Hoffman D.M., Singh B., Thomas J.H. (Eds). Handbook of Vacuum Science and Technology
Подождите немного. Документ загружается.


552 Chapter 4.8: Aluminum-Based
Vacuum
Systems
of A2219, A5083, and A6063 aluminum alloys.
Welding
in the World (Pergamon Press) 31(2)
(1993) 128.
97.
J. H. Dudas and F. R. Collins, Preventing weld cracks in high-strength aluminum alloys.
Weld-
ing
Journal,
June 1966, p. 3.
98.
Ibid.
99.
Ibid., p. 10.
100.
Welding Aluminum Theory
and Practice, p. 5.3.
101.
Ibid., p. 10.14.
102.
Daniel Van Lanen, Senior Welder, Argonne National Laboratory, Verbal interview Novem-
ber 4, 1994. Provided most of the specific suggestions and insights regarding welding tech-
niques based on his many years of experience.
103.
Joseph Gagliano, manager of
APS
welding, Argonne Laboratory, private communication, 1994.
104.
ANSI/AWS D1.2, Structural welding code —
aluminum,
American Welding Society, 550 N.W.
LeJeune Rd., Miami, FL 33126 (Tel: 800/334-9353).
105.
ANSI/AWS A5.10-92, Specification for bare aluminum and aluminum alloy welding elec-
trodes and rods, American Welding Society, 550 N.W. LeJeune Rd., Miami, FL 33126 (Tel:
800/334-9353).
106.
Qualification Standard for Structural Welding of Aluminum, Publication 25, the Aluminum
Association, 900-19th St., N.W, Washington, DC 20006 (Tel: 301/645-0756).
107.
MIL-STD-2219, Fusion welding for aerospace applications, U.S. Department of Defense,
Available at no charge through Naval Publications (Tel: 215/697-2179).
108.
Van Lanen.

CHAPTER
4.9
Preparation and Cleaning
of Vacuum Surfaces
Donald M. Mattox
Management
Plus,
Inc.
Surfaces that are in contact with the vacuum environment are called vacuum sur-
faces.
These surfaces may be associated with the vacuum system, fixturing asso-
ciated with a vacuum-related processing or the parts related to vacuum processing.
Thus they may be a metal, ceramic, glass, semiconductor, or polymer. Surfaces
used for vacuum applications should be prepared such that they do not contribute
to contamination in the system
[1-5].
Some surfaces, such as the fixtures, can be
easily or routinely removed for cleaning, while others, such as the vacuum cham-
ber and associated feedthroughs, are nonremovable and must be cleaned in place.
Often the surface areas of the fixtures and parts are greater than that of the vac-
uum system, and these surfaces may determine the pumping performance and the
residual gas and vapor contamination in the vacuum system. This type of contam-
ination can be called "introduced contamination" or "process-related" contami-
nation as converse to "system-related" contamination associated with nonremov-
able surfaces.
Surface preparation includes cleaning and surface modification. Cleaning
means reducing the contamination level on the surface to an acceptable level for
application of the vacuum environment. Surface modification can involve chang-
ing the surface morphology to be more rough or smooth, changing the chemical
composition of the surface, changing the outgassing or outdiffusion properties of
the material, or changing the mechanical properties of the surface.
ISBN 0-12-325065-7 Copyright © 1998 by Academic Press
^^^•^^ All rights of repnwJuclioH in any form reserved.
553

554 Chapter 4.9: Preparation and Cleaning of Vacuum Surfaces
Surface roughness is important to the vacuum performance of a vacuum mate-
rial.
Surface roughness not only increases the surface area available for adsorp-
tion of contaminants, it also traps small particulates, making them more difficult
to remove. A rough or porous surface also allows capillary condensation of water,
making it more difficult to remove water vapor from the system. During fabrication
of surfaces for use in a vacuum environment, the surface should be made as smooth
as possible. For metals this means light machine cuts, sharp machining tools, cut-
ting lubricants, etc. Fully dense ceramics and glasses can be formed with smooth
surfaces by using fine-grit grinding and polishing materials in the final stages of
forming. After fabrication the surface can be further smoothed by mechanical [6],
chemical, electrochemical [7,8] or chemical/mechanical [9] polishing.
