Higgins R.A. Engineering Metallurgy: Applied Physical Metallurgy
Подождите немного. Документ загружается.

COMPOSITION
Fig.
9.5.
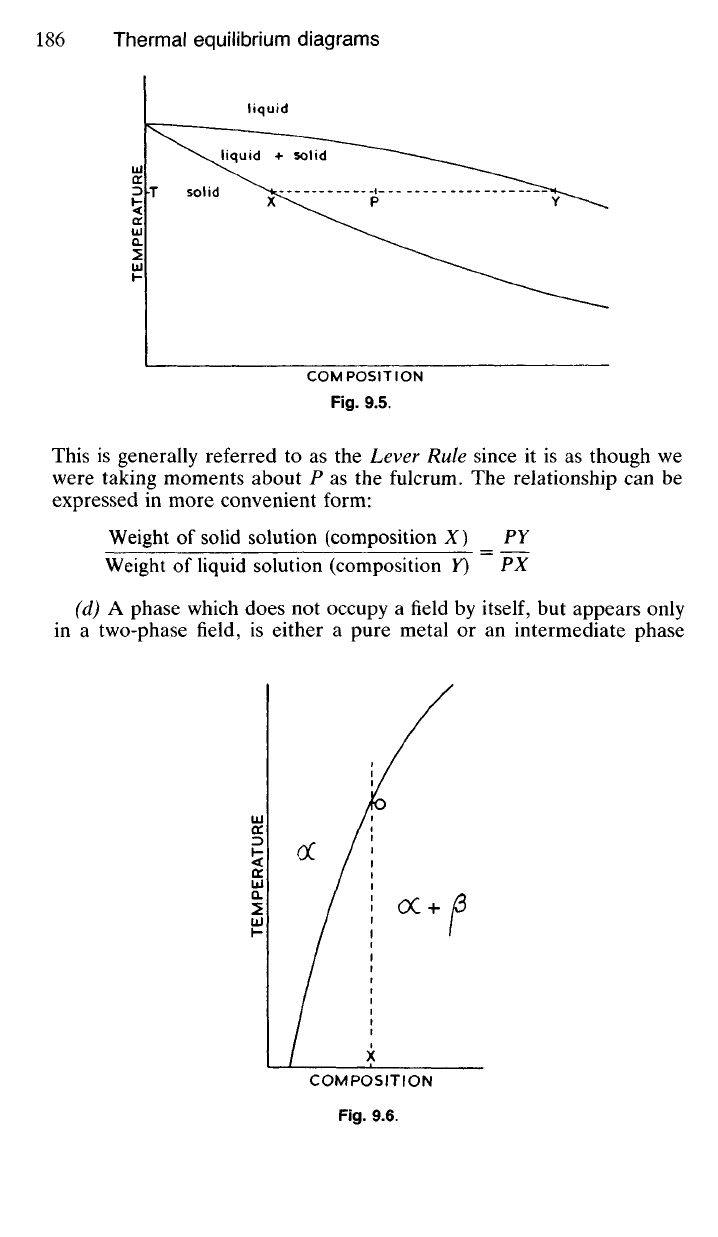
This is generally referred to as the Lever Rule since it is as though we
were taking moments about P as the fulcrum. The relationship can be
expressed in more convenient form:
Weight of solid solution (composition X) PY
Weight of liquid solution (composition Y) PX
(d) A phase which does not occupy a field by
itself,
but appears only
in a two-phase field, is either a pure metal or an intermediate phase
TEMPERATURE
TEMPERATURE
liquid
liquid 4- solid
solid
COMPOSITION
Fig.
9.6.

(usually an intermetallic compound) of invariable composition. In fact
the phase 'field' will be infinitely narrow such as is represented by the
100%
bismuth ordinate in Fig. 9.4 or the Mg
2
Sn ordinate in Fig. 9.15.
(e) If we draw a vertical line on a diagram this will represent the
composition of a given alloy (X in Fig. 9.6). If, on following along this
line,
a phase boundary is crossed (at O) the number of phases will change
by one at this point, ie a phase (|3) will be either precipitated or absorbed.
(In this instance (3 will be absorbed when the alloy X is heated above O
and precipitated again when the alloy X cools below O.)
We will now consider the main types of thermal equilibrium diagram
which are of use in studying metallic alloy systems. Generally a useful alloy
will only be formed when the two metals are completely soluble in each
other in the liquid state; but in some instances the two metals are only
partially soluble as liquids. We will begin with a brief study of one such
case.
Case
I—Two
metals which are only partially
soluble in each other in the liquid state
9.30 Fig. 9.7 represents the phase equilibrium conditions for the zinc-
lead system above 500
0
C. As in all of these equilibrium diagrams the base
line shows the composition varying between 100% of one component at
one side of the diagram to 100% of the other component at the opposite
side.
