Hoffman D.M., Singh B., Thomas J.H. (Eds). Handbook of Vacuum Science and Technology
Подождите немного. Документ загружается.

734 Chapter 5.6: Common Analytical Methods for Surface and Thin Film
Electron spectroscopy provides qualitative analysis and quantitative analysis of
material surfaces, thin-film elemental depth profiling, chemical surface analysis,
and elemental mapping. These features and the instrumentation required to pro-
duce these effects are discussed in the following sections.
5.6.2.2 Qualitative Analysis
Qualitative analysis is performed through the analysis of the electron cloud emit-
ted from a material surface excited by an electron beam or a low-energy X-ray
source such as Mg K^ or Al K^ X-rays (1253 or 1468 eV, respectively). Materials
that can be analyzed include metals (conductors), insulators (polymers, inorganic
insulators, etc.), and semiconductors. Some organic materials can be damaged by
electron or X-ray irradiation, but in general, most materials can be reliably quali-
tatively analyzed. As described next, analysis is straightforward, and spectral in-
formation can be obtained from well-documented handbooks
[1-9}.
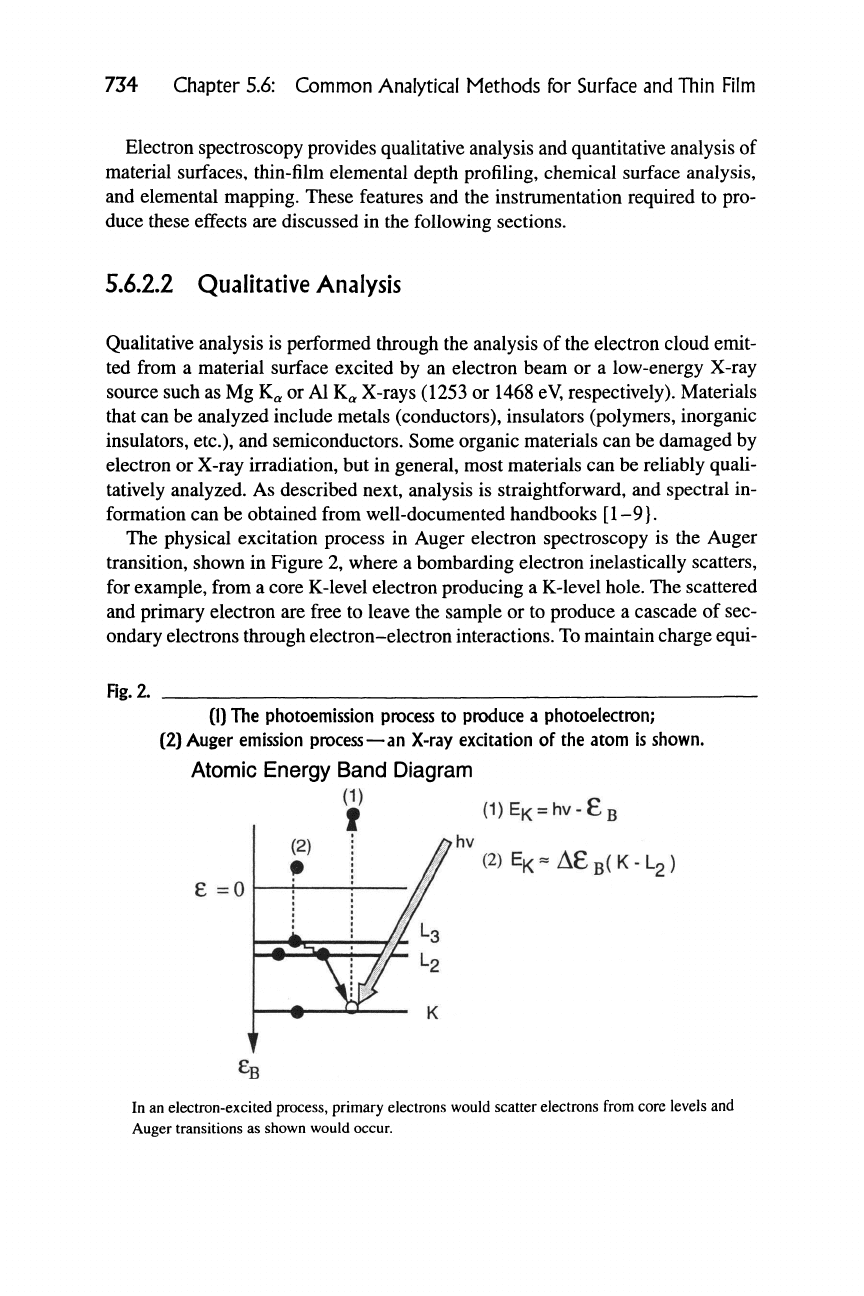
The physical excitation process in Auger electron spectroscopy is the Auger
transition, shown in Figure 2, where a bombarding electron inelastically scatters,
for example, from a core K-level electron producing a K-level hole. The scattered
and primary electron are free to leave the sample or to produce a cascade of sec-
ondary electrons through electron-electron interactions. To maintain charge equi-
Rg.2.
