Higgins R.A. Engineering Metallurgy: Applied Physical Metallurgy
Подождите немного. Документ загружается.

at as low a temperature as possible. In fact a little more incompatibility
would lead to a complete 'divorce' to the extent that the metals would
separate forming a eutectic, probably of two solid solutions. We shall in
fact deal with this type of system in the next case.
Case IV—Two metals mutually soluble in all
proportions in the liquid state but only partially
soluble in the solid state
9.60 This case is in effect intermediate between the two dealt with in
Cases II and III and the thermal equilibrium diagram is, in consequence,
a sort of hybrid of these two. It represents a stage where incompatibility
has exceeded that suggested in Fig. 9.11. As in the cadmium-bismuth
system (where the metals are completely incompatible in the solid state)
a eutectic is formed, but in this case it is a eutectic of two solid solutions
instead of two pure metals. It is in fact the 'general' case and purists may
argue that, as such, it should have been dealt with before either of the
preceding particular cases. The systems have, however, been dealt with in
order of complexity rather than logical classification in order to assist the
student who may be encountering the subject for the first time.
The only new feature of this system, as compared with those foregoing,
is that we have phase boundaries occurring below the solidus, indicating
that phase changes can take place in the solid. On the tin-lead thermal-
equilibrium diagram (Fig. 9.12), which we shall use as an example of this
type of system, such phase boundaries as these are indicated by the lines
BC and FG. A line such as BC or FG is termed a solvus and indicates a
change in solubility of one metal in another in the solid state. Part of the
tin-lead system was used in Chapter 7 as an introduction to equilibrium
diagrams. We will now consider it in its entirety.
9.61 As the diagram shows, we have two solid solutions in the system,
since:
(a) Tin will dissolve up to a maximum of 2.6% lead at the eutectic
temperature, forming the solid solution a.
(b) Lead will dissolve up to a maximum of 19.5% tin at the eutectic
temperature, giving the solid solution (3.
(The different phases present in an alloy series are generally lettered from
left to right across the diagram, using letters of the Greek alphabet for
convenient reference.)
The slope of the phase boundaries BC and FG indicates that both the
solubility of lead in tin (a) and the solubility of tin in lead (|3) decrease as
the temperature falls. This is a normal phenomenon with solid solutions
as it is with liquid solutions. In Fig. 9.13 the solubility of a salt in water at
different temperatures is compared with the solid solubility of one metal
in another at different temperatures. In each case solubility increases as
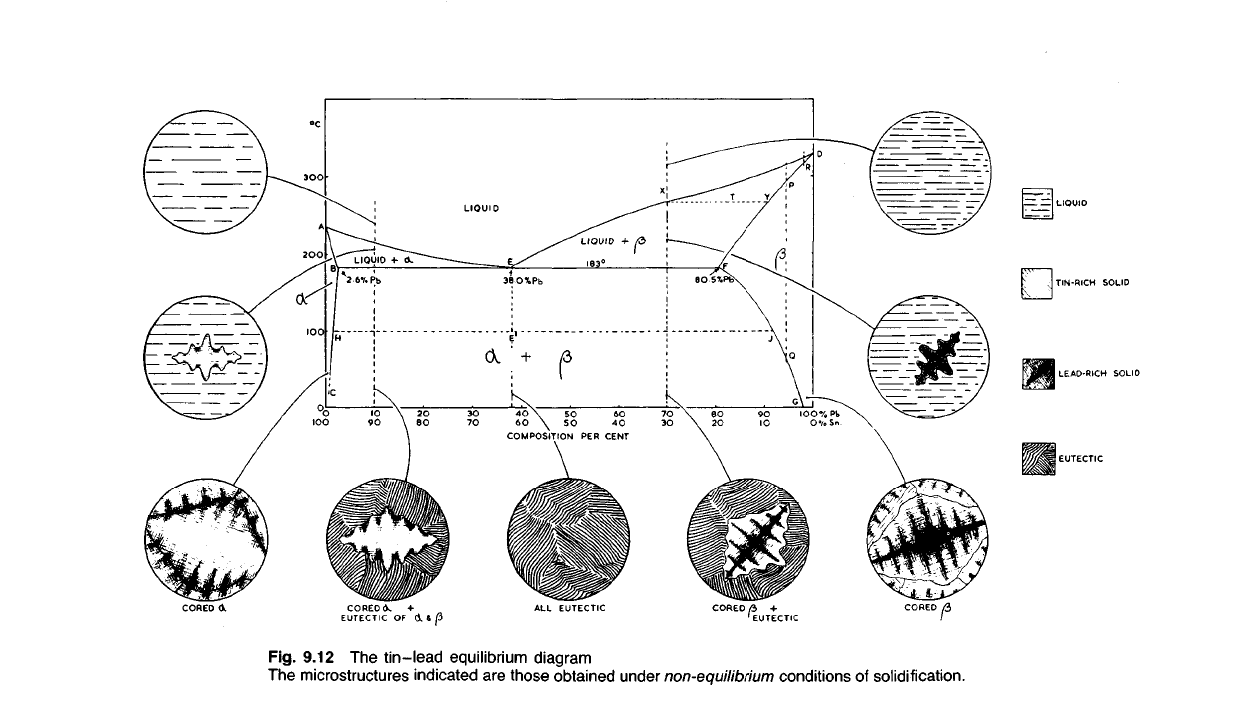
Fig. 9.12 The tin-lead
equilibrium diagram
The
microstructures indicated
are
those obtained under
non-equilibrium
conditions
of
solidification.