In most diagrams the percentages are by weight but in some cases it
is more relevant to indicate percentages in terms of numbers of atoms.
The ordinate represents temperature. Thus point P represents a mixture
consisting of 70% by weight of zinc and 30% by weight of lead at a tempera-
ture of 700
0
C.
9.31 The diagram (Fig. 9.7) shows that at 500
0
C molten zinc will dis-
solve no more than 2% lead (point A) giving a single solution of lead in
zinc.
At the same temperature molten lead will dissolve about 5% zinc
(point B) thus giving a single solution of zinc in lead. However, any alloy
at 500
0
C which has a composition given by some point between A and B
will consist of two separate layers of two different liquid solutions—the
upper layer will be zinc containing 2% dissolved lead (composition A) and
the lower layer will be lead containing 5% dissolved zinc (composition B).
9.32 The slope of the line ADJC indicates that as the temperature rises
lead becomes increasingly soluble in molten zinc. Similarly the slope of
BEC shows that, under similar conditions, the solubility of zinc in molten
lead also increases. These two solubility curves meet at 798°C indicating
that at temperatures above this, the two metals become completely soluble
in each other so that a single liquid phase will be present in the mixture
whatever its overall composition.
9.33 Consider a molten mixture consisting of 70% zinc and 30% lead
(composition H). At 500
0
C this will consist of a saturated layer of lead in
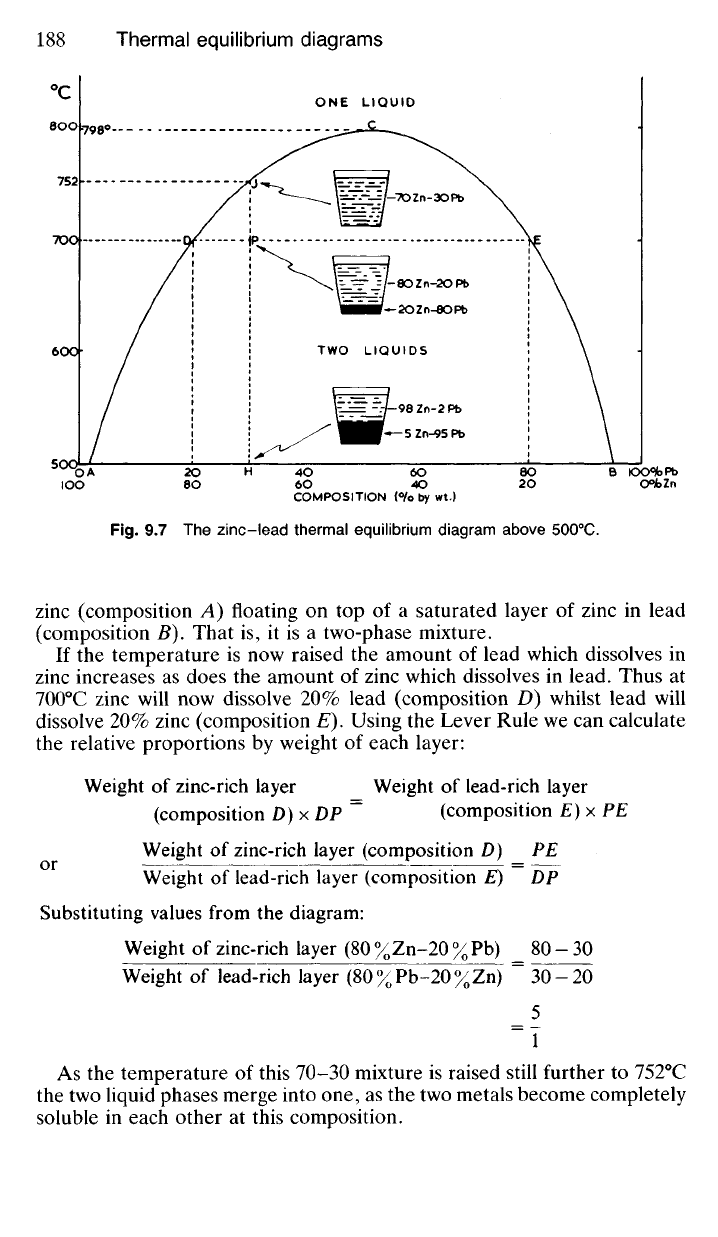
COMPOSITION {%> by wt.)
Fig.
9.7 The zinc-lead thermal equilibrium diagram above 500
0
C.
zinc (composition A) floating on top of a saturated layer of zinc in lead
(composition B). That is, it is a two-phase mixture.
