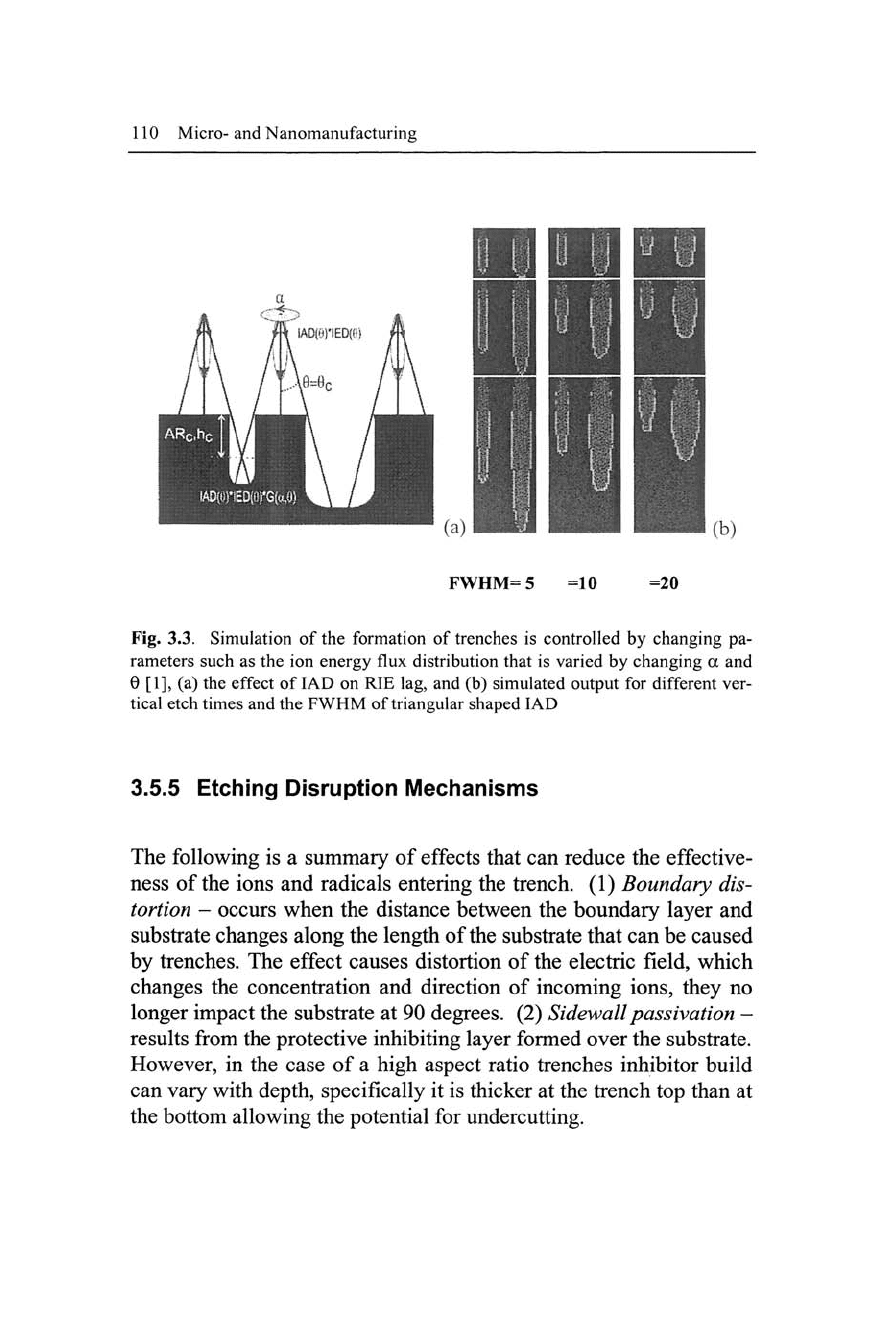
Figure 3.3 shows the simulation of the formation of trenches by
changing the ion energy flux distribution. It is possible for the im-
age forces at the wall to give extra energy to the incoming ions, in
this case they have enough energy to remove the sidewall inhibitor
and etching can occur. When deciding what factors cause RIE lag
there are three possible causes, inhibitor depletion, radical depletion
and ion depletion. Inhibitor depletion can be eliminated from ex-
perimental evidence. Radical depletion can be tested in such a way
that the effects of ions, and inhibitors are eliminated. In these ex-
periments RIE lag is not observed, therefore this mechanism is also
eliminated. Therefore, ion depletion must be the mechanism respon-
sible,
since ion depletion is reliant on contributions from the ion dis-
tribution and image force. A simulation has been developed that ex-
amines ion depletion due to sidewall capturing. It was found that the
average ion energy decreases with increasing incident angle. The
Manufacturing High Aspect Ratio Microstructures 109
particular ion angular distribution (IAD) associated with this effect
usually results in bottling, and also predicts RIE lag. Simulated re-
sults are backed up by experiment. Etch rates can be predicted if the
aspect ratio rather than the feature size is scaled. The aspect ratio is
time dependent because the trench becomes deeper as time in-
creases. As the trench deepens radical supply become restricted
therefore it can be observed that etch rates reduce as the aspect ratio
increases. The image force mechanism predicts RIE lag will increase
as feature size decreases; it is thought RIE lag is significant only in
high aspect ratio sub-quarter microscale trenches. Figure 3.4 illus-
trates the etching mechanisms associated with ion and radical etch-
ing of sihcon.
/ input:
mask layout,
vafues of angle B,
stepsize of angle
os.,
MAXtTER /
X
ion beam
^
^^^^^^masR
19-
5
hit materia! elements
in diresctian defined by
0
and
a
Fig. 3.2. Flowchart showing incremental steps in determining the etching charac-
teristics of
a
trench [1]
110 Micro-and Nanomanufacturing
FWHM=5 =10
=20
Fig. 3.3. Simulation of the formation of trenches is controlled by changing pa-
rameters such as the ion energy flux distribution that is varied by changing a and
6 [1], (a) the effect of IAD on RIE lag, and (b) simulated output for different ver-
tical etch times and the FWHM of triangular shaped IAD
3.5.5 Etching Disruption IVIechanisms
The following is a summary of effects that can reduce the effective-
ness of the ions and radicals entering the trench. (1) Boundary dis-
tortion - occurs when the distance between the boundary layer and
substrate changes along the length of the substrate that can be caused
by trenches. The effect causes distortion of the electric field, which
changes the concentration and direction of incoming ions, they no
longer impact the substrate at 90 degrees. (2) Sidewall passivation -
results from the protective inhibiting layer formed over the substrate.
However, in the case of a high aspect ratio trenches inhibitor build
can vary with depth, specifically it is thicker at the trench top than at
the bottom allowing the potential for undercutting.
