Hoffman D.M., Singh B., Thomas J.H. (Eds). Handbook of Vacuum Science and Technology
Подождите немного. Документ загружается.


572 Chapter 4.9: Preparation
and
Cleaning of Vacuum Surfaces
However, because of the toxicity and carcinogenic properties of some of these
materials, they should be used in well-ventilated areas or the vapors should be
contained in a closed recycling system. Volatile organic compounds (VOCs) are
those that have boiling points below 138°C. The discharge of VOCs into the en-
vironment is regulated by local, state, and federal laws. To comply with these reg-
ulations it may be necessary to recycle the material by condensation of the vapors
or to thermally destroy the vapors by burning [58], that is, instead of releasing
them into the atmosphere.
Chlorofluorocarbon (CFC) solvents are more stable and less toxic than the
chlorinated solvents; however, the well-substantiated atmospheric ozone deple-
tion and the controversial increase in the greenhouse effect have caused their use to
be banned. Since January 1, 1996, CFC solvents have not been produced in the
United States. Other possible solvents for removal of greases are N-methyl-2-
pyrrolidone-based solvents [59,60], terpene-based solvents, as well as the Fluoro-
inerts®, which do not contain chlorocarbon bonds. Reports indicate that the ter-
penes may be as effective as the CFCs in many instances, though they have a
greater tendency to leave residues. Terpenes suffer from the fact that they have
low flashpoints (about 120°F) and reduced lower explosive limits (LELs) than
the CFCs. Other approaches use nonlinear alcohols and purely aqueous cleaning
[61].
This area of solvent development is rapidly changing.
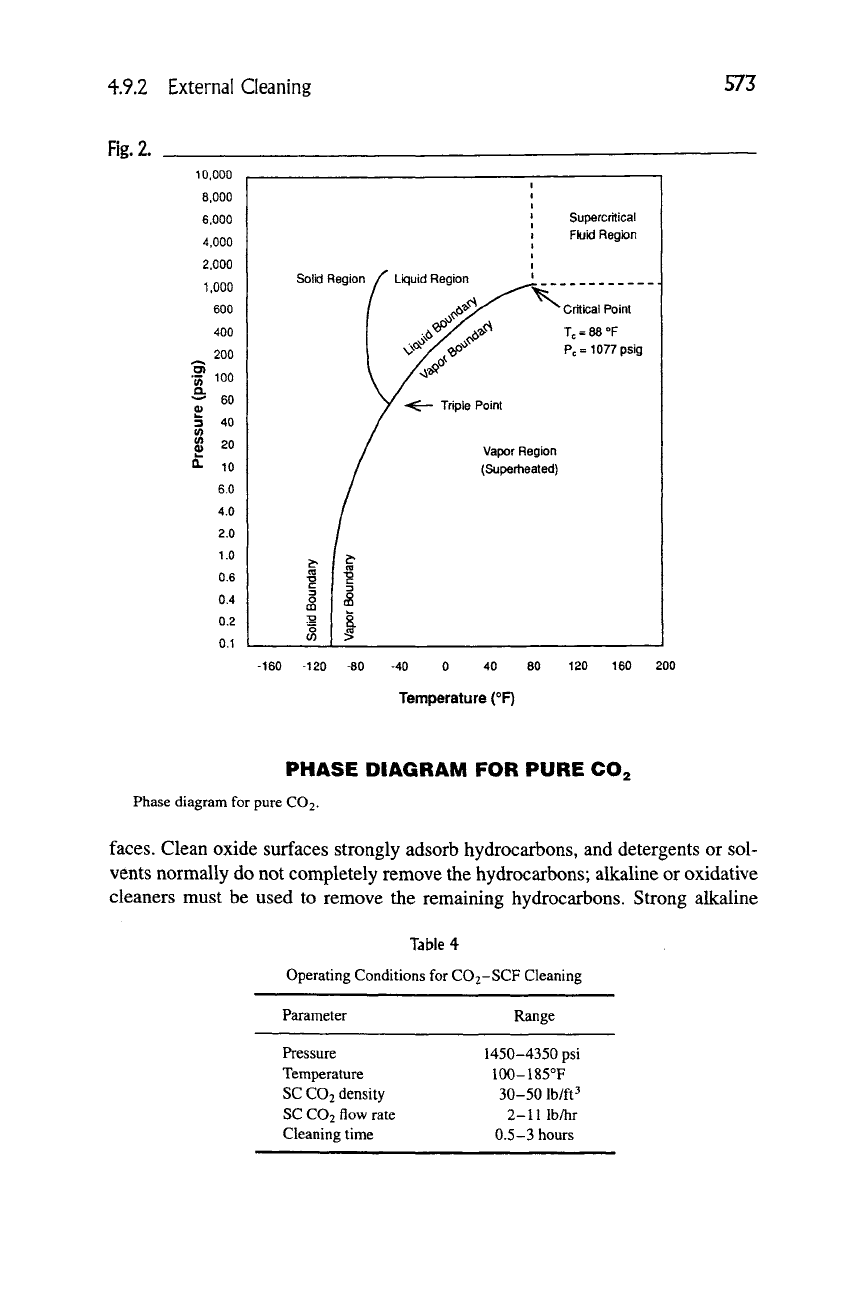
Another type of solvents are the supercritical fluids. If a gas such as CO2 is
compressed to its "critical pressure" (CO2 = 1077 psi), it liquefies to become
a "critical fluid." If it is also heated above its "critical temperature" (CO2 =
31.1°C), it becomes a supercritical fluid (SCF), as shown in Figure 2. Critical
fluids and supercritical fluids are good solvents for many medium-molecular-
weight, nonpolar or slightly polar organics. The more dense they are, the better