If the temperature is now raised the amount of lead which dissolves in
zinc increases as does the amount of zinc which dissolves in lead. Thus at
700
0
C zinc will now dissolve 20% lead (composition D) whilst lead will
dissolve 20% zinc (composition E). Using the Lever Rule we can calculate
the relative proportions by weight of each layer:
Weight of zinc-rich layer Weight of lead-rich layer
(composition D)xDP
=
(composition E) x PE
Weight of zinc-rich layer (composition D) PE
Weight of lead-rich layer (composition E) DP
Substituting values from the diagram:
Weight of zinc-rich layer (80%Zn-20%Pb) _ 80-30
Weight of lead-rich layer (80%Pb-20%Zn) ~ 30-20
_5
~T
As the temperature of this 70-30 mixture is raised still further to 752°C
the two liquid phases merge into one, as the two metals become completely
soluble in each other at this composition.
KX)<*>Pb
O°A>Zn
°C
ONE LIQUID
TWO LIQUIDS
Case II—Two metals mutually soluble in all
proportions in the liquid state becoming
completely insoluble in the solid state
9.40 It is extremely doubtful whether such a situation as this really exists
in practice since most solid metals appear to dissolve quantities, however
small, of other metals. However, in the case of bismuth and cadmium the
mutual solid solubility is so small that we can neglect it for the sake of our
argument and assume that the metals are completely insoluble in the solid
state.
Bismuth, a heavy, brittle, slightly pink metal, is one of the least
'metallic' of metals as indicated by its position near to the non-metals in
the Periodic Table. It forms a complex rhombic-type crystal structure in
which the metallic bond is partly replaced by a mixture of covalent bonds
and van der Waals forces. Cadmium on the other hand crystallises as a
normal CPH structure so that it is not surprising that these two metals with
such unlike lattice structures do not form mixed crystals but separate out
almost completely on solidification. The bismuth-cadmium thermal-
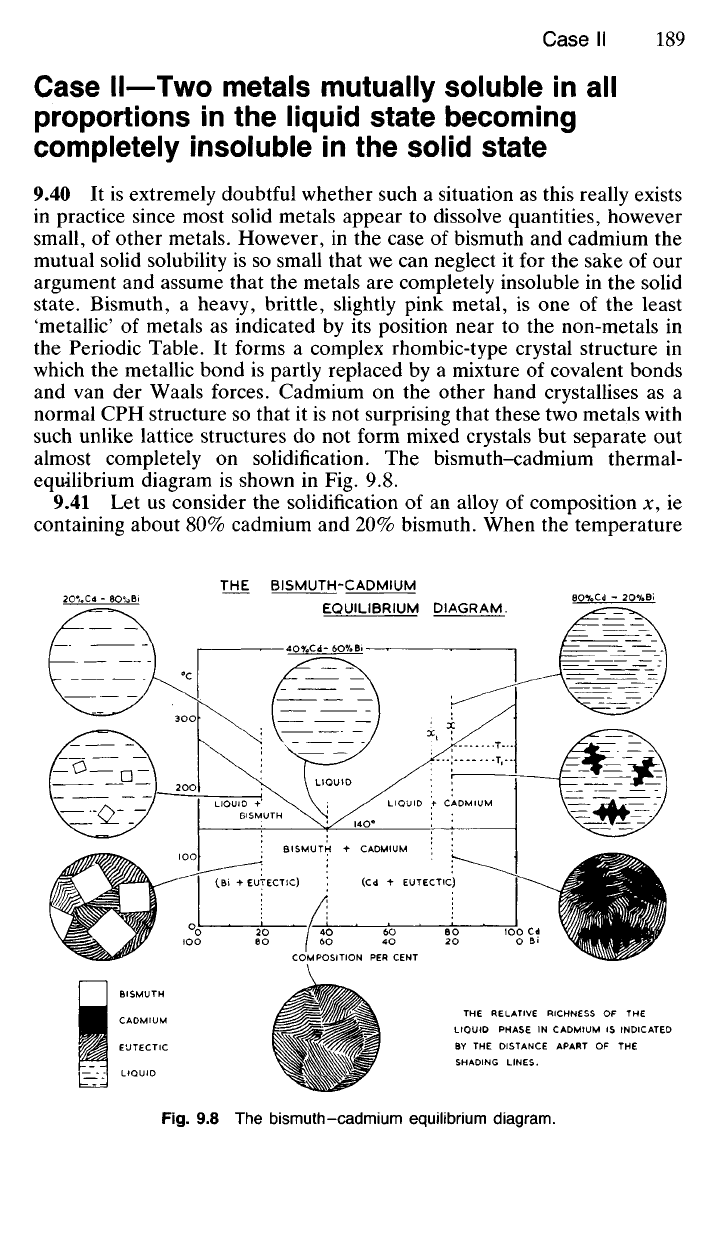
equilibrium diagram is shown in Fig. 9.8.
