Higgins R.A. Engineering Metallurgy: Applied Physical Metallurgy
Подождите немного. Документ загружается.

COMPOSITION (%>)
Fig.
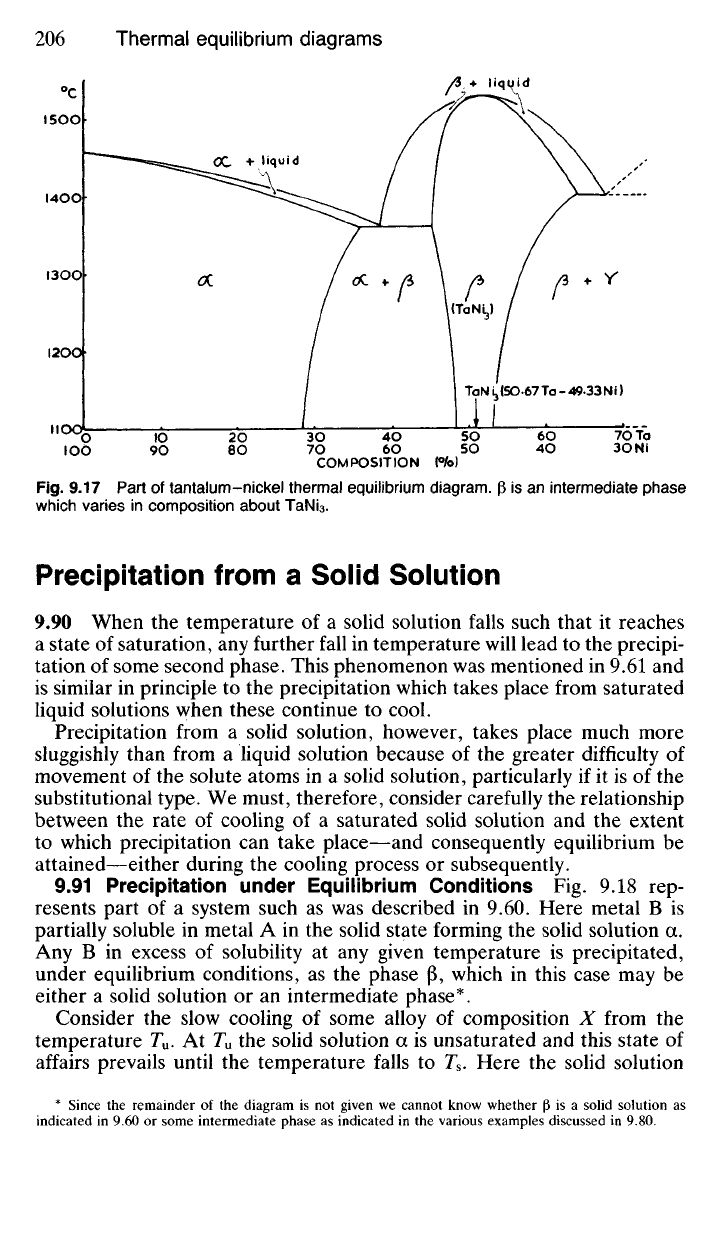
9.17 Part of tantalum-nickel thermal equilibrium diagram. |3 is an intermediate phase
which varies in composition about TaNi
3
.
Precipitation from a Solid Solution
9.90 When the temperature of a solid solution falls such that it reaches
a state of saturation, any further fall in temperature will lead to the precipi-
tation of some second phase. This phenomenon was mentioned in 9.61 and
is similar in principle to the precipitation which takes place from saturated
liquid solutions when these continue to cool.
Precipitation from a solid solution, however, takes place much more
sluggishly than from a liquid solution because of the greater difficulty of
movement of the solute atoms in a solid solution, particularly if it is of the
substitutional type. We must, therefore, consider carefully the relationship
between the rate of cooling of a saturated solid solution and the extent
to which precipitation can take place—and consequently equilibrium be
attained—either during the cooling process or subsequently.
9.91 Precipitation under Equilibrium Conditions Fig. 9.18 rep-
resents part of a system such as was described in 9.60. Here metal B is
partially soluble in metal A in the solid state forming the solid solution a.
Any B in excess of solubility at any given temperature is precipitated,
under equilibrium conditions, as the phase (3, which in this case may be
either a solid solution or an intermediate phase*.
Consider the slow cooling of some alloy of composition X from the
temperature T
n
. At T
u
the solid solution a is unsaturated and this state of
affairs prevails until the temperature falls to T
s
. Here the solid solution
* Since the remainder of the diagram is not given we cannot know whether (3 is a solid solution as
indicated in 9.60 or some intermediate phase as indicated in the various examples discussed in 9.80.