LIQUID
TIN-RICH SOLID
LEAD-RICH
SOLID
EUTECTIC
CORED
/3 +
'EUTECTIC
ALL EUTECTIC
LIQUID
+• /S>
LIQUID
COMPOSITION
PER
CENT
CORED(^
+
EUTECTIC
OF U)3
CORED
O.
LIQUID
+ <y.
a + /3
CORED
/3
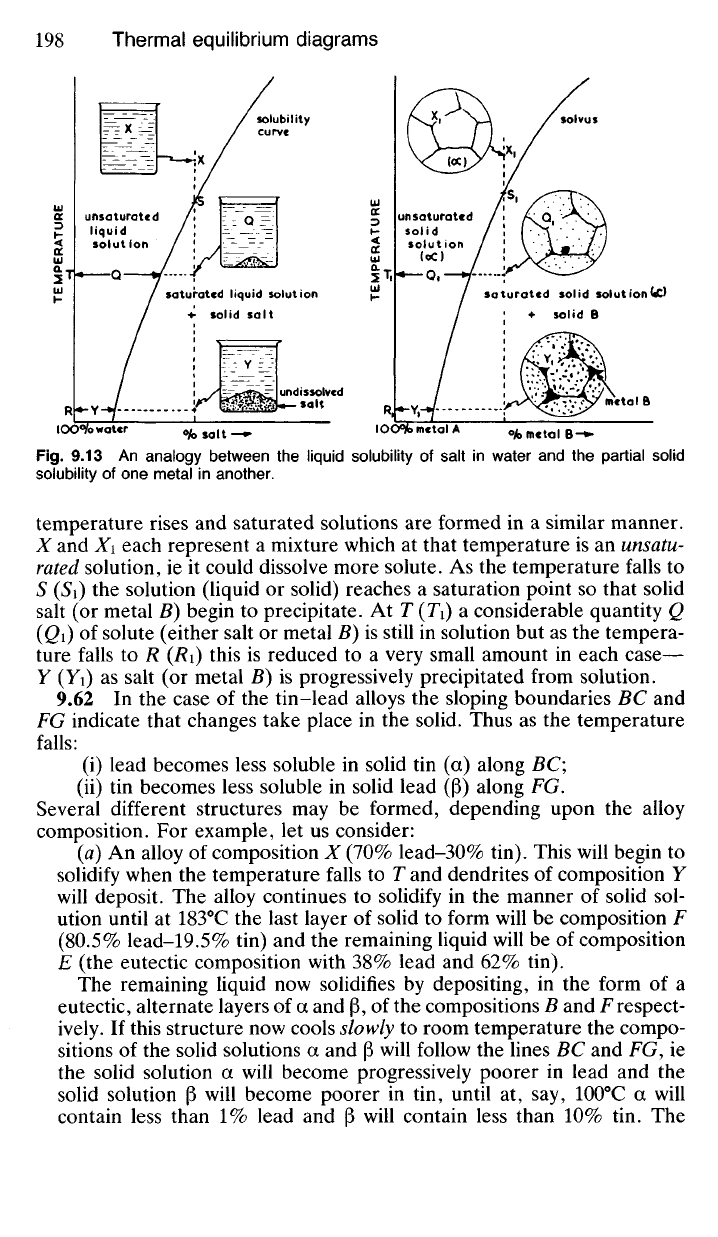
Fig.
9.13 An analogy between the liquid solubility of salt in water and the partial solid
solubility of one metal in another.
temperature rises
and
saturated solutions
are
formed
in a
similar manner.