9.41 Let us consider the solidification of an alloy of composition x, ie
containing about 80% cadmium and 20% bismuth. When the temperature
THE BISMUTH-CADMIUM
EQUILIBRIUM DIAGRAM.
LIQUID +
BISMUTH
LIQUID
LIQUID + CADMIUM
BISMUTH + CADMIUM
(Bi + EUTECTIC)
(Cd + EUTECTIC)
COMPOSITION PER CENT
BISMUTH
CADMIUM
EUTECTIC
LIQUID
THE RELATIVE RICHNESS OF THE
LIQUIO PHASE IN CADMIUM IS INDICATED
BY THE DISTANCE APART OF THE
SHADING LINES.
Fig.
9.8 The bismuth-cadmium equilibrium diagram.

falls to T, crystal nuclei of pure cadmium begin to form in accordance with
rule (b) stated in 9.24. (The temperature horizontal or tie-line, T, cuts the
liquidus at the chosen composition, x, and the other phase boundary is the
100%
cadmium ordinate.) Since pure cadmium is deposited, it follows
that the liquid which remains becomes correspondingly richer in bismuth.
Therefore the composition of the liquid moves to the left—say, to x\—
and, as indicated by the diagram, no further deposition of cadmium takes
place until the temperature has fallen to Ti. When this happens more
cadmium is deposited, and dendrites begin to develop from the nuclei
which have already formed. The growth of the cadmium dendrites, on the
one hand, and the consequent enrichment of the remaining liquid in bis-
muth, on the other, continues until the temperature has fallen to 140
0
C.
The remaining liquid then contains 40% cadmium and 60% bismuth, ie
the eutectic point E has been reached.
At this point the two metals are in equilibrium in the liquid, but, due to
the momentum of crystallisation, the composition swings a little too far
past the point E, resulting in the deposition of a little too much cadmium.
In order that equilibrium shall be maintained, a swing back in composition
across the eutectic point takes place by the deposition of a layer of bismuth.
In this way the composition of the liquid oscillates about E by depositing
alternate layers of cadmium and bismuth, whilst the temperature remains
at 140
0
C until the remaining liquid has solidified. Thus the final structure
will consist of primary crystals of cadmium which formed between the
temperature T and 140
0
C, and a eutectic consisting of alternate layers of
cadmium and bismuth which formed at 140
0
C.
9.42 Had the original liquid contained less than 40% cadmium, then
crystals of pure bismuth would have formed first, causing the composition
of the remaining liquid to move to the right until ultimately the point E
was reached as before, and the final liquid contained 40% cadmium and
60%
bismuth. This remaining liquid would solidify as eutectic in the
manner already described.
9.43 If the original liquid contained exactly 40% cadmium and 60%
bismuth at the outset, then no solidification whatever would occur until
the temperature had fallen to 140
0
C. Then a structure composed entirely
of eutectic would be formed as outlined above.
9.44 In all three cases mentioned, the eutectic part of the structure will
be of constant composition and will always contain 40% cadmium and 60%
bismuth. Any variation either side of this in the overall composition of the
alloy will be compensated for by first depositing appropriate amounts of
either primary cadmium or primary bismuth, whichever is in excess of the
eutectic composition. It is important to realise that there is no question
of solid solubility existing in any way in the final structure, whatever its
composition. With the aid of a microscope, we can see the two pure metals
cadmium and bismuth as separate constituents in the microstructure. In
other words, this is a case of complete insolubility in the solid state.