0) The photoemisslon process to produce a photoelectron;
(2) Auger emission process—an X-ray excitation of the atom is shown.
Atomic Energy Band Diagram
•^ (1)E,^ = hv-8i
(1)EK = hv-8B
(2)EK«A8B(K-L2)
In an
electron-excited
process,
primary
electrons
would
scatter
electrons
from
core
levels
and
Auger transitions
as
shown
would
occur.

5.6.2 The Electron Spectroscopies 735
librium in the atom, one of two relaxation processes may occur. An electron from
a shallower core level, Li, will lose its energy by filling the deep-level hole in the
K core level (generated by the scattering event) emitting an X-ray of energy E^ ~
EL-
Another process, known as the Auger process, can occur where the K hole is
filled by the electron from the L2 level by losing energy. The energy loss is syn-
chronously coupled to an electron in a nearby level,
L3.
The
L3
electron is excited
to a kinetic energy approximately equal to the energy loss,
EK
~
^L
and may leave
the atom. The excited Auger electron is designated by the three levels involved in
the process, KLL. The energy associated with the Auger transition is unique to the
atom of origin and is used for chemical analysis. Typically, the core level is des-
ignated by standard spectroscopic notation (K, L12, L23,
Note that Auger processes can also be excited by X-rays.
Since a large number of secondary and primary electrons are observed in the
Auger spectrum, the signal is generally differentiated with respect to the kinetic
energy. This removes a large portion of the background and produces a very sen-
sitive method of observing the Auger transitions.
If the material is excited by nearly monochromatic X-rays, core-level electrons
can be excited. The excitation process must conserve energy, and hence the ki-
netic energy of the excited electron
Ey^
— hv
—
E^ where E^ is the binding en-
ergy of the core-level electron designated by spectroscopic notation, K, Li, L2,
M3,
M4, etc. The core-level excited electron has a unique binding energy, de-
pending on the atom of origin, and is used for chemical identification.
5.6.2.3 Surface Sensitivity Mechanism
When an electron leaves an excited atomic level in a material, it loses its origi-
nal kinetic energy primarily through electron-electron interactions and thermal
losses (phonons). The original electron kinetic energy is indicative of the atom of
origin (qualitative analysis). After the electron loses energy, it is no longer readily
identified with the original excitation and is called a loss electron. The range the
electron traverses before losing its first quanta of energy through some process in
the material, is known as the "electron inelastic mean free path" and is generally
of the order of a few atomic layers. This range depends on material and kinetic
energy. Electrons that escape from the surface can have energies equal to or less
than its kinetic energy {hv - E^). To identify surface species, the excited elec-
trons that leave the surface must not lose kinetic energy. Only "elastically" scat-
tered electrons leaving the surface add to the detected atomic core-level transition
(either electron- or photon-excited spectra). Therefore, both Auger electron and
photoelectron spectroscopy are surface sensitive for the same reason.
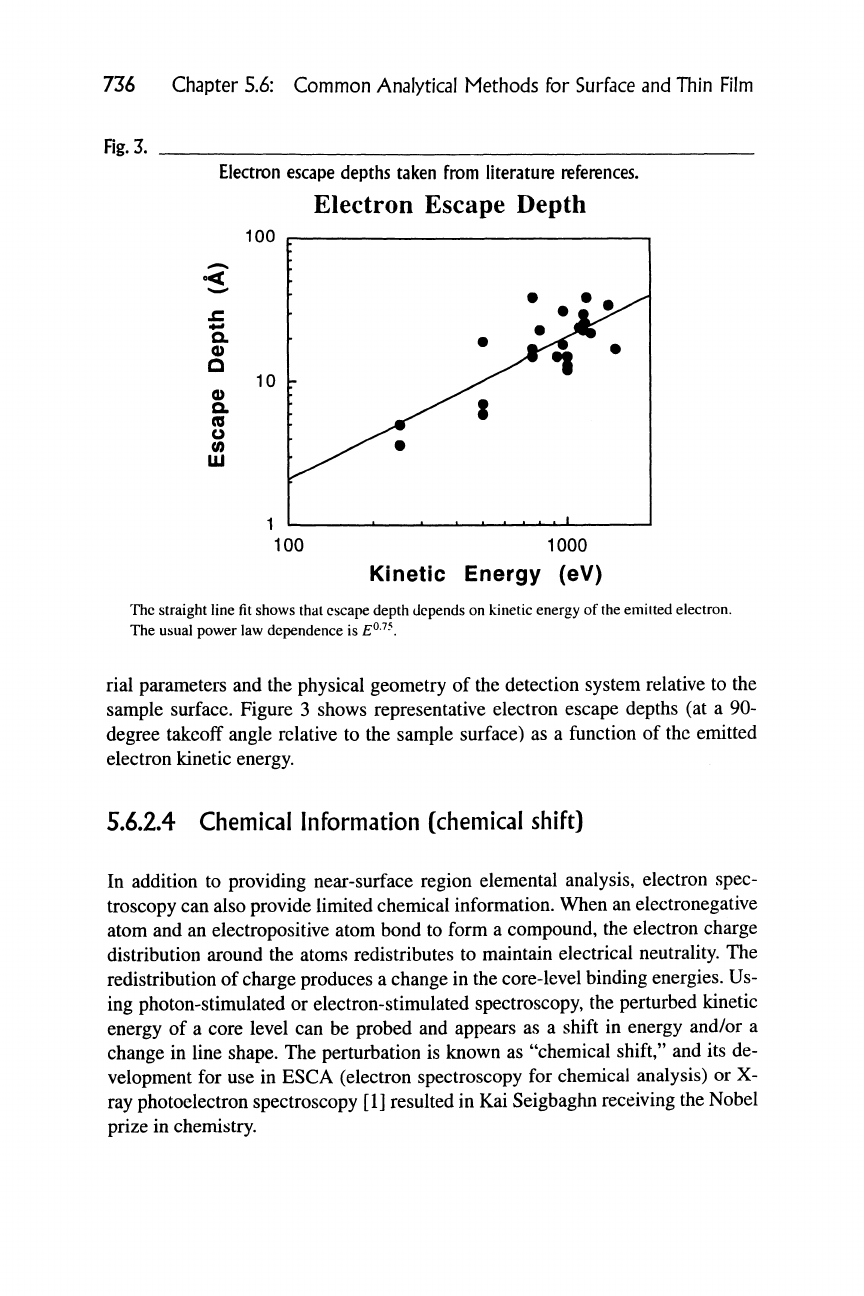
The electron "escape depth" is a function of electron kinetic energy, the mate-
736 Chapter
5.6:
Common Analytical Methods
for
Surface
and
Thin Film
Fig.