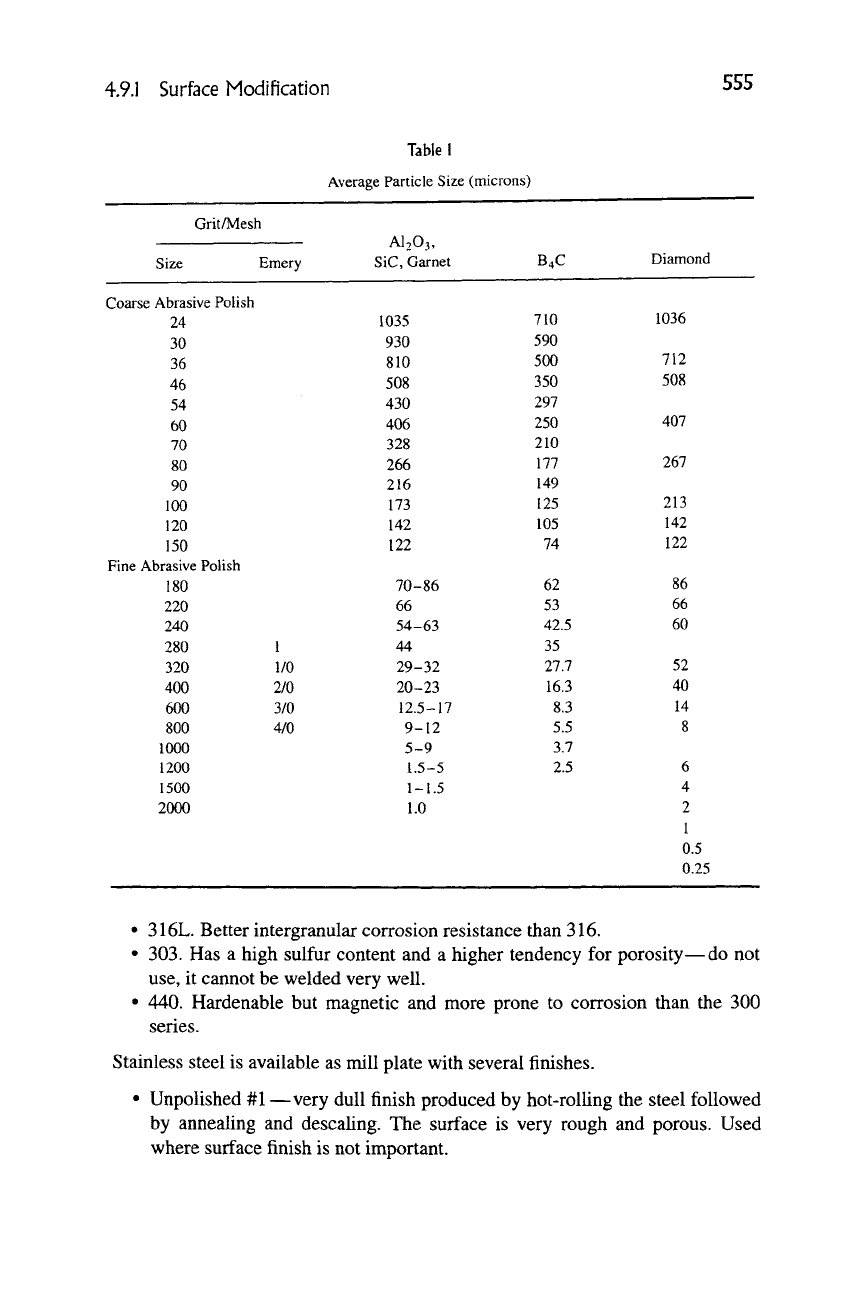
Mechanical polishing can be done by machine or by hand using a wet or dry
polishing procedure. The polishing material may be in the form of a powder, con-
tained in a slurry, loosely held in a polishing cloth, or rigidly held on a solid ma-
terial such as a paper. Table 1 lists a number of common abrasive and polishing
compounds and their size and nomenclature.
The cleaning and treatment of the surfaces should be spelled out in the opera-
tion and maintenance instructions for the system. This is particularly true for
aluminum.
4.9.1
SURFACE MODIFICATION
4.9.1.1 Nonremovable Surfaces
Nonremovable surfaces are vacuum surfaces that, once assembled, cannot be re-
moved or even reached without great difficulty.
STAINLESS STEEL
Stainless steel is the preferred material for nonremovable metal surfaces since it
forms a natural passive oxide and is resistant to corrosion. There are many stain-
less steel alloys, such as
• 304. Common machinable alloy, nonmagnetic—carbides can precipitate in
weld areas, which can result in galvanic corrosion if electrolytes are present.