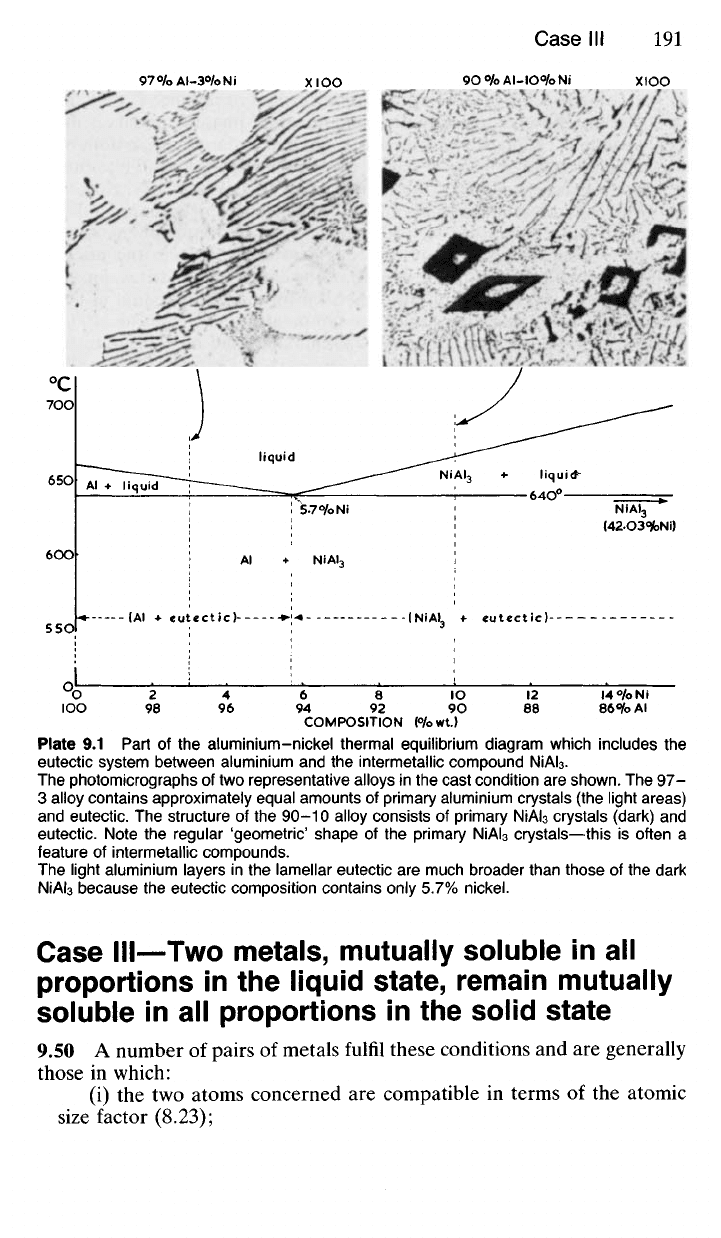
COMPOSITION (°/owt.)
Plate
9.1
Part
of the
aluminium-nickel thermal equilibrium diagram which includes
the
eutectic system between aluminium
and the
intermetallic compound
NiAI
3
.
The photomicrographs
of
two representative alloys
in the
cast condition
are
shown.
The 97-
3 alloy contains approximately equal amounts
of
primary aluminium crystals
(the
light areas)
and eutectic.
The
structure
of the
90-10 alloy consists
of
primary
NiAI
3
crystals (dark)
and
eutectic. Note
the
regular 'geometric' shape
of the
primary
NiAI
3
crystals—this
is
often
a
feature
of
intermetallic compounds.
The light aluminium layers
in the
lamellar eutectic
are
much broader than those
of the
dark
NiAI
3
because
the
eutectic composition contains only 5.7% nickel.
Case Ill—Two metals, mutually soluble
in all
proportions
in the
liquid state, remain mutually
soluble
in all
proportions
in the
solid state
9.50
A
number
of
pairs
of
metals fulfil these conditions
and are
generally
those
in
which:
(i)
the two
atoms concerned
are
compatible
in
terms
of the
atomic
size factor (8.23);
0
C
liquid
NiAI
3
+ liquid-
Al + liquid
(Al + eutectic)
(NiAI
3
• eutectic)
Al + NiAI
3
NiAI
3
(ii) the electrochemical properties of the two metals are very similar;
(iii) the crystal structures of the two pure metals are similar in pattern.
If these conditions are fulfilled it is reasonable to expect atoms of the
second metal to be able to replace atoms of the first in all proportions to
form a disordered solid solution. Such is the case in the alloy systems: Ag-
Au; Ag-Pd; Au-Pt; Bi-Sb; Co-Ni; Cu-Ni; Cu-Pt; Fe-Pt; Ni-Pt;
and Ta-Ti. (In some of these systems further phase changes occur in the
solid state due to polymorphic transformations (3.14)). Since the copper-