0
C
OC + liquid
/3 • liquid
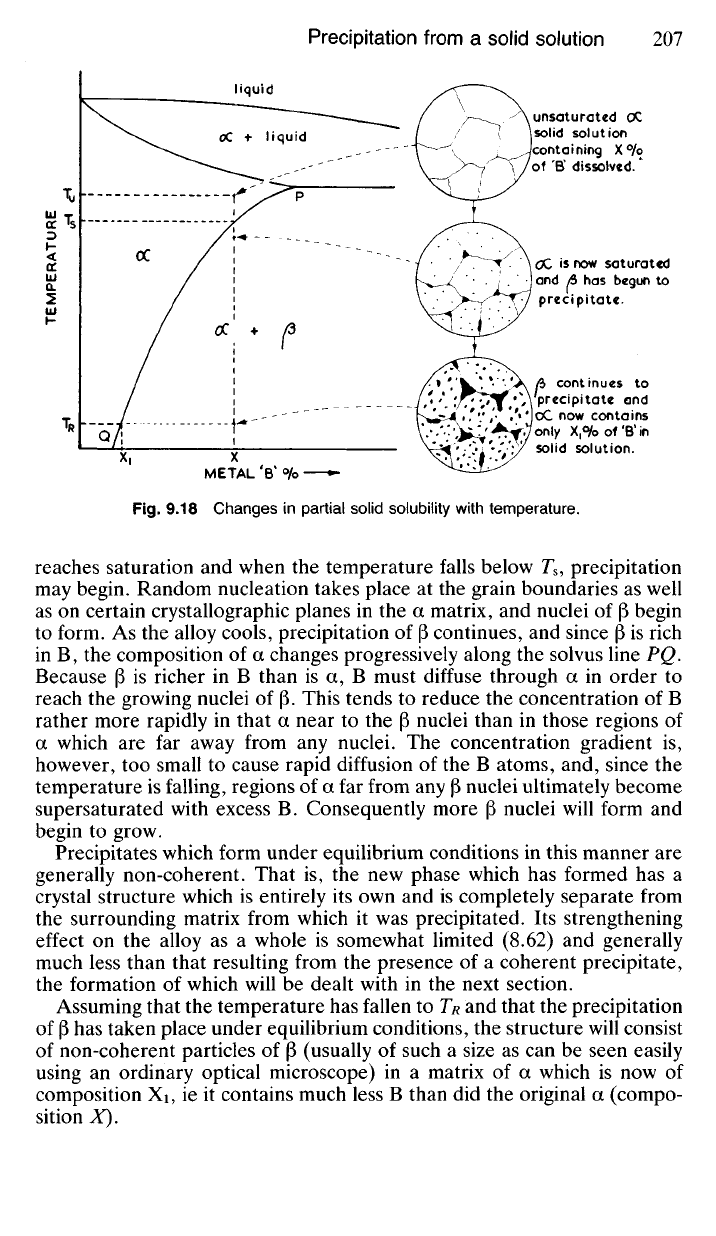
Fig.
9.18 Changes in partial solid solubility with temperature.
reaches saturation and when the temperature falls below T
8
, precipitation
may begin. Random nucleation takes place at the grain boundaries as well
as on certain crystallographic planes in the a matrix, and nuclei of [3 begin
to form. As the alloy cools, precipitation of |3 continues, and since |3 is rich
in B, the composition of a changes progressively along the solvus line PQ.
Because (3 is richer in B than is a, B must diffuse through a in order to
reach the growing nuclei of (3. This tends to reduce the concentration of B
rather more rapidly in that a near to the (3 nuclei than in those regions of
a which are far away from any nuclei. The concentration gradient is,
however, too small to cause rapid diffusion of the B atoms, and, since the
temperature is falling, regions of a far from any
(3
nuclei ultimately become
supersaturated with excess B. Consequently more (3 nuclei will form and
begin to grow.
Precipitates which form under equilibrium conditions in this manner are
generally non-coherent. That is, the new phase which has formed has a
crystal structure which is entirely its own and is completely separate from
the surrounding matrix from which it was precipitated. Its strengthening
effect on the alloy as a whole is somewhat limited (8.62) and generally
much less than that resulting from the presence of a coherent precipitate,
the formation of which will be dealt with in the next section.
Assuming that the temperature has fallen to T
R
and that the precipitation
of
(3
has taken place under equilibrium conditions, the structure will consist
of non-coherent particles of (3 (usually of such a size as can be seen easily
using an ordinary optical microscope) in a matrix of a which is now of
composition Xi, ie it contains much less B than did the original a (compo-
sition X).
UJ
CC
3
<
CC
UJ
a
UJ
K-
liquid
CC + liquid
METAL 'S" °/o
unsaturated CC
solid solution
containing X°/o
of 'B' dissolved."