their solvency power.
Solvents can be densified most easily when they are in the supercritical state.
Carbon dioxide has been shown to have a Hildebrand solubility parameter that
can vary from 0 in the gas to 10 under high-pressure supercritical conditions [62].
Values of 6 to 8 are typical, which is about the same as hexane and carbon tetra-
chloride. Supercritical CO2 fluid (SCF-CO2, critical point 31°C, 74 bar pressure)
has the advantage that it is stable, has low toxicity, minimal cost, and is a solvent
for many organic materials and has shown promise as a solvent cleaning tech-
nique [63]. Table 4 shows typical operating parameters for SCF cleaning.
Saponifiers
Alkaline cleaners (generally silicate- and phosphate-based) are saponifiers that
convert organic fats to water-soluble soaps. Alkaline cleaners have a pH of about
11 and are generally used hot
[64].
After using alkaline cleaners, the surface should
receive an acid dip prior to the water rinse since alkali salts adhere strongly to sur-
4.9.2 External Cleaning
573
Rg.2.
10,000
8.000
6.000
4.000
2.000
1,000
600
400
^ 200
f 100
^ 60
3 40
8 20
^ 10
6.0
4.0
2.0
1.0
0.6
0.4
0.2
0.1
Solid Region
n
T3
3
01
8.
/^ Liquid Region
^
V -<— Triple Point
1 Supercritical
1 Fluid Region
Critical Point
Tc =
88 '^F
Pc= 1077 psig
Vapor Region
(Superheated)
-120 -80
-40
40 80
160 200
Temperature (°F)
PHASE DIAGRAM FOR PURE CO2
Phase diagram for pure CO2.
faces.
Clean oxide surfaces strongly adsorb hydrocarbons, and detergents or sol-
vents normally do not completely remove the hydrocarbons; alkaline or oxidative
cleaners must be used to remove the remaining hydrocarbons. Strong alkaline
Table 4
Operating Conditions for CO2-SCF Cleaning
Parameter
Range
Pressure
Temperature
SC CO
2
density
SC CO2 flow rate
Cleaning time
1450-4350 psi
100-185°F
30-50 Ib/ft^
2-lllb/hr
0.5-3 hours
574 Chapter 4.9: Preparation and Cleaning of Vacuum Surfaces
cleaners can etch aluminum and oxide surfaces, particularly glasses, so solution
strength (pH), temperature, and exposure times should be carefully controlled.
Detergent Cleaning
Detergent (soap) cleaning is a comparatively mild cleaning technique
[65].