X
and
X\
each represent
a
mixture which
at
that temperature
is an
unsatu-
rated solution,
ie it
could dissolve more solute.
As the
temperature falls
to
S
(Si) the
solution (liquid
or
solid) reaches
a
saturation point
so
that solid
salt
(or
metal
B)
begin
to
precipitate.
At T
(Ti)
a
considerable quantity
Q
(Qi)
of
solute (either salt
or
metal
B) is
still
in
solution
but as the
tempera-
ture falls
to R (Ri)
this
is
reduced
to a
very small amount
in
each case—
Y
(Yi) as
salt
(or
metal
B) is
progressively precipitated from solution.
9.62
In the
case
of the
tin-lead alloys
the
sloping boundaries
BC and
FG indicate that changes take place
in the
solid. Thus
as the
temperature
falls:
(i) lead becomes less soluble
in
solid
tin (a)
along
BC;
(H)
tin
becomes less soluble
in
solid lead
(|3)
along
FG.
Several different structures
may be
formed, depending upon
the
alloy
composition.
For
example,
let us
consider:
(a)
An
alloy
of
composition
X
(70% lead-30%
tin).
This will begin
to
solidify when
the
temperature falls
to T
and
dendrites
of
composition
Y
will deposit.
The
alloy continues
to
solidify
in the
manner
of
solid
sol-
ution until
at
183°C
the
last layer
of
solid
to
form will
be
composition
F
(80.5%
lead-19.5%
tin) and the
remaining liquid will
be of
composition
E
(the
eutectic composition with
38%
lead
and
62%
tin).
The remaining liquid
now
solidifies
by
depositing,
in the
form
of a
eutectic, alternate layers
of a
and
|3,
of
the
compositions
B
and
F
respect-
ively.
If
this structure
now
cools slowly
to
room temperature
the
compo-
sitions
of the
solid solutions
a and |3
will follow
the
lines
BC and
FG,
ie
the solid solution
a
will become progressively poorer
in
lead
and the
solid solution
(3
will become poorer
in tin,
until
at, say,
100
0
C
a
will
contain less than
1%
lead
and (3
will contain less than
10% tin. The
TEMPERATURE
TEMPERATURE
solubility
curve
solvus
unsaturatcd
solid
solution
j
(cC)
/
saturated solid solutionWC)
+ solid
B
metal
B
% metal
B
IOOfcmctalA
undissolved
salt
°A> salt
10O
4
Yo water
saturated liquid solution
+ solid salt
unsaturatcd
liquid
solution
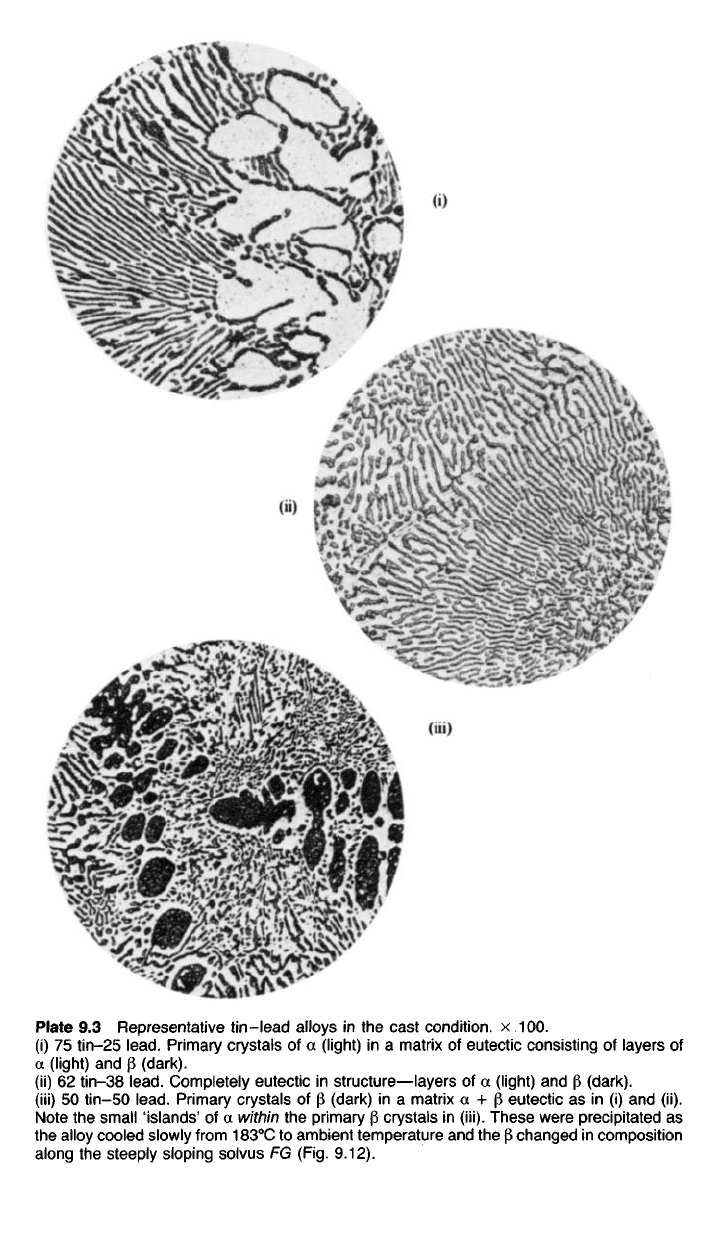
Plate 9.3 Representative tin-lead alloys in the cast condition, x 100.