nickel alloys are the only ones of the group mentioned above which are of
use in engineering it is proposed to deal with this system. The cop-
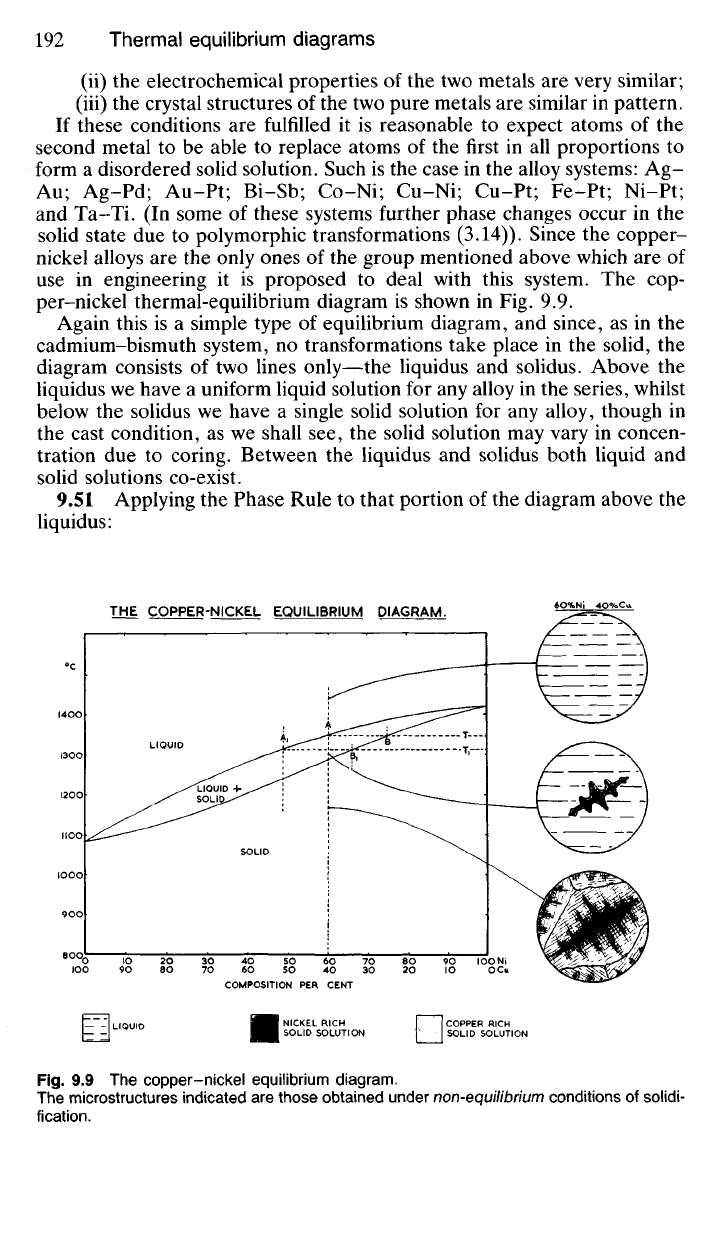
per-nickel thermal-equilibrium diagram is shown in Fig. 9.9.
Again this is a simple type of equilibrium diagram, and since, as in the
cadmium-bismuth system, no transformations take place in the solid, the
diagram consists of two lines only—the liquidus and solidus. Above the
liquidus we have a uniform liquid solution for any alloy in the series, whilst
below the solidus we have a single solid solution for any alloy, though in
the cast condition, as we shall see, the solid solution may vary in concen-
tration due to coring. Between the liquidus and solidus both liquid and
solid solutions co-exist.
9.51 Applying the Phase Rule to that portion of the diagram above the
liquidus:
THE COPPER-NICKEL EQUILIBRIUM DIAGRAM.
LIQUID
0
C
LIQUID
SOLID
SOLID
COMPOSITION PER CENT
IOO Ni
OC.
LIQUID
NICKEL RICH
SOLID SOLUTION
COPPER RICH
SOLID SOLUTION
Fig.
9.9 The copper-nickel equilibrium diagram.
The microstructures indicated are those obtained under non-equilibrium conditions of solidi-
fication.
p + F= C+ 1
1+F =2+1
and F = 2
since there are two degrees of freedom both temperature and composition
can be altered over fairly wide limits without upsetting the stability of the
single phase. However, for a point between the liquidus and solidus, ie in
'liquid + solid' field:
2 + F=2+ 1
and F = 1
There is thus one degree of freedom, and if either temperature or compo-
sition are altered independently of the other, then the system becomes
unstable so that some solid will melt or some liquid will solidify. Thus any
alteration in temperature must be accompanied by a change in overall
composition if the proportions of liquid and solid are to remain the same.