In de-
tergent cleaning, the detergent surrounds contaminants, taking them into suspen-
sion without actually dissolving the material. This action is assisted by wetting
agents and surfactants, which loosen the contaminants from the surface. Liquid
dishwasher soap is an excellent detergent for many applications such as cleaning
polymer surfaces. A major problem with soaps is that metal ions, such as the cal-
cium and magnesium, which are found in hard water, make the soaps insoluble,
thus leaving a residue. Deionized (DI) water should always be used for residue-
free detergent cleaning. Many detergents contain phosphates, which can be envi-
ronmentally harmful and subject to pollution regulations.
Surface Tension
The ability to wet a surface depends on the relative surface tensions of the surface
and the fluid. The lower the surface tension, the better the fluid can wet a surface
and penetrate into small spaces. The surface tension of clean oxide surfaces is
close to 1000 dyne/cm. The surface tension of water can be lowered by the addi-
tion of chemicals. Table 5 shows the surface tension of some water solutions:
Table 5
Surface Tension of Fluids
Material
Pure H2O
/z-propanol
H2O + 30 vol% «-propanol
Ethyl alcohol
H2O
-H
50 vol% ethanol
1000 g H2O -f 34 g NH4OH
1000gH2O+ 17.7 gHCl
1000 g H2O + 14 g NaOH
1000gH2O + 6gNaCl
at 18°C
at50°C •
at 100"C
at
25X
at 18°C
at 30°C
at 30°C
at 18X
at 20°C
at 18°C
at
20X
Surface Tension (in air)
mJ/m^ (dyne/cm)
= 73.05
= 67.91
= 58.9
= 23.32
= 26.9
= 21.5
= 27.5
= 57.05
= 65.75
= 101.05
= 82.55

4.9.2 External Cleaning 575
Surfactants
Surfactants are the generic name for surface-active agents that reduce the inter-
facial energy of materials in contact. Surfactants used with water have both
hydrophobic ("water hating") and hydrophilic ("water loving") groups. They dis-
solve in water by virtue of their hydrophilic groups and lower the surface energy
of water to about 30 mJ/m^. The surfactant collects at the interface between im-
miscible substances, such as oil and water, and lowers the interfacial energy. Sur-
factants should best be used in deionized water.
pH Adjusters and Chelating Agents
In solutions, pH adjusters are used to aid in the cleaning action. Generally it is
found that basic solutions clean better than acidic solutions if chemical etching is
not involved. The pH of the cleaning solution is often adjusted to basic using am-
monia or anMnonium hydroxide. Chelating agents keep in solution the normally
insoluble phosphates that are formed in hard-water detergent cleaning. Glass-
cleaning solutions often use chelating agents such as ethylene diamine tetraacetic
acid (EDTA) and citric acid with salts containing hydroxy
1
and amine substitutes.
Reactive Cleaning
Reactive cleaning uses liquids, gases, vapors, or plasmas to react with a contami-
nant to form a volatile or soluble reaction product.
Fluids
Reactive cleaning liquids are often oxidizing solutions. Many acid-based systems
can be used as oxidants. One system commonly used in the semiconductor indus-
try is the "piranha solution." The piranha solution is hot (>50°C) concentrated
sulfuric acid plus ammonium persulfate [66]. The addition of the solid anrnio-
nium persulfate to the hot sulfuric acid produces peroxydisulfuric acid, which re-
acts with water to form H2SO5 (Carols acid) which further decomposes to form
free atomic oxygen. The anmionium persulfate should be added just prior to the
immersion of the part into the solution. The effectiveness of this oxidation tech-
nique can be shown by first placing a piece of paper in the hot sulfuric acid where
it is carbonized, then adding the ammonium persulfate and watching the carbon
disappear. This treatment is sometimes followed by a brief dip in a 10:1 solution of
water and HF or inmiersion for 20 minutes in a hot solution of hydrogen peroxide
and ammonium hydroxide in the ratio H2O : H2O2 (30%) : NH4OH (29%) at
80°C.
Another similar oxidizing solution uses stabilized sulfuric acid and hydro-
gen peroxide.