(i) 75 tin-25
lead.
Primary crystals of a (light) in a matrix of eutectic consisting of layers of
a (light) and p (dark).
(ii) 62 tin—38
lead.
Completely eutectic in structure—layers of a (light) and P (dark).
(iii) 50 tin-50
lead.
Primary crystals of p (dark) in a matrix a + p eutectic as in (i) and (ii).
Note the small 'islands' of a within the primary |3 crystals in (iii). These were precipitated as
the alloy cooled slowly from 183°C to ambient temperature and the P changed in composition
along the steeply sloping solvus FG (Fig. 9.12).
(iii)
(ii)
(i)
proportions of a and (3 will also vary from EFIEB at 183°C to E'JIE'H
at 100
0
C. In the same way, provided we assume that the structure cooled
slowly enough to allow the primary dendrites of (3 to reach a uniform
composition F, these dendrites will now alter in composition to / at
100
0
C,
and in so doing precipitate some a of composition H in order to
adjust their own composition. The a precipitated will join that already
present in the eutectic.
(b) If the alloy contained, say, 95% lead, and was cooled slowly
enough to prevent coring, solidification would be complete at P and a
uniform solid solution |3 would result. On continuing to cool slowly,
further solid changes would begin at Q with the precipitation of small
amounts of a at the grain boundaries of the (3. This a would increase in
amount as the temperature fell, and the |3 became progressively poorer
in tin. Hence the final structure would consist of crystals of uniform (3
containing about 98% lead with small amounts of a precipitated at the
crystal boundaries.
(c) If the original alloy contained more than 98% lead and was cooled
slowly, the structure would remain entirely
(3
throughout, after its solidi-
fication had been completed at R.
9.63 In actual practice it is unlikely that cooling would be slow enough
to prevent some coring from taking place. Since the initial |3 dendrites
would then be relatively rich in lead, this could lead to the formation of
small amounts of a at the (3 grain boundaries in (c) and more than the
expected amount of a in (b). In case (c) this a would be absorbed on
annealing. 'Solution annealing' is an important process used in the treat-
ment of many alloys to absorb some constituent which has been precipi-
tated due to coring. After treatment the alloy will be soft and ductile and
able to receive cold-work. Solution annealing is also an integral part of
most strengthening processes based on precipitation hardening, the prin-
ciples of which will be discussed later (9.92).