3.
Electron escape depths taken from literature references.
Electron Escape Depth
100
Q,
0)
D
0)
Q.
(D
O
(0
UJ
100 1000
Kinetic Energy (eV)
The straight line fit shows that escape depth depends on kinetic energy of
the
emitted electron.
The usual power law dependence is
E^-^-.
rial parameters and the physical geometry of the detection system relative to the
sample surface. Figure 3 shows representative electron escape depths (at a 90-
degree takeoff angle relative to the sample surface) as a function of the emitted
electron kinetic energy.
5,6.2.4
Chemical Information (chemical shift)
In addition to providing near-surface region elemental analysis, electron spec-
troscopy can also provide limited chemical information. When an electronegative
atom and an electropositive atom bond to form a compound, the electron charge
distribution around the atoms redistributes to maintain electrical neutrality. The
redistribution of charge produces a change in the core-level binding energies. Us-
ing photon-stimulated or electron-stimulated spectroscopy, the perturbed kinetic
energy of a core level can be probed and appears as a shift in energy and/or a
change in line shape. The perturbation is known as "chemical shift," and its de-
velopment for use in ESCA (electron spectroscopy for chemical analysis) or X-
ray photoelectron spectroscopy [1] resulted in Kai Seigbaghn receiving the Nobel
prize in chemistry.
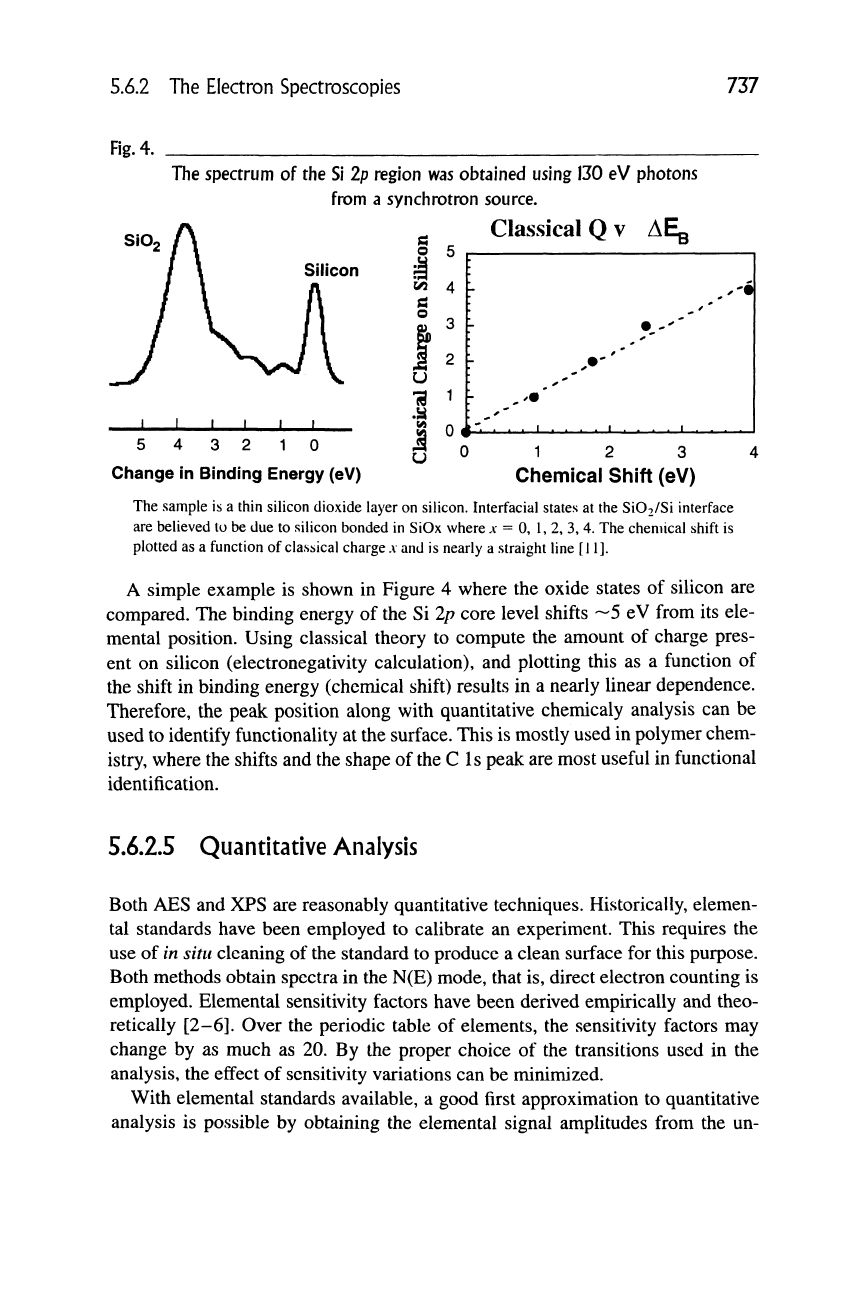
5.6.2 The Electron Spectroscopies
737
Fig.