^f> Chapter 4.9: Preparation and Cleaning of
Vacuum
Surfaces
Hydrogen peroxide (H2O2) is a good oxidizing solution for cleaning glass. Of-
ten boiling 30% unstabilized H2O2 is used. The most common hydrogen peroxide
has been stabilized, which reduces the release of free oxygen. Unstabilized H2O2
must be stored in a refrigerator to slow decomposition. Hydrogen peroxide is
sometimes used with ammonium hydroxide, to increase the complexing of sur-
face contaminants, and is used at a ratio of
8 (30% H2O2): 1 (NH4OH): 1 (H2O)
However, the decomposition rate of the H2O2 is greatly increased by combina-
tion with ammonium hydroxide.
Oxidative cleaning can be performed using chlorine-containing chemicals. For
example, a water slurry of sodium dichloroisocyanurate (i.e., swinmiing pool chlo-
rine),
which has 63% available chlorine, can be used to scrub an oxide surface to
remove hydrocarbon contamination. This combines mechanical scrubbing with
oxidation and improves the cleaning action.
Gases
Reactive gas cleaning uses a reactive gas at high temperature to form a volatile
material. For example, air firing of an oxide surface oxidizes all the hydrocarbons,
and they are volatilized. High-temperature air fire is an excellent way to clean sur-
faces that are not degraded by high temperature [67,68]. For example, alumina
can be cleaned of hydrocarbons by heating to 1000°C in air. Some care must be
taken in furnace firing, because particulate generation from the furnace liner can
be a source of undesirable particulates and sodium from the insulating material
may be an undesirable contaminant for semiconductor device fabrication.
Self-
cleaning kitchen ovens clean by oxidation at about 425°C.
The oxidation by ozone (O3) created by ultraviolet radiation (UV) at atmo-
spheric pressure and low temperature is useful for removing hydrocarbons. The
ultraviolet radiation also causes bond scission of the hydrocarbon contaminants,
which aids in cleaning. The UV/O3 cleaning has greatly simplified the produc-
tion, storage, and maintenance of hydrocarbon-free surfaces [69,70].
The UV is produced by a mercury vapor lamp in a quartz envelope so that both
the 1849 A and the 2537 A radiation is transmitted. The short-wavelength radia-
tion generates ozone and causes bond scission in the hydrocarbon contaminants.
The mercury lamps can be custom made to a variety of shapes for specific appli-
cations. Ozone adsorbs UV so the surfaces should be as close as possible to the
UV source. UV radiation intensity should be maintained to about 1-10 milli-
watts/cm^ at the surface. In the UV/O3 chamber the air may be stagnant or flow-
ing. If flowing air is used, the air should be filtered. In a correctly operating sys-
tem, ozone can be detected by smell when the chamber is opened. The smell is
similar to that of the air after a lightning storm and indicates that the ozone con-

4.9.2 External Cleaning 577
centration is less than 10 parts per million by volume (ppmbv). Higher concen-
trations of ozone deaden the olfactory nerves and are harmful.
Typical exposure times for UV/O3 cleaning are from a few minutes to remove
a few monolayers of hydrocarbon contamination to hours or days or weeks for
storage of cleaned surfaces. The UV/O3 cleaning technique has the advantage
that it can be used as a dry in-line cleaning technique [71]. The UV/O3 cleaning
technique is also useful for cleaning holes (vias) in surfaces [72].
Plasmas
Plasma cleaning can be done in a plasma system separate from the deposition sys-
tem. Reactive plasma cleaning uses a reactive species in the plasma to react with
the contaminants to form a volatile species, which leave the surface at much
lower temperatures than those necessary for reactive gas cleaning. The additional
requirement on reactive plasma cleaning is that it does not leave a residue. Oxy-
gen (from pure ["medical"] air) and hydrogen (pure or as "forming gas") [73] are
the most common materials used for plasma-cleaning vacuum surfaces both as an
external cleaning process and as an in situ cleaning technique (Section 4.9.4.2)
[74].