• 304L. Low carbon — used for better intergranular corrosion resistance than
obtained with 304. Use for fluid lines and gas lines containing moisture.
• 316. For general corrosion resistance—do not mix 304 and 316 when used
in fluid transport because of galvanic corrosion at joints.
4.9.1 Surface Modification
555
Grit/Mesh
Size Emery
Coarse Abrasive Polish
24
30
36
46
54
60
70
80
90
100
120
150
Fine Abrasive Polish
180
220
240
280
320
400
600
800
1000
1200
1500
2000
1
1/0
2/0
3/0
4/0
Table 1
Average Particle Size
AI2O3,
SiC,
Garnet
1035
930
810
508
430
406
328
266
216
173
142
122
70-86
66
54-63
44
29-32
20-23
12.5-17
9-12
5-9
1.5-5
1-1.5
1.0
(microns)
B4C
710
590
500
350
297
250
210
177
149
125
105
74
62
53
42.5
35
27.7
16.3
8.3
5.5
3.7
2.5
Diamond
1036
712
508
407
267
213
142
122
86
66
60
52
40
14
8
6
4
2
1
0.5
0.25
• 316L. Better intergranular corrosion resistance than 316.
• 303. Has a high sulfur content and a higher tendency for porosity—do not
use,
it cannot be welded very well.
• 440. Hardenable but magnetic and more prone to corrosion than the 300
series.
Stainless steel is available as mill plate with several finishes.
• Unpolished
#1
—very dull finish produced by hot-rolling the steel followed
by annealing and descaling. The surface is very rough and porous. Used
where surface finish is not important.

5^6 Chapter 4.9: Preparation and Cleaning of Vacuum Surfaces
• Unpolished #2D —Dull finish produced by a final cold roll after the hot
rolling but before annealing and descaling. Used for deep drawing where the
surface roughness retains the drawing lubricant.
• Unpolished #2B—Bright finish obtained by a light cold roll after anneal-
ing and descaling. Grain boundary etching due to descaling still present.
General-purpose finish.
• Polished #3—Intermediate polish using 50 or 80 grit abrasive compound.
^max of 140 microinches. Heavy polishing grooves.
• Polished #4—General-purpose surface obtained with 100-150 grit abra-
sives.
Z^niax
of
45
microinches. Lighter polishing grooves.
• Buffed #6—Polished with 200 grit abrasive.
• Buffed #7—Polished with 200 grit abrasive with a top dressing using chrome
oxide rouge. R^ of 8-20 microinches.
• Buffed #8—Polished with 320 grit abrasive (or smaller) with an extensive
top dressing using chrome oxide rouge. /?a of ^-14 microinches.* To the
eye,
the surface appears to be free of grinding lines.
Stainless steel plate is often ground to form a flat surface and then plates are
welded to form a chamber. Stainless steel plates may also be shaped by deforma-
tion, which will create a wrinkled surface morphology and may trap lubricants in
the subsurface region. Deformation or machining of stainless steel will work-
harden the surface. This is desirable where the surface must provide a cutting
edge such as when used on CF flanges or for providing a deformation and wear-
resistant surface. Heating to above 450°C will anneal the work-hardened surface,
and the hardness will be lost.
The surface of the steel can be mechanically polished to improve the smooth-
ness.
It can then be chemically polished or electropolished to make it more smooth.
Electropolishing [10] decreases the R^ by about a factor of
2
and eliminates many
of
the
microcracks, asperities, and crevices in the polished surface. Typically elec-
tropolishing is done in an electrolyte containing phosphoric acid and the smooth
areas are protected by a thin phosphate layer, causing the peaks to be removed.
This phosphate layer should be removed using an HCl rinse and then the surface
rinsed to an acid-free condition prior
to
use.
Directed streams of electrolyte ("jets")
can be used to selectively electropolish local areas of a surface [11]. Commercial
suppliers provide electropolishing services to the vacuum industry either at their
plants or on site at the customer's plant.
*The center line average (CLA) or
R^
is the average peak height above the center line of a profile
over the surface. The center line is defined as the line that divides the profile in such a way as the net
deviation is zero. The
R^
tells nothing about maximum peak heights (/?,„ax)' slopes or the lateral di-
mensions of
the
peaks (waviness).