Case V—A system in which a peritectic
transformation is involved
9.70 Sometimes in an alloy system two phases which are already present
interact at a fixed temperature to produce an entirely new phase. This is
known as a peritectic transformation. The term is derived from the Greek
'peri',
which means 'around', since, during the transformation process, the
original solid phase becomes surrounded or coated by the transformation
product. Frequently one of the interacting phases is a liquid though this is
not a precondition and sometimes two solids will take part—albeit very
slowly—in a peritecto/d transformation. A peritectic transformation occurs
between the phase 6 and the remaining liquid (producing austenite) in
the iron-carbon system (11.20) but here we will consider the peritectic
transformation which takes place in the platinum-silver system, part of
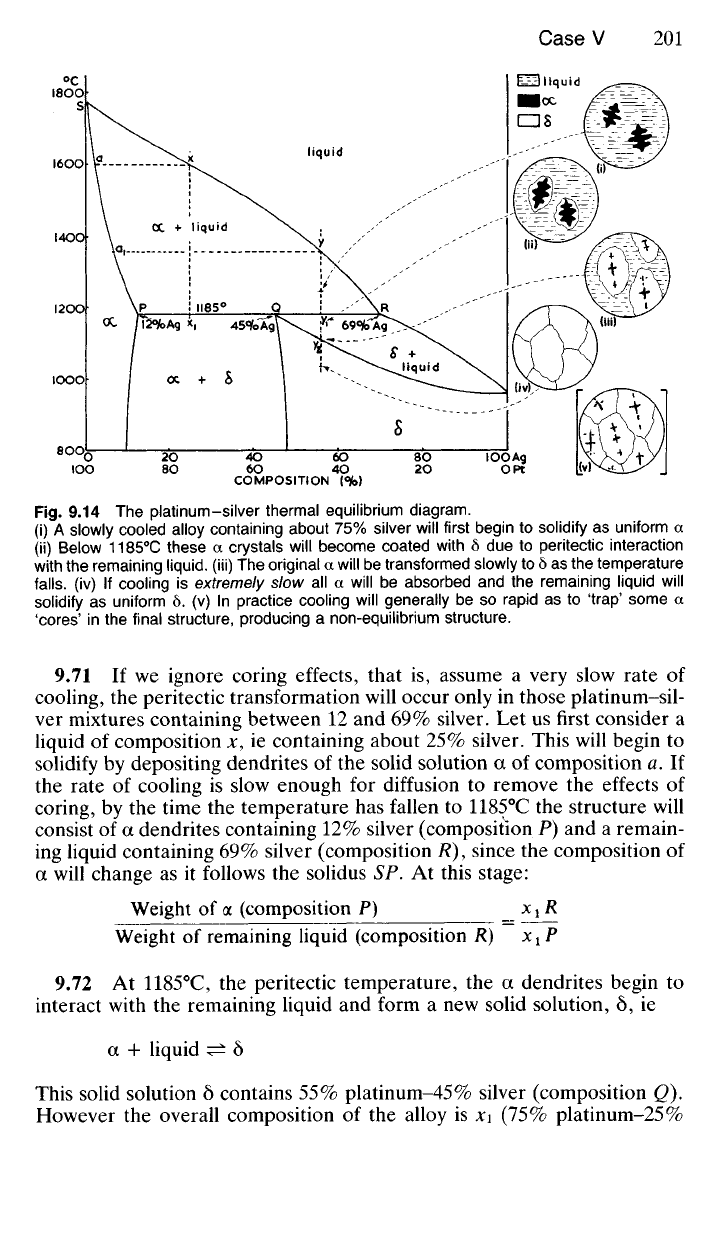
which is shown in Fig. 9.14.
COMPOSITION (°/o)
Fig.
9.14 The platinum-silver thermal equilibrium diagram.
(i) A slowly cooled alloy containing about 75% silver will first begin to solidify as uniform a
(ii) Below 1185°C these a crystals will become coated with 6 due to peritectic interaction
with the remaining liquid, (iii) The original a will be transformed slowly to 6 as the temperature
falls,
(iv) If cooling is extremely slow all a will be absorbed and the remaining liquid will
solidify as uniform 6. (v) In practice cooling will generally be so rapid as to 'trap' some a
'cores'
in the final structure, producing a non-equilibrium structure.
9.71 If we ignore coring effects, that is, assume a very slow rate of
cooling, the peritectic transformation will occur only in those platinum-sil-
ver mixtures containing between 12 and 69% silver. Let us first consider a
liquid of composition x, ie containing about 25% silver. This will begin to
solidify by depositing dendrites of the solid solution a of composition a. If
the rate of cooling is slow enough for diffusion to remove the effects of
coring, by the time the temperature has fallen to 1185°C the structure will
consist of a dendrites containing 12% silver (composition P) and a remain-
ing liquid containing 69% silver (composition R), since the composition of
a will change as it follows the solidus SP. At this stage:
Weight of a (composition P) X
1
R
Weight of remaining liquid (composition R) X
1
P
9.72 At 1185°C, the peritectic temperature, the a dendrites begin to
interact with the remaining liquid and form a new solid solution, 6, ie
a + liquid ^ 5
This solid solution b contains 55% platinum-45% silver (composition Q).