Oxygen (or air) plasmas are very effective in removing hydrocarbons and ab-
sorbed water vapor from surfaces. The reaction of the oxygen with carbon on the
surface can be monitored using a mass spectrometer to monitor the CO and CO2
that is produced [75]. Hydrogen plasmas can be used to remove hydrocarbon con-
tamination when oxygen plasmas are unacceptable. This technique has been used
to clean vacuum surfaces (stainless steel) in nuclear fusion reactors [76]. Hydro-
gen plasmas have been used to clean metals [77] and semiconductor materials [78].
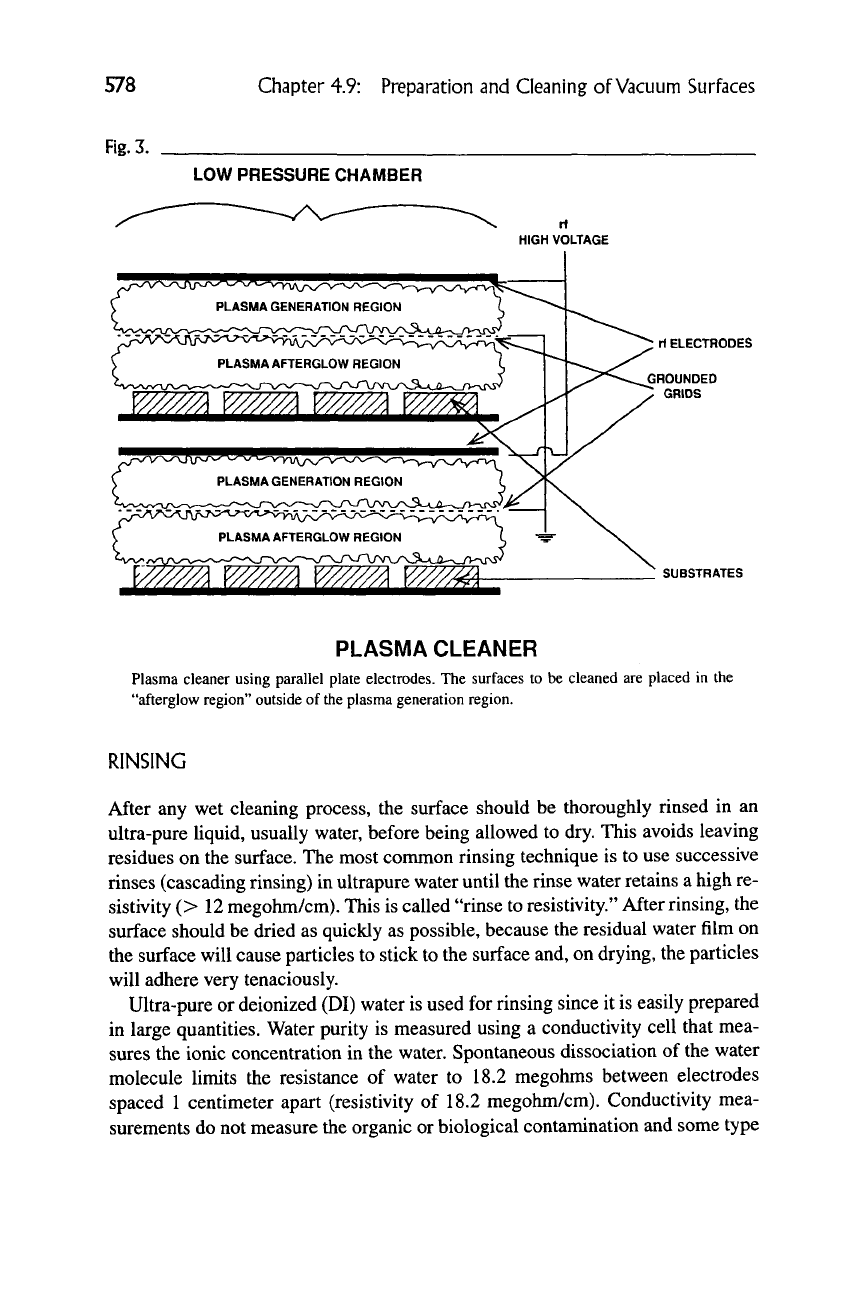
Figure 3 shows a typical plasma cleaner where the plasma is generated by an
RF discharge and the surfaces to be cleaned are in a "remote" or "afterglow" lo-
cation and not in the plasma generation region. Figure 4 shows the processes that
occur on a surface exposed to a plasma. The surface attains a potential (sheath po-
tential) that is negative with respect to the plasma, and ions are accelerated from
the plasma to the surface. For the case of a "cold plasma," which has low energy
particles, this sheath potential will only be a few volts. When the plasma particles
are more energetic or the electrons are accelerated to the surface, the sheath po-
tential can be tens of
volts.