4.
The spectrum of the Si 2p region was obtained using 130 eV photons
from a synchrotron source.
Classical Q v AEg
S 5 r-
Silicon
J-
5 4 3 2 10
Change in Binding Energy (eV)
1 2 3
Chemical Shift (eV)
The sample is a thin silicon dioxide layer on silicon. Interfacial states at the Si02/Si interface
are believed to be due to silicon bonded in SiOx where
A-
= 0, 1, 2, 3, 4. The chemical shift is
plotted as a function of classical charge
A
and is nearly a straight line [11].
A simple example is shown in Figure 4 where the oxide states of silicon are
compared. The binding energy of the Si Ip core level shifts ~5 eV from its ele-
mental position. Using classical theory to compute the amount of charge pres-
ent on silicon (electronegativity calculation), and plotting this as a function of
the shift in binding energy (chemical shift) results in a nearly linear dependence.
Therefore, the peak position along with quantitative chemicaly analysis can be
used to identify functionality at the surface. This is mostly used in polymer chem-
istry, where the shifts and the shape of the C Is peak are most useful in functional
identification.
5.6.2.5 Quantitative Analysis
Both AES and XPS are reasonably quantitative techniques. Historically, elemen-
tal standards have been employed to calibrate an experiment. This requires the
use of in situ cleaning of the standard to produce a clean surface for this purpose.
Both methods obtain spectra in the N(E) mode, that is, direct electron counting is
employed. Elemental sensitivity factors have been derived empirically and theo-
retically
[2-6].
Over the periodic table of elements, the sensitivity factors may
change by as much as 20. By the proper choice of the transitions used in the
analysis, the effect of sensitivity variations can be minimized.
With elemental standards available, a good first approximation to quantitative
analysis is possible by obtaining the elemental signal amplitudes from the un-
738 Chapter 5.6: Common Analytical Methods for Surface and Thin Film
known and standard spectrum (usually by integrating the spectral region digi-
tally).
The unknown concentration is then obtained from the ratio of the unknown
to the standard.
When standards are not available, quantitative analysis is still possible. By
measuring all the elemental peak areas of the unknown, the normalized atomic
concentration is given by
X?,
= (/«>«)-Y/y^M (1)
where X is the normalized atomic concentration of element a in the matrix m, / is
the amplitude of the /-th element in the matrix m, and a is the elemental sensitiv-
ity factor. The sensitivity factors can be improved by including the element in a
material similar to what is being analyzed. Generally, this expression provides a
reasonably good normalized concentration. For further information, the reader is
referred to References
[2-7].
As an example of the application of this methodology. Figure 5 shows a typical
XPS spectrum of an unknown surface. The elements are identified from peak bind-
ing energies. With the aid of software, the peak areas are integrated. The elemen-
tal peak areas are placed in a table and the normalization procedure, as shown in
Equation (1), is performed. After the computation is performed, an elemental sur-
face compositional table is available, as shown next for the unknown.
Compare this with what is expected from the chemical formula
CH3
I
-esio-^„
I
CH3
where the O to Si to C ratio is expected to be
1:1:2.
The experimental data are
found to be in reasonable agreement. Therefore, the unknown is identified as sili-
cone (polydimethylsiloxane).
AES quantitation is very similar. The exception is when the data are obtained
in the derivative mode, that is, the spectral data are A^(^) • dNIdE versus E. In-
stead of measuring the peak area, the peak-to-peak signal amplitude is measured,
and the appropriate elemental sensitivity factors for derivative data are applied.