However the overall composition of the alloy is x\ (75% platinum-25%
0
C
liquid
CC + liquid
S
+
liquid
OC + S
S
OC
liquid
CC
8
silver) and it follows that not all of the a will be used up, since it is in
excess of that required to produce a structure entirely of 5. Therefore, all
of the liquid is used up and some a remains, so that the final structure will
consist of a containing 12% silver (composition P), and 5 containing 45%
silver (composition Q). These phases will be in a ratio given by:
Weight of a (composition P) X
1
Q
Weight of 3 (composition Q) X
1
P
9.73 Let us now consider a liquid alloy of initial composition y. This
will begin to solidify by depositing crystals of a of composition ai, and if
we again neglect coring, the crystals will gradually change in composition
to P when the temperature has fallen to 1185
0
C, and again the composition
of the remaining liquid will be R. At this stage—
Weight of a (composition P) ^
1
R
Weight of remaining liquid (composition R) y
x
P
When the peritectic transformation takes place, we shall find that this time
there is an excess of liquid because yi lies between Q and R. The a will
therefore be completely used up, and just below 1185°C we shall be left
with the new solid solution, 8, and some remaining liquid in the ratio—
Weight of S (composition Q) y
x
R
Weight of remaining liquid (composition R) y
x
Q
As the temperature continues to fall slowly, this remaining liquid will
solidify as 5, which will change in composition along Qy
2
. At y
2
solidifi-
cation will be complete and the structure will consist of crystals of uniform
5 of the same composition as that of the original liquid. In practice it is
unlikely that the rate of cooling would be slow enough to permit equilib-
rium being reached. Consequently, though the equilibrium structure is
indicated as being completely b (in the case of alloy y), remnants of the
original a dendrites may be present in the rapidly-cooled structure. The
original a dendrites, produced at temperatures above 1185°C, became
coated with a protective envelope of 8 as the interaction with the liquid
began and subsequent diffusion was far too slow to promote dispersal of
the remaining a.
Case Vl—Systems containing one or more
intermediate phase
9.80 Frequently two metals will not only form limited solid solutions with
each other but, in other suitable proportions, will also interact to form one
or more intermediate phases which may vary in nature from secondary
solid solutions to true intermetallic compounds. The formation of any
intermediate phase further complicates
the
thermal equilibrium diagram
by introducing extra boundary lines
and
since some alloy systems include
a number
of
intermediate phases, formed
at
different compositions
of the
two metals,
the
resulting diagram becomes increasingly complex.
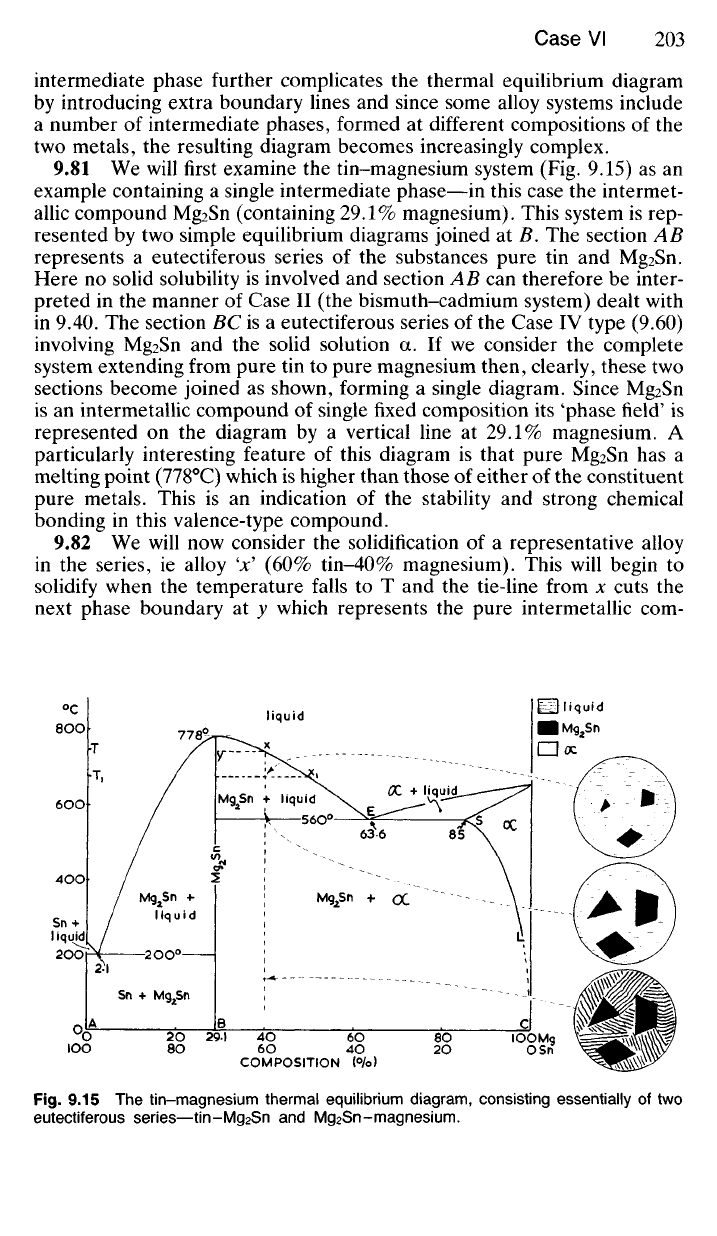
9.81
We
will first examine
the
tin-magnesium system
(Fig. 9.15) as an
example containing
a
single intermediate phase—in this case
the
intermet-
allic compound Mg
2
Sn (containing 29.1% magnesium). This system
is rep-
resented
by two
simple equilibrium diagrams joined
at B. The
section
AB
represents
a
eutectiferous series
of the
substances pure
tin and
Mg
2
Sn.