4.9.1 Surface Modification 557
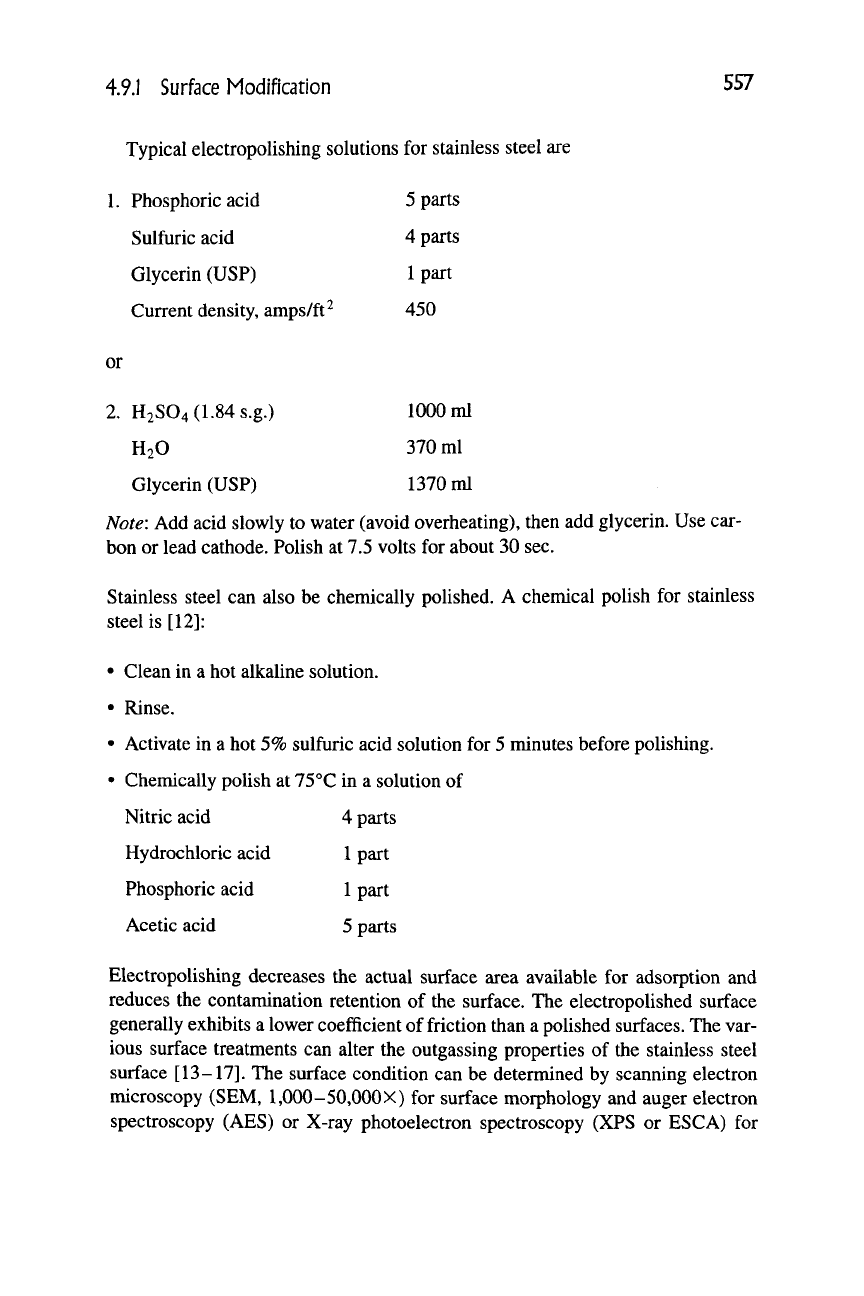
Typical electropolishing solutions for stainless steel are
. Phosphoric acid
Sulfuric acid
Glycerin (USP)
Current density, amps/ft^
5 parts
4 parts
1 part
450
or
2.
H2S04(1.84s.g.) 1000 ml
H2O 370 ml
Glycerin (USP) 1370 ml
Note: Add acid slowly to water (avoid overheating), then add glycerin. Use car-
bon or lead cathode. Polish at 7.5 volts for about 30 sec.
Stainless steel can also be chemically polished. A chemical polish for stainless
steel is [12]:
• Clean in a hot alkaline solution.
• Rinse.
• Activate in a hot 5% sulfuric acid solution for 5 minutes before polishing.