5.6.2.6 Elemental Depth Profiling
and Cliemicai Depth Profiling
Both AES and XPS are typically equipped with ion milling or sputtering using
rare gases (He, Ne, Ar, Xe). Ion gun designs permit a focused beam of rare gas
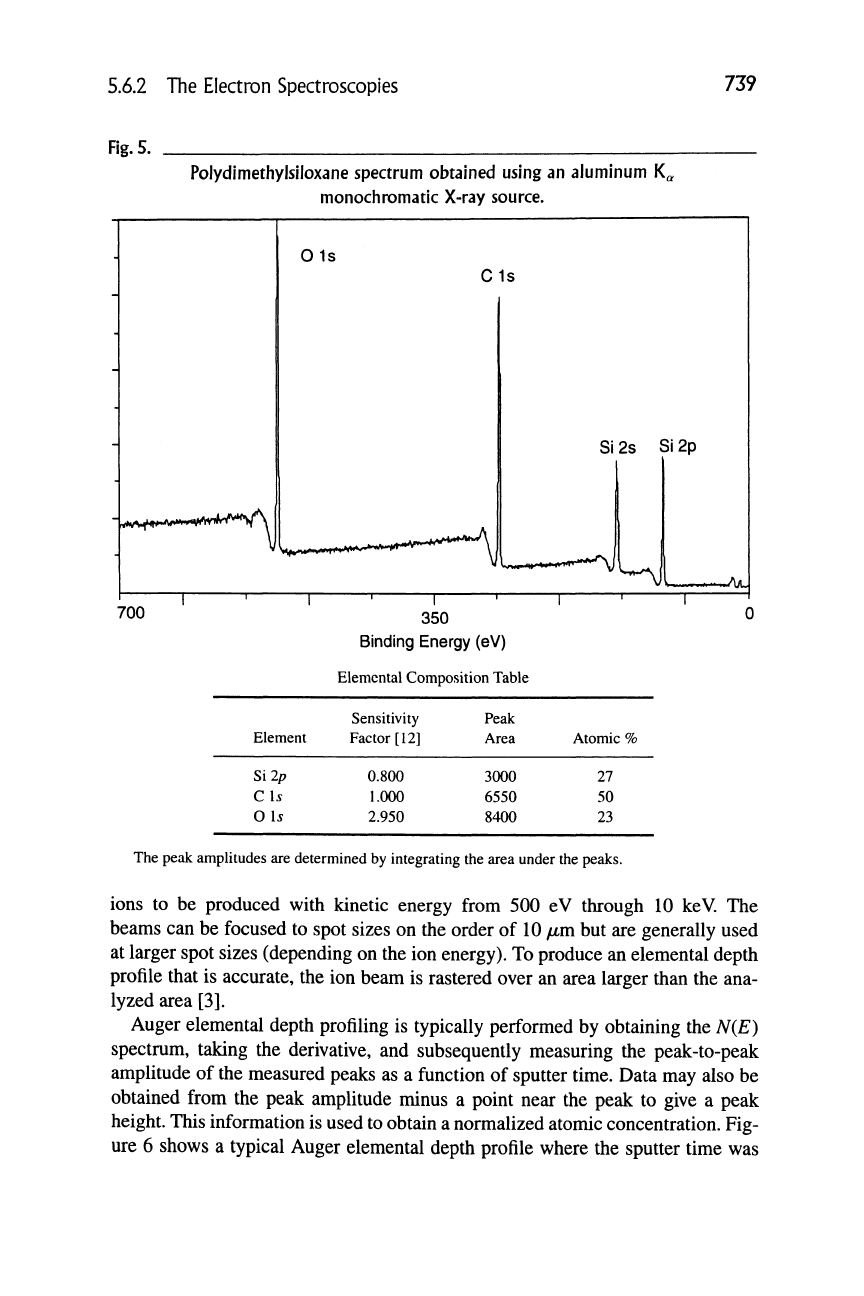
5.6.2 The Electron Spectroscopies
739
Fig.
5.
Polydimethylsiloxane spectrum obtained using an aluminum K^
monocliromatic X-ray source.
Element
Si2p
C \s
0 Is
350
Binding Energy (eV)
Elemental Composition Table
Sensitivity
Factor [12]
0.800
1.000
2.950
Peak
Area
3000
6550
8400
Atomic %
27
50
23
The peak amplitudes are determined by integrating the area under the peaks.
ions to be produced with kinetic energy from 500 eV through 10 keV. The
beams can be focused to spot sizes on the order of 10 /im but are generally used
at larger spot sizes (depending on the ion energy). To produce an elemental depth
profile that is accurate, the ion beam is rastered over an area larger than the ana-
lyzed area [3].