Here
no
solid solubility
is
involved
and
section
AB can
therefore
be
inter-
preted
in the
manner
of
Case
II (the
bismuth-cadmium system) dealt with
in
9.40. The
section
BC is a
eutectiferous series
of the
Case
IV
type (9.60)
involving Mg
2
Sn
and the
solid solution
a. If we
consider
the
complete
system extending from pure
tin to
pure magnesium then, clearly, these
two
sections become joined
as
shown, forming
a
single diagram. Since Mg
2
Sn
is
an
intermetallic compound
of
single fixed composition
its
'phase field'
is
represented
on the
diagram
by a
vertical line
at 29.1%
magnesium.
A
particularly interesting feature
of
this diagram
is
that pure Mg
2
Sn
has a
melting point (778°C) which
is
higher than those
of
either
of the
constituent
pure metals. This
is an
indication
of the
stability
and
strong chemical
bonding
in
this valence-type compound.
9.82
We
will
now
consider
the
solidification
of a
representative alloy
in
the
series,
ie
alloy
'x' (60%
tin-40% magnesium). This will begin
to
solidify when
the
temperature falls
to T and the
tie-line from
x
cuts
the
next phase boundary
at y
which represents
the
pure intermetallic
com-
0
C
liquid
liquid
Mg
2
Sn
OC
liquid
OZ +
liquid
liquid
liquid
Fig.
9.15 The
tin-magnesium thermal equilibrium diagram, consisting essentially
of two
eutectiferous series—tin-Mg
2
Sn
and
Mg
2
Sn-magnesium.
COMPOSITION
(%>)
pound Mg
2
Sn. Therefore crystals of Mg
2
Sn begin to form. Since these
crystals are relatively tin-rich (70.9% tin) the composition of the remaining
liquid moves to the right to 'x{ and as the temperature falls to T\ more
Mg
2
Sn solidifies. Mg
2
Sn continues to solidify in this way until at 560
0
C the
remaining liquid contains 63.6% magnesium-36.4% tin—the composition,
E, of the Mg
2
Sn +a eutectic.
The remaining liquid then solidifies as eutectic at 560
0
C by depositing
alternate layers of Mg
2
Sn and the solid solution a, containing 85% mag-
nesium-15%
tin (composition S). When completely solid the structure will
consist of primary crystals of the intermetallic compound Mg
2
Sn sur-
rounded by a eutectic of Mg
2
Sn and a. If the solid alloy is then allowed to
cool slowly below 560
0
C, a will change in composition along the solvus line
SL by depositing particles of Mg
2
Sn which will join the Mg
2
Sn constituent
already present in the eutectic.
9.83 The large size of lead atoms compared with those of many other
metals contributes to the fact that lead forms relatively few solid solutions.