• Chemically polish at 75 °C in a solution of
Nitric acid 4 parts
Hydrochloric acid
1
part
Phosphoric acid
1
part
Acetic acid 5 parts
Electropolishing decreases the actual surface area available for adsorption and
reduces the contamination retention of the surface. The electropolished surface
generally exhibits a lower coefficient of friction than a polished surfaces. The var-
ious surface treatments can alter the outgassing properties of the stainless steel
surface [13-17]. The surface condition can be determined by scanning electron
microscopy (SEM,
1,000-50,000
X) for surface morphology and auger electron
spectroscopy (AES) or X-ray photoelectron spectroscopy (XPS or ESCA) for

558 Chapter 4.9: Preparation and Cleaning of Vacuum Surfaces
surface composition. The chemical composition of electropolished surfaces can
be specified for critical applications [18,19].
Electropolishing, as well as acid treatments, "charge" the steel surface with hy-
drogen, and for UHV applications the stainless steel should be vacuum-baked at
1000°C for several hours to outgas hydrogen taken up by the surface [13]. The
surface of stainless steel will form a natural passive oxide layer when dried and
exposed to the ambient. The passive layer can be improved by heating in air. How-
ever, control of the temperature and dewpoint is very important. A smooth oxide
film is formed on 316L stainless steel at 450°C and a dewpoint of -100°C but
small nodules and surface coarsening result when the oxidation is done above
550°C in air with this dew point [20]. Type 304 and 316 stainless steels are more
easily passivated than are the 400 series (hardenable) stainless steels [21].
The natural oxide on stainless steel can be removed thus:
• Vapor clean in trichloroethane (5 minutes).
• Rinse in cold water.
• Hot alkaline cleaner 5 minutes.
• Rinse in hot water.
• Potassium permanganate (100 ml DI water -f 50 g NaOH + 5 g KMn04 at
95
°C)
— soak to condition oxide scale.
• Hydrochloric acid dip to sensitize surface (remove natural oxide passivation).
• Pickling solution (30 vol% HNO3 4- 3 vol % HF) at room temperature for
30 minutes.
• Rinse in hot, deionized water.
LOW-CARBON STEEL
Low-carbon or mild steel (such as 1018 steel) is low cost with respect to stainless
steel and is often used in applications involving large vacuum vessels. Sometimes
the steel is porous, usually due to "stringers" in the metal plate. This problem is
generally masked by painting the exterior of the chamber to reduce the real leak
rate.
Removal of this paint can open up the porosity and increase the leak rate.
Generally, when using such steels, the surface is used in the as-machined condi-
tion. Low-carbon steel can be cleaned by wiping with neutral pH perchloroethane
or trichloroethane, followed by an acetone wipe followed by drying with anhy-
drous methanol. Water-based cleaning should be avoided because it can contrib-
ute to rusting of the steel.
Carbon steel and low alloy steels can be cleaned by pickling in a hydrochloric
acid bath (8-12 wt %) at 40°C for 5-15 min to strip the oxide from the surface
[22].
A simple technique for removing localized iron rust is (1) solvent clean,
(2) soak (or wet) in acetic acid, (3) brush away residue, and (4) repeat as neces-
sary. Low-carbon steel can also be electropolished, but this is not typically done.

4.9.1 Surface Modification 559
ALUMINUM ALLOYS
Aluminum alloys are not normally used for vacuum processing systems because
they are soft and easily corroded, especially by chlorine-containing chemicals.
With proper fabrication, aluminum has proven to be a good high- and ultra-high-
vacuum material [23]. However, mill-rolled aluminum has an outgassing rate
-100 times that of mill-rolled stainless steel [24]. A dense, thin oxide with good
outgassing properties can be formed on aluminum surfaces by (1) machining un-
der a dry, chlorine-free argon/oxygen gas, (2) machining under pure anhydrous
ethanol, or (3) extrusion under a dry, chlorine-free argon/oxygen gas [25-27].
The natural oxide on aluminum can be removed (stripped) before polishing. A
chemical strip for the oxide on aluminum is as follows:
• Soak in solution of
5%
NaOH by weight at 70-75°C.
• Soak in a solution of 1 part concentrated HNO3 to 1 part deionized water
at 20°C, followed by a dip in a solution of
1
part concentrated HNO3 with
64 g/liter NH4HF2 at 20°C (desmutting procedure—Cu and Si).
• Rinse well.