In addition to being bombarded by ions, the surface in
contact with the plasma will be bombarded by "activated species," excited spe-
cies,
and other high-energy species. Ions and excited species release their ener-
gies of ionization or excitation when they impinge on the surface. For example,
when a singly charged argon ion impinges on a surface, it will give up the kinetic
energy it attained by acceleration through the sheath potential and the ionization
energy, which is 15.7 eV. For a high ion flux, this release of heat can result in an
appreciable temperature rise at the surface.
578
Chapter 4.9: Preparation and Cleaning of Vacuum Surfaces
Fig.
3.
LOW PRESSURE CHAMBER
HIGH VOLTAGE
rf ELECTRODES
^^j
^^ ^^ P^^
C PLASMA GENERATION REGION 1
^-^^^^^^
SUBSTRATES
PLASMA CLEANER
Plasma cleaner using parallel plate electrodes. The surfaces to be cleaned are placed in the
"afterglow region" outside of the plasma generation region.
RINSING
After any wet cleaning process, the surface should be thoroughly rinsed in an
ultra-pure liquid, usually water, before being allowed to dry. This avoids leaving
residues on the surface. The most common rinsing technique is to use successive
rinses (cascading rinsing) in ultrapure water until the rinse water retains a high re-
sistivity (> 12 megohm/cm). This is called "rinse to resistivity." After rinsing, the
surface should be dried as quickly as possible, because the residual water film on
the surface will cause particles to stick to the surface and, on drying, the particles
will adhere very tenaciously.
Ultra-pure or deionized (DI) water is used for rinsing since it is easily prepared
in large quantities. Water purity is measured using a conductivity cell that mea-
sures the ionic concentration in the water. Spontaneous dissociation of the water
molecule limits the resistance of water to 18.2 megohms between electrodes
spaced 1 centimeter apart (resistivity of 18.2 megohm/cm). Conductivity mea-
surements do not measure the organic or biological contamination and some type

4.9.2 External Cleaning
579
Fig.
4.
PLASMA
(+)
SURFACE
(-]
^ "^
PARTICLES
IONS (+)
ELECTRONS
(-)
NEUTRALS
Atoms/Molecules
Excited Species
Metastable Species
Radicals
PHOTONS
IR
Optical
UV
Soft X-ray
lt«^^^
VJ
r-^ RECOMBINATION
Jon
^.
* Electron
Q ADSORBED SPECIES
«9\s Cb REACTED SPECIES
O ABSORBED SPECIES
PLASMA SHEATH
&
SHEATH POTENTIAL
PLASMA — SURFACE INTERACTION
(LOW ENERGY IONS)
Plasma-surface interactions.
of residue analysis must be used to measure these impurities. There are a number
of techniques for determining the nature of the contaminants in water [79]. A
simple technique is to let some of the water evaporate on a clean glass surface and
then look for a "haze" or for loss of contact angle (discussed later).