Thus lead and gold are to all intents and purposes insoluble in each other
in the solid state but, due to differences in the electrochemical properties
of the metals two intermediate phases, Au
2
Pb and AuPb
2
are formed at
the appropriate compositions. These two intermediate phases are produced
as a result of peritectic interactions and the resultant phase diagram is
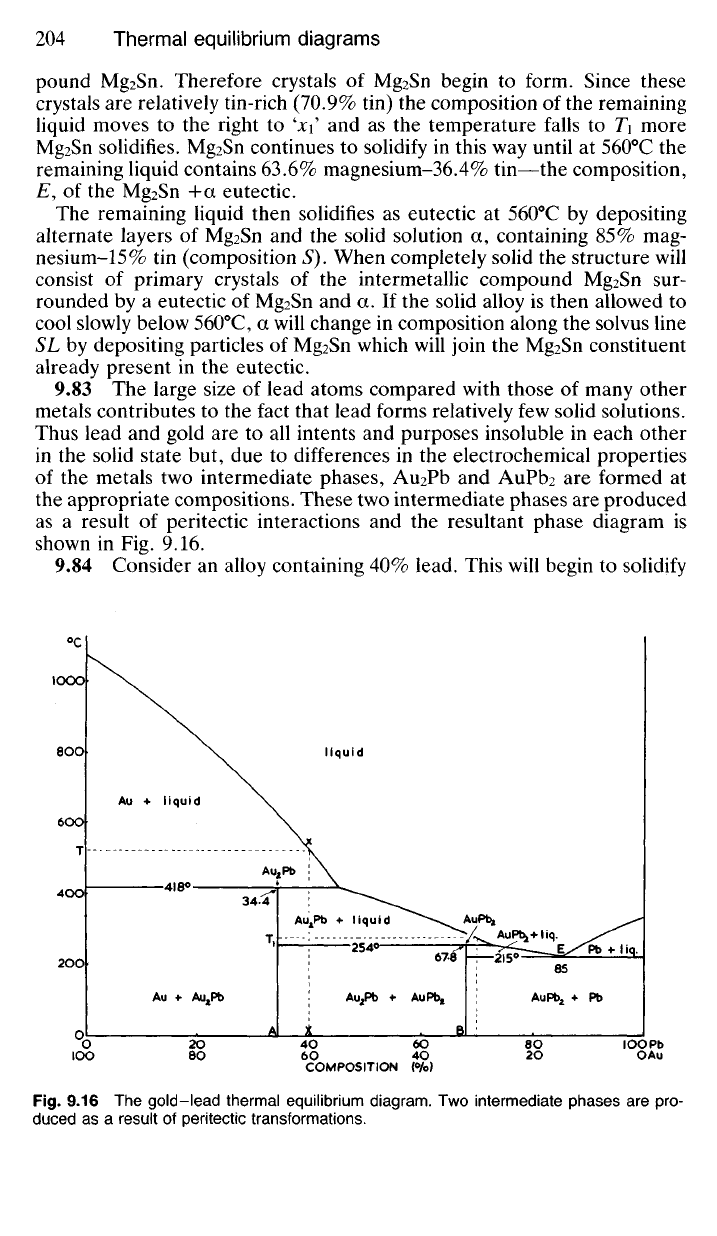
shown in Fig. 9.16.
9.84 Consider an alloy containing 40% lead. This will begin to solidify
°c
liquid
Au + liquid
Au
1
Pb + liquid
Fig.
9.16 The gold-lead thermal equilibrium diagram. Two intermediate phases are pro-
duced as a result of peritectic transformations.
COMPOSITION (o/o)
at about 500
0
C (T) by forming dendrites of gold, but on cooling to 418°C,
the first peritectic temperature, these gold dendrites undergo a peritectic
interaction with the remaining liquid to produce crystals of the intermedi-
ate phase, Au2Pb:
Au + liquid ^ AU2 Pb
These crystals of Au
2
Pb continue to grow as the temperature falls, but at
254°C a further peritectic interaction occurs resulting in the formation of
another intermediate phase AuPb
2
:
Au
2
Pb + liquid ^± AuPb
2
During this second transformation all of the liquid is used up first and some
Au
2
Pb remains along with the new phase AuPb
2
. Assuming that the system
has cooled slowly to room temperature these two phases will be present
in the ratio:
Weight OfAu
2
Pb _ BX
Weight of AuPb
2
~~
~AX
_ 67.8-40
" 40 - 34.4
_27.8
_5
~T
9.85 An alloy containing 70% lead will begin to solidify at about 280
0
C
(Ti) by depositing crystals of Au
2
Pb but at 254°C a peritectic interaction
results in the formation of AuPb
2
. Here all of the Au
2
Pb is used up and
the remaining liquid continues to solidify as the new phase AuPb
2
until at
215°C the remaining liquid is of composition E (85% lead-15% gold). A
eutectic consisting of alternate layers of AuPb
2
and pure lead is then
formed so that the final structure consists of primary AuPb
2
in a eutectic
of AuPb
2
and lead.
In both of these cases it has been assumed that cooling has been slow
enough to permit equilibrium to be attained throughout so that peritectic
transformations proceed to completion. In practice this may not be so.
Thus in the 40% lead alloy more than the indicated quantity of Au
2
Pb may
be present in the microstructure whilst in the 70% lead alloy some Au
2
Pb
cores may be present even though the diagram indicates a AuPb
2
-lead
structure.
9.86 Sometimes an intermediate phase is of variable composition. Thus
the phase (3 in the tantalum-nickel phase diagram (Fig. 9.17) is based on
TaNi
3
but it may be thought of as being able to dissolve either excess
tantalum or excess nickel so that the phase field occupies width on the
diagram.