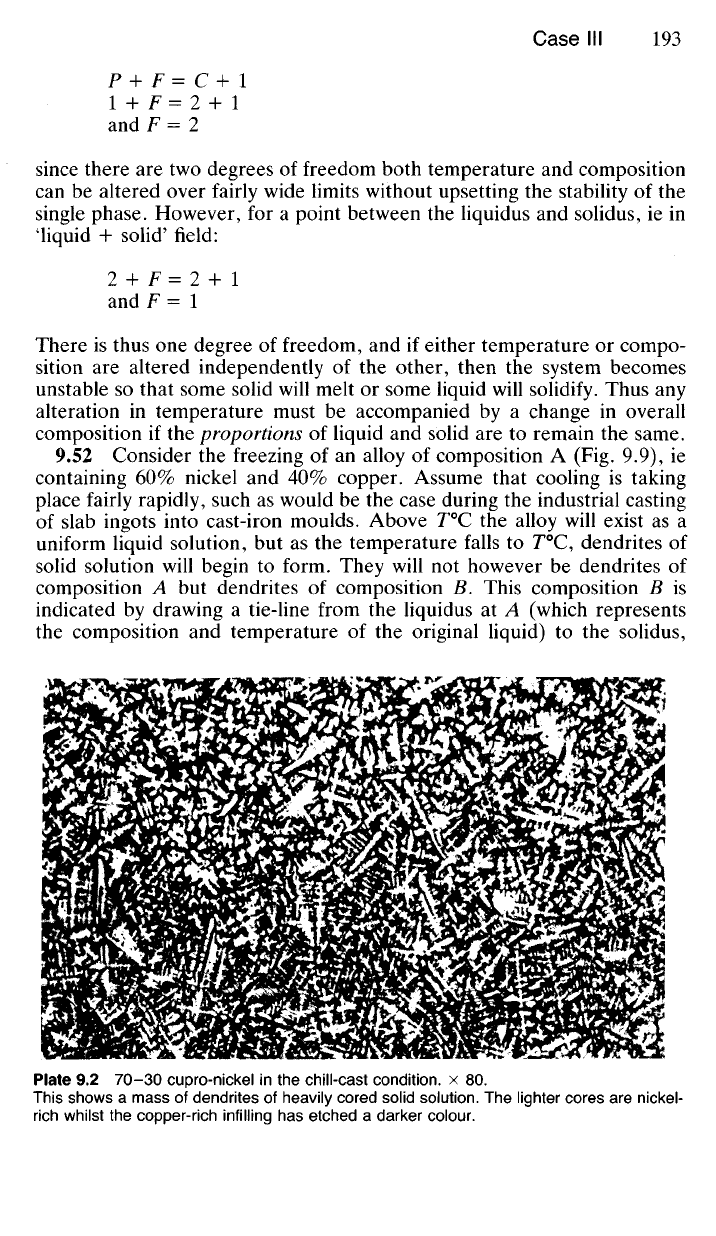
9.52 Consider the freezing of an alloy of composition A (Fig. 9.9), ie
containing 60% nickel and 40% copper. Assume that cooling is taking
place fairly rapidly, such as would be the case during the industrial casting
of slab ingots into cast-iron moulds. Above T
0
C the alloy will exist as a
uniform liquid solution, but as the temperature falls to T
0
C, dendrites of
solid solution will begin to form. They will not however be dendrites of
composition A but dendrites of composition B. This composition B is
indicated by drawing a tie-line from the liquidus at A (which represents
the composition and temperature of the original liquid) to the solidus,
Plate 9.2 70-30 cupro-nickel in the chill-cast condition, x 80.
This shows a mass of dendrites of heavily cored solid solution. The lighter cores are nickel-
rich whilst the copper-rich infilling has etched a darker colour.
which it cuts at B. Thus the dendrites which form will contain approxi-
mately 75% nickel (composition B) and, since the original liquid contained
only 60% nickel (composition A), it follows that the remaining liquid will
contain an even lower proportion of nickel. Hence its composition will
move to the left, say to A\.
Solidification will continue when the temperature falls further to T
1
, and
this time a layer of solid of composition B
1
will be deposited. This is less
rich in nickel than the original seed crystals and, as crystallisation proceeds,
successive layers will contain less and less nickel, and consequently more
and more copper, until ultimately the liquid is used up.
Clearly, then, a non-uniform solid solution is formed, and whilst its
overall composition will be 60% nickel and 40% copper, due to the coring
effect the initial skeleton of a crystal will contain about 75% nickel and its
outside fringes only about 50% nickel.
9.53 The situation is further influenced, however, by diffusion (8.23),
which is taking place simultaneously with crystallisation. Due to the fact
that successive layers of the alloy which deposit are richer in nickel than
the remaining liquid, a concentration gradient is set up which tends to
make copper atoms diffuse inwards towards the centre of the dendrite,
whilst nickel atoms move outwards towards the copper-rich liquid. In the
above case we have assumed very rapid cooling so that little or no diffusion
has been possible, and that under these conditions the structure has been
unable to reach equilibrium so that a heavily cored structure forms. Since
we are dealing with non-equilibrium conditions, only a limited amount of
information can be obtained from the equilibrium diagram—in this case a
guide as to why the cored structure develops. If cooling were slow, dif-
fusion could take place extensively so that coring would be slight in the
final crystals. Annealing at a sufficiently high temperature will also permit
diffusion to proceed (8.24), resulting in the formation of almost completely
uniform solid-solution crystals.
9.54 We will now consider the cooling of the same alloy under con-
ditions which are ideally slow so that complete equilibrium is attained at
each stage of solidification. The liquid (composition A, Fig. 9.10) will begin
to solidify at temperature T by depositing nuclei of composition B. This
causes the composition of the remaining liquid to move to the left, but,
due to the prevailing slow cooling, diffusion is able to keep pace with
TEMPERATURE
LIQUID
LIQUID + SOLID
SOLID
COMPOSITION PER CENT
Fig.
9.10.