The aluminum can be chemically polished thus:
1.
• SoakinlO%HCL
• Rinse in deionized water.
or
2.
• Make a solution of
H3PO4 80%
CH3COOH 15%
HNO3 5%
• Raise to temperature 90 to 110°C.
• Dip for 2-4 min.
Heavily corroded aluminum surfaces can be electrocleaned thus:
• Pickle in 5% NaOH solution at 75°C
• Washin30%HNO3.
• Dip in 12% H2SO4;
• Follow with an anodic electroetch at 90°C in a solution of 100 g H3BO3 and
0.5 g borax in 1 liter deionized water starting at 50 volts and increasing to
600 volts.

560 Chapter 4.9: Preparation and Cleaning of Vacuum Surfaces
Anodized Aluminum
In special cases where the surface hardness must be increased or chemical corro-
sion resistance is necessary (e.g., plasma etching with chlorine), anodized alu-
minum surfaces can be useful [28,29]. Alloying elements, impurities and heat
treatment can influence the nature and quality of the anodized coating — typi-
cally, the purer the aluminum alloy, the better the anodized layer. To build up a
thick anodized layer on aluminum, it is necessary for the electrolyte to continu-
ously corrode the oxide, giving a porous oxide layer. ASTM Specification B-580-
73 designates seven thicknesses (up to 50 microns) for anodization with letter
designations of A through G. Anodization baths for the various thicknesses are
• Oxalic anodize — very thick films (50 microns)
• Sulfuric acid — thick films (80% aluminum oxide, 18% aluminum sulfate,
2%
water—15% porosity)
• Chromic acid—thin films (1-2 microns)
• Phosphoric acid—very porous films (base for organic coatings)
After formation, the porous aluminum oxide can be "sealed" by hydration, which
swells the amorphous oxide. Sealing is done in hot (95-100°C) deionized water
(sulfuric acid anodize only) or by the use of sodium dichromate solution (improve
corrosion resistance) or with nickel or cobalt acetate solutions. Sealing reduces
the hardness of the anodized film. Steam sealing can be used to avoid the use of
nickel-containing hot water to prevent the possibility of nickel contamination in
semiconductor manufacturing. For vacuum use, the anodized surface should be
vacuum-baked before use. To increase the corrosion protection or lubricity of the
anodized surface, other materials can be deposited in the porous surface. Examples
are the Magnaplate® coating to improve corrosion protection and Tufram® coat-
ing using to improve the frictional properties of anodized aluminum surfaces.
Anodized aluminum does not provide a good surface for sealing with elas-
tomer seals. In anodized systems, the sealing surfaces are often machined to re-
veal the underlying aluminum. These surfaces can be protected from corrosion
with a thin layer of a chemically resistant grease such as Krytox®.
Aluminum can be anodized with
a
dense oxide (barrier anodization) [30] but this
technique has not been evaluated for vacuum application, because it is rather thin.
COPPER
Copper is often used in vacuum systems as an electrical conductor or as a shear-
sealing material. The polishing of copper often means the removal of the oxide
layer. Copper can be polished (smoothed) thus:
• Inmierse in a solution of
60 ml phosphoric acid (s.g. 1.75)

4.9.1 Surface Modification 561
10 ml nitric acid (s.g. 1.42)
10 ml acetic anhydride and
8 ml water
for 4 minutes at room temperature
The oxide can be removed from copper thus:
1.
• Clean in perchloroethylene.
• Ultrasonic clean in alkaline detergent (pH = 9.7) at 60T for 5 to
10 minutes.
• Rinse.
• Deoxidize in 50 vol % HCl at room temperature for 5 to 10 minutes.
• Rinse.
2.
• Solvent clean.
• Inmierse in solution of
60 ml phosphoric acid (s.g. 1.75)
10 ml nitric acid (s.g. 1.42)
10 ml acetic anhydride
8 ml water
for 4 minutes at room temperature
• Rinse.
CERAMICS AND GLASSES
Ceramics and glasses develop surface microcracks when ground or polished.
These microcracks reduce the strength of the material as well as contribute to sur-
face retention of contamination. Oxide ceramics and glasses can be etched in a
solution of hydrofluoric acid (HF) or ammonium bifluoride, which will mildly
etch the surface and blunt the microcracks.
POLYMERS
The use of polymers in the vacuum system should be minimized. If used, the
polymers should be outgassed by vacuum baking before being installed in the sys-