Auger elemental depth profiling is typically performed by obtaining the N(E)
spectrum, taking the derivative, and subsequently measuring the peak-to-peak
amplitude of the measured peaks as a function of sputter time. Data may also be
obtained from the peak amplitude minus a point near the peak to give a peak
height. This information is used to obtain a normalized atomic concentration. Fig-
ure 6 shows a typical Auger elemental depth profile where the sputter time was
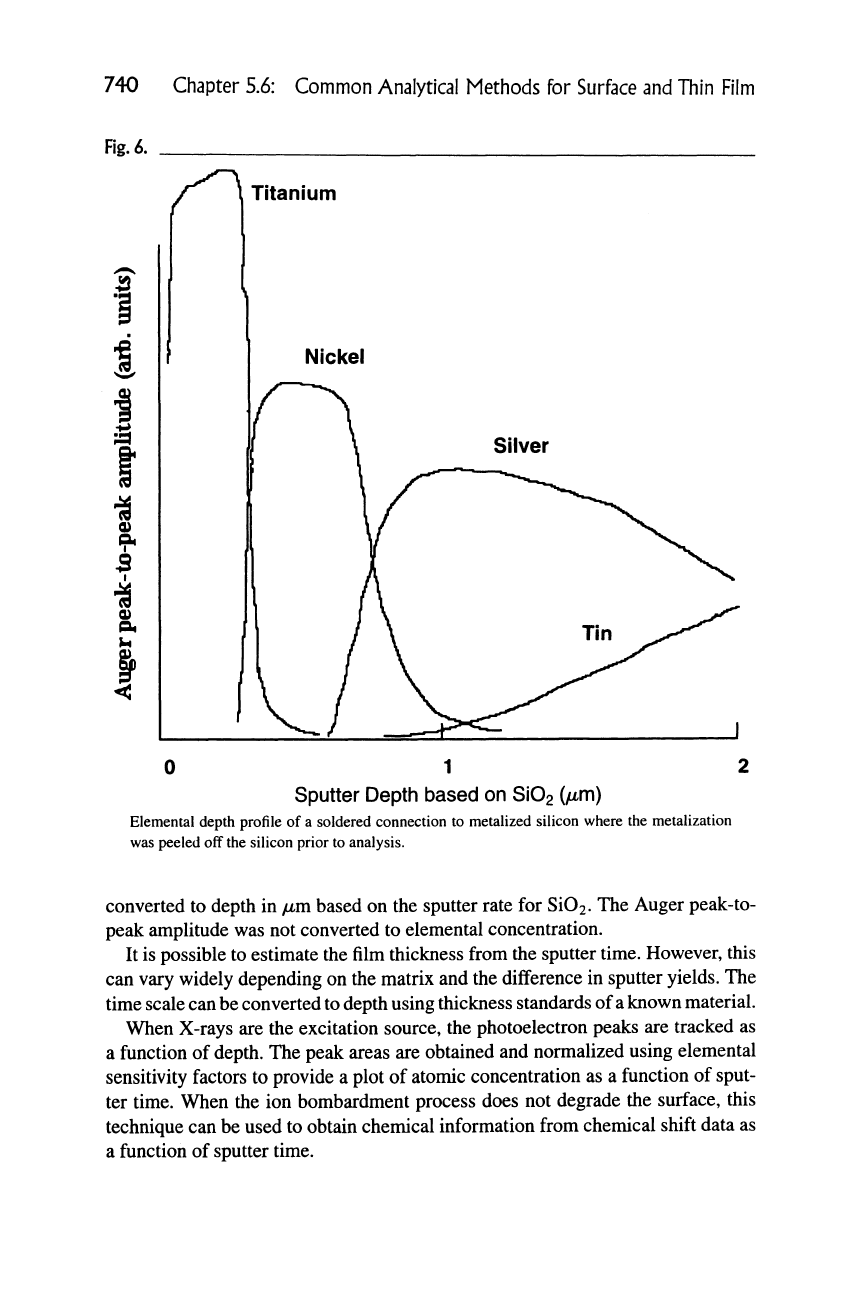
740 Chapter 5.6: Common Analytical Methods for Surface and Thin Film
Fig.
6.
'^
0)
9*
/""]. Titani
Titanium
0 1 2
Sputter Depth based on Si02
(jmm)
Elemental depth profile of a soldered connection to metalized silicon where the metalization
was peeled off the silicon prior to analysis.
converted to depth in
/mm
based on the sputter rate for Si02. The Auger peak-to-
peak amplitude was not converted to elemental concentration.
It is possible to estimate the film thickness from the sputter time. However, this
can vary widely depending on the matrix and the difference in sputter yields. The
time scale can be converted to depth using thickness standards of
a
known material.
When X-rays are the excitation source, the photoelectron peaks are tracked as
a function of depth. The peak areas are obtained and normalized using elemental
sensitivity factors to provide a plot of atomic concentration as a function of sput-
ter time. When the ion bombardment process does not degrade the surface, this
technique can be used to obtain chemical information from chemical shift data as
a function of sputter time.

5.6.2 The Electron Spectroscopies 741
5.6.2.7 Auger Elemental Mapping
One of the main features of using Auger electron spectroscopy as a surface ana-
lytical method is the use of the very finely focused electron beam obtainable with
modern electron columns (similar to scanning electron microscopes). The elec-
tron sources are typically LaB^ (single-crystal) or field emission sources. These
sources provide a high brightness beam at very small spot sizes (<100 A diame-
ter beams).
Elemental mapping is accomplished by rastering the electron beam across the
surface where at each point (pixel), the elemental signal amplitude is measured
relative to the background. The background amplitude is used to correct for some
of the topographic effects encountered in the analysis. These data are stored in a
data file on a computer where it can later be manipulated using software. The pic-
ture can also be obtained in an analog fashion by photographing the Auger signal
amplitude displayed as Z-axis modulation on an oscilloscope.