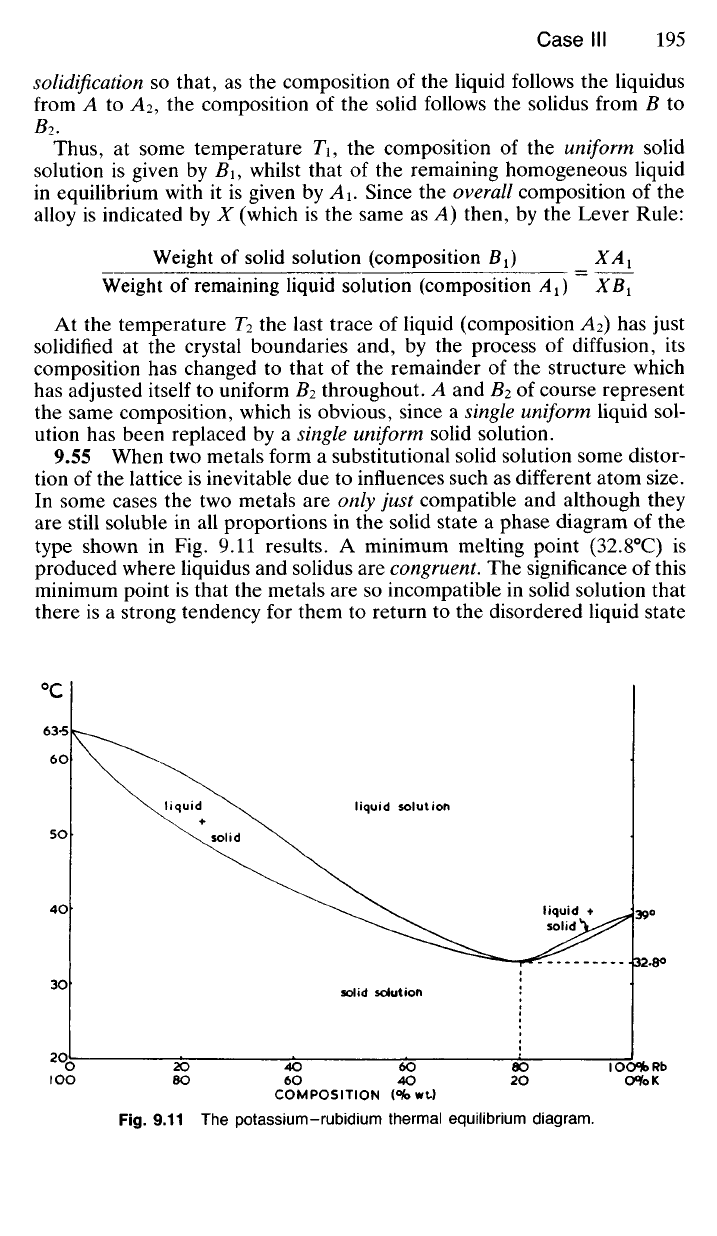
solidification
so
that,
as the
composition
of the
liquid follows
the
liquidus
from
A to A
2
, the
composition
of the
solid follows
the
solidus from
B to
B
2
.
Thus,
at
some temperature
Ti, the
composition
of the
uniform solid
solution
is
given
by B\,
whilst that
of the
remaining homogeneous liquid
in equilibrium with
it is
given
by A\.
Since
the
overall composition
of the
alloy
is
indicated
by X
(which
is the
same
as A)
then,
by the
Lever Rule:
Weight
of
solid solution (composition
B
1
) XA
1
Weight
of
remaining liquid solution (composition
A
1
) XB
1
At
the
temperature
T
2
the
last trace
of
liquid (composition
A
2
) has
just
solidified
at the
crystal boundaries
and, by the
process
of
diffusion,
its
composition
has
changed
to
that
of the
remainder
of the
structure which
has adjusted itself
to
uniform
B
2
throughout.
A and B
2
of
course represent
the same composition, which
is
obvious, since
a
single uniform liquid
sol-
ution
has
been replaced
by a
single uniform solid solution.
9.55 When
two
metals form
a
substitutional solid solution some distor-
tion
of the
lattice
is
inevitable
due to
influences such
as
different atom size.
In some cases
the two
metals
are
only just compatible
and
although they
are still soluble
in all
proportions
in the
solid state
a
phase diagram
of the
type shown
in Fig. 9.11
results.
A
minimum melting point (32.8
0
C)
is
produced where liquidus
and
solidus
are
congruent.
The
significance
of
this
minimum point
is
that
the
metals
are so
incompatible
in
solid solution that
there
is a
strong tendency
for
them
to
return
to the
disordered liquid state
0
C
liquid solution
liquid
+
solid
solid solution
liquid
solid
COMPOSITION (^>wt)
Fig.
9.11 The potassium-rubidium thermal equilibrium diagram.