Typical semiconductor specifications for ultra-pure water for end use are
• Resistivity—18 megohm/cm continuous at 25°C
• Particle count—less than 500 particles (0.5 microns or larger) per liter
• Bacteria count—less than one colony (cultured) per cc
• Organics—less than one part per million

580 Chapter 4.9: Preparation and Cleaning of Vacuum Surfaces
• Total electrolytes—less than 5 parts per billion NaCl equivalent
• Quantity requirements
• Peak-level usage
High volumes of ultrapure water are made by
• Pretreatment—pH adjustment, coagulation, filtration
• Reverse osmosis — semipermeable membrane (pore size of
10 ~-^
to
10 ~^
microns), which rejects salts, dissolved solids (90-98%) and organics (99%),
and requires 400 to 600 psi feedwater
• Degasification—removes dissolved CO2
• Ion exchange (anion and cation)—ion exchange resins remove ions by ex-
changing
H "^
for cations and OH
~
for anions
• Absorption materials (activated carbon)—remove organics
• Filtration—removes particulates and biological matter (0.2 microns filter
pore size, for bacterial, 1.0 microns pore size for prefilter)
• Ultraviolet radiation or ozone bubbling—kills bacteria on filters
• Endpoint filtration—0.2 micron filter pore size
Smaller amounts of ultra-pure water can be prepared by the same process steps
beginning with the ion exchange process. Triply distilled water can also be used
but it is relatively expensive. Note that in the production of "soft water" anions
and cations are exchanged for Na"^ and Cl~, and the water will leave a residue.
APPLICATION OF FLUIDS
Fluids are often used in cleaning processes. Fluid baths should be continuously
filtered and monitored so as to replace or replenish the active ingredients as they
are used or become contaminated. In cases of contaminants that are less dense
than the fluid, the surface of the solution can be "skimmed" as contaminants rise
to the surface, or the container can be of an "overflow" type where the fluid flows
over the lip of the container. There are a number of ways to apply the fluids to the
surface to be cleaned.
Soaking
Soaking involves immersing the part in a solution for an extended time, and there-
fore soaking has not been a desirable technique for production. This may change
in the future when less aggressive cleaning chemicals must be used because of en-
vironmental concerns. Immersion of a surface in a stagnant solution is generally
a poor technique, because the contaminants that are taken into solution are con-
centrated near the surface and must diffuse away.

4.9.2 External Cleaning 581
Mechanical Disturbance
Mechanical disturbance uses agitation, wiping, brushing, or scrubbing in a fluid
environment to break up the stagnant fluid layer near the surface, loosen particles,
and aid in carrying contamination away from the surface. Care must be taken to
ensure that any material that is used in a fluid does not produce particulates and is
compatible with the fluid and surfaces it contacts. When using any mechanical
rubbing, care should be taken to prevent contamination by abrasive transfer from
the rubbing media—gentle pressure should be used. There are a variety of brush
materials used in fluids including polypropylene. Teflon®, and Nylon®. If wiping
or scrubbing with a cloth is used, care should be taken that the cloth is lint-free
and desized by multiple washing before use. Special particulate-free sponge, and
cloth materials are available for wiping. In semiconductor technology, mechani-
cal scrubbing combined with high-pressure fluid jets (2000-3000 psi) and spin-
ning are standard cleaning procedures.
Spraying
Liquid sprays can be used to remove contaminants from surfaces. Spray pressures
can be low, at less than a hundred psi, or high, at several thousand psi. Spraying
parameters include the type of fluid, pressure, angle of incidence, and volume of
fluid. Sprays should be directed at an oblique angle to the surface. Spray systems
often use copious amounts of material so the fluid should be recycled. The spray-
ing fluid can also contain suspended particles that aid in cleaning by adding a
mild abrasion to the cleaning. For example, CeO polishing compound is often
added to fluids in cleaning glass for mirrors [49].
The fluid should be monitored by residue analysis, and when it is contaminated
above a given level it should be replaced. With increasing concern about solvent
vapors, many of the newer solvent spray systems are self-contained with con-
densers to trap the solvent vapors as used with vapor cleaning. Some systems al-
low the purification of the solvents by distillation. It should be noted that spraying
can induce resonant vibrations that can cause component failure or deterioration.
Vapor Cleaning
In vapor cleaning, a cold surface is held in a hot vapor of the solvent. The solvent
condenses on the surface, dissolves the contaminants and flows off into a hot
"sump"
[80,81]. When the part reaches the temperature of the vapor, condensa-
tion ceases and the cleaning action stops. The vaporization of the solvent in the
sump provides continuous distillation of the cleaning fluid. Parts should never
be immersed in the sump fluid. Fluid in the sump should be changed when it