An Auger elemental map of silicon and carbon in silicon carbide fibers in a ti-
tanium matrix is shown in Figure 7. The fibers consist of a graphite core sur-
rounded by SiC and a graphite coating encapsulating the SiC.
5.6.2.8 Instrumentation
Electrostatic analysis of the emitted electrons is performed using a number of
conmion analyzers. In the following, three of the most common analyzers are
briefly described.
The cylindrical mirror analyzer (CMA) is used mainly in AES systems. This
instrument consists of concentric cylinders. The size of the outer cylinder is fixed
depending on the inner cylinder diameter. Electrons are accepted into the struc-
ture where the outside cylinder is biased negative, and this voltage is scanned.
The geometrical ratio of the cylinders fixes the energy resolution to —0.2-0.5%
of the kinetic energy.
The excitation source, generally an electron gun, is mounted coaxially in the
inner cylinder of the analyzer but can be mounted externally to the CMA. Both
assemblies are at ground potential. The electron gun can be a high-quality mag-
netically focused gun or an electrostatically focused electron gun. Both instru-
ments are available on the commercial market. The coaxial geometry provides a
small-size structure where the source is axially aligned with the analyzer. The
sample for analysis must be at the proper location relative to the analyzer to angu-
lar restrictions. A typical simple AES system based on this analyzer is shown in
Figure 8. Electrons are collected from a 360-degree cone at an angle of —42 de-
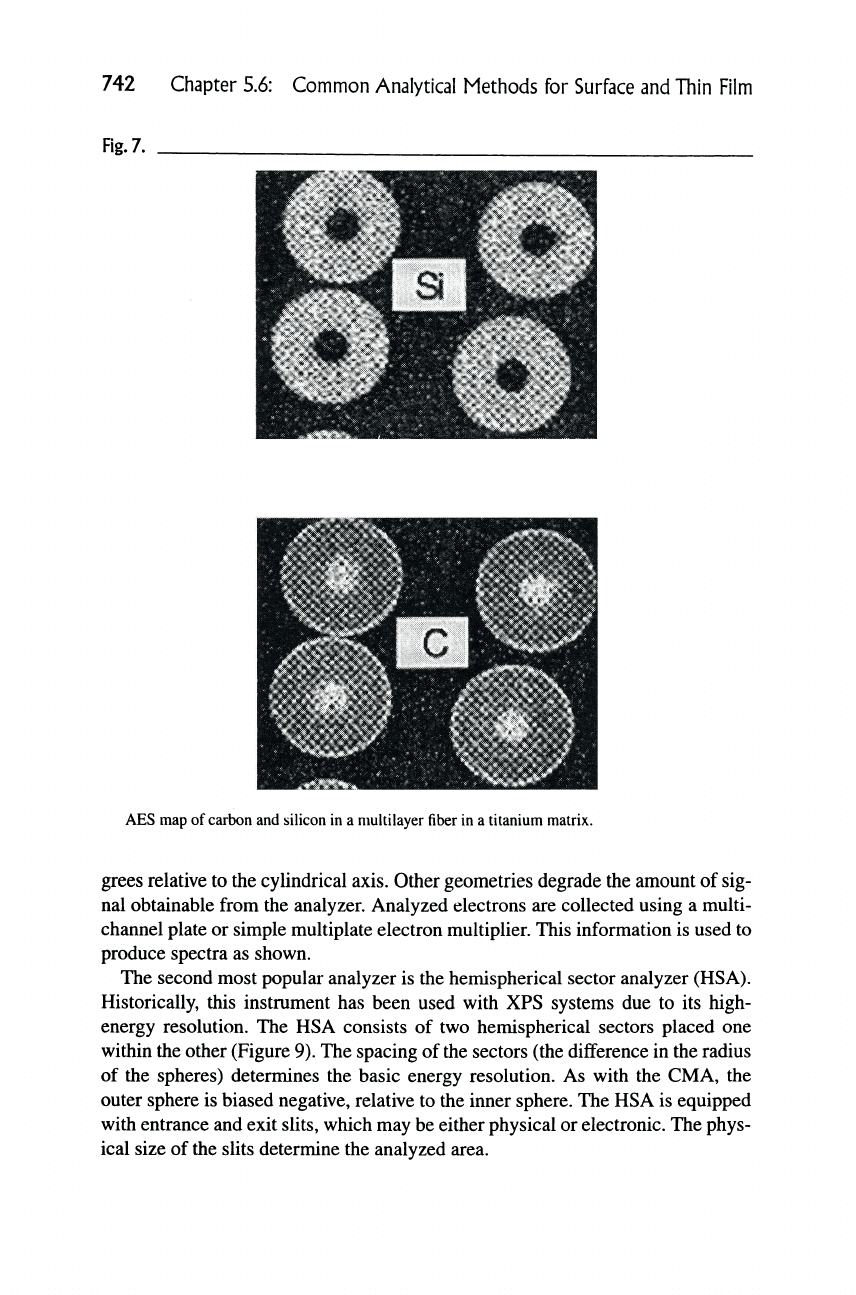
742 Chapter 5.6: Common Analytical Methods for Surface and Thin Film
Fig.
7.
AES map of
carbon
and silicon in a multilayer
fiber
in a titanium matrix.
grees relative to the cylindrical axis. Other geometries degrade the amount of sig-
nal obtainable from the analyzer. Analyzed electrons are collected using a multi-
channel plate or simple multiplate electron multiplier. This information is used to
produce spectra as shown.
The second most popular analyzer is the hemispherical sector analyzer (HSA).
Historically, this instrument has been used with XPS systems due to its high-
energy resolution. The HSA consists of two hemispherical sectors placed one
within the other (Figure 9). The spacing of the sectors (the difference in the radius
of the spheres) determines the basic energy resolution. As with the CMA, the
outer sphere is biased negative, relative to the inner sphere. The HSA is equipped
with entrance and exit slits, which may be either physical or electronic. The phys-
ical size of the slits determine the analyzed area.
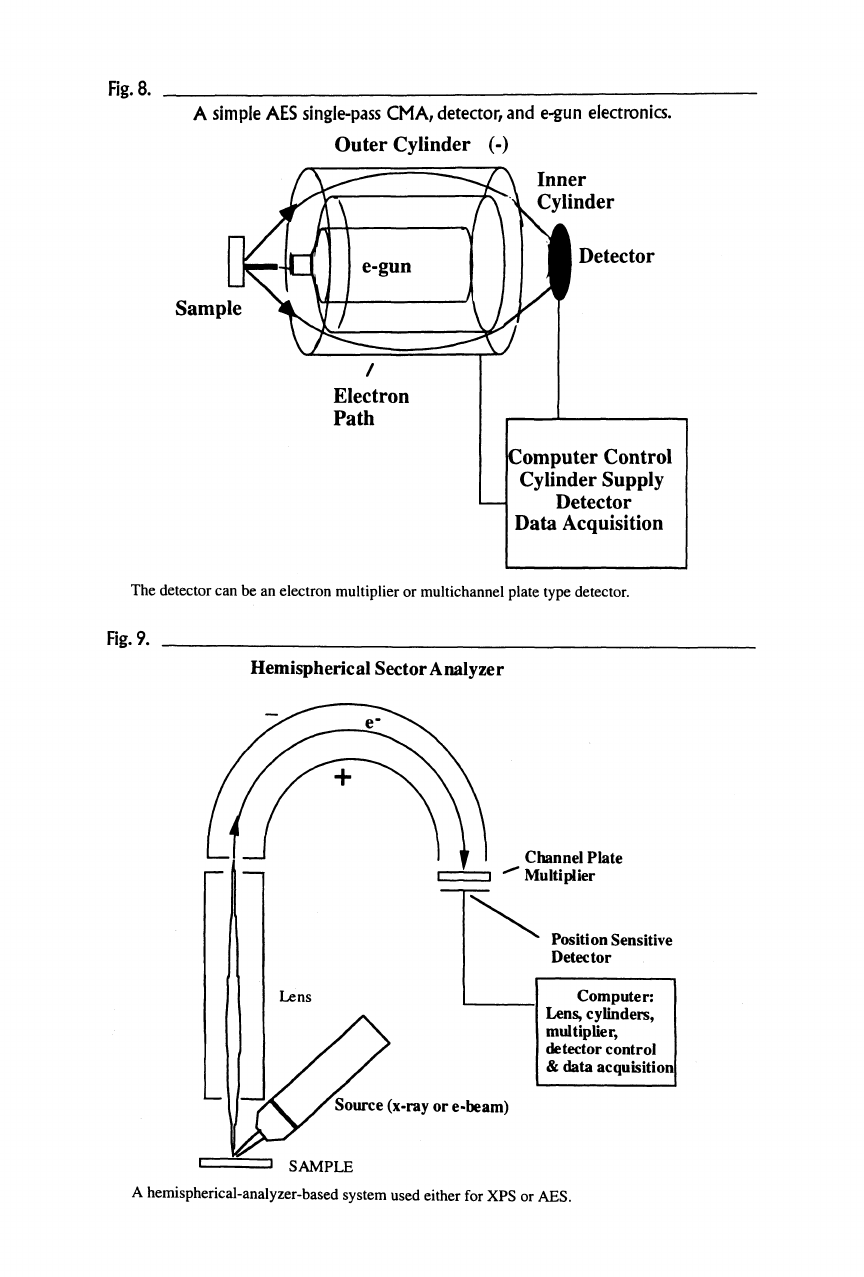
Fig.
8.
A simple
AES
single-pass
CMA,
detector, and
e-gun
electronics.
Outer Cylinder (-)
Inner
A Cylinder
Sample
Detector
jComputer Control
Cylinder Supply
Detector
Data Acquisition
The detector can be an electron multiplier or multichannel plate type detector.
Fig.
9.
Hemispherical Sector Analyzer
Channel Plate
\ ' I '^ Multiplier
Position Sensitive
Detector
Computer:
Lens,
cylinders,
multiplier,
detector control
& data acquisition!
Source (x-ray or
e-beam)
' "" ' SAMPLE
A hemispherical-analyzer-based system used either for XPS or AES.