OC is now saturated
and A has begun to
precipitate.
CC
+ /3
OC
A continues to
precipitate and
oC now contains
only X
1
^o of'B'in
solid solution.
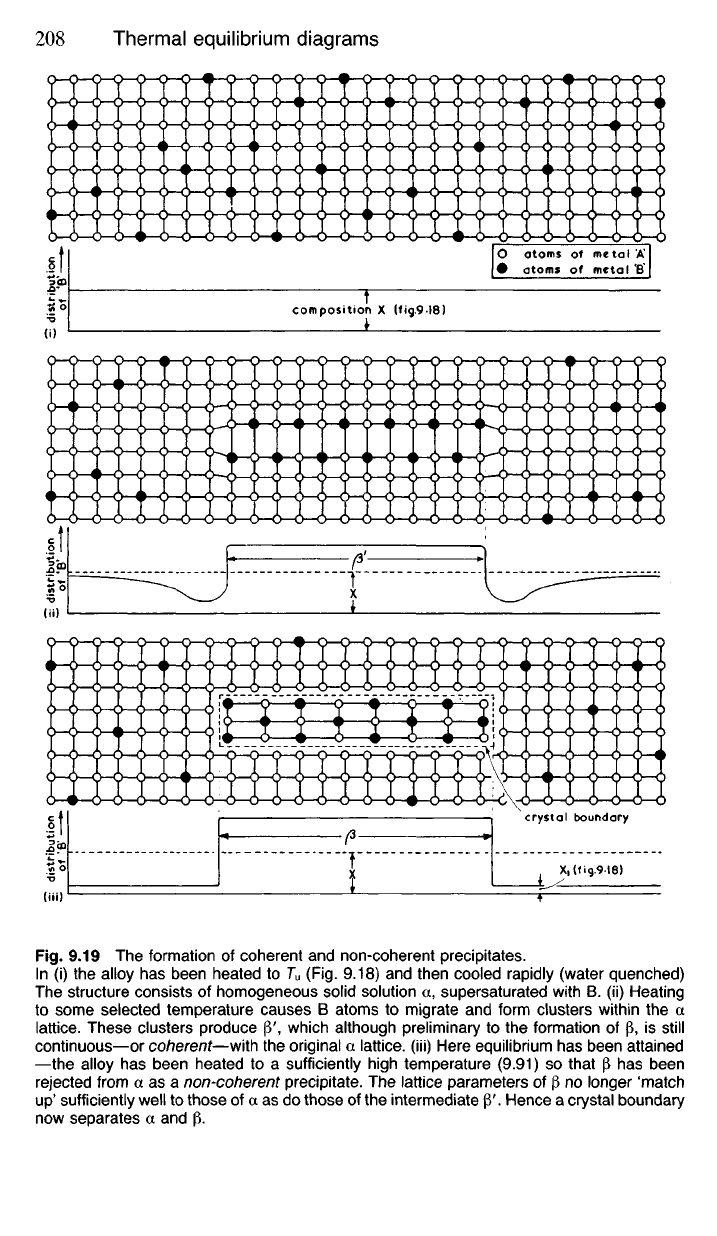
Fig.
9.19 The formation of coherent and non-coherent precipitates.
In (i) the alloy has been heated to T
u
(Fig. 9.18) and then cooled rapidly (water quenched)
The structure consists of homogeneous solid solution a, supersaturated with B. (ii) Heating
to some selected temperature causes B atoms to migrate and form clusters within the a
lattice.
These clusters produce |3\ which although preliminary to the formation of p, is still
continuous—or coherent—with the original a lattice, (iii) Here equilibrium has been attained
—the alloy has been heated to a sufficiently high temperature (9.91) so that |3 has been
rejected from a as a non-coherent precipitate. The lattice parameters of (3 no longer 'match
up'
sufficiently well to those of a as do those of the intermediate P'. Hence a crystal boundary
now separates a and p.
distribution
of
1
B"
distribution
of
'B'
distribution
of
'B
%
atoms of metal
1
A'
atoms of metal B'
composition X (fig.918)
crystal boundary
X,(fig.9l8)
9.92 Precipitation under Non-equilibrium Conditions
We
will now
assume that
the
alloy
X,
which
has
been retained
at
temperature
Tu,
long
enough
for its
structure
to be
completely homogeneous
a, is
cooled very
rapidly
to
room temperature
(T
R
).
This could
be
achieved
by
quenching
it
in cold water.
In
most cases treatment such
as
this will prevent any precipi-
tation from taking place and we
are
left with
a
solid solution which,
at T
R
,
is now super-saturated with B. The structure will
not be in
equilibrium
and
is said
to be in a
metastable state.
It has an
urge
to
return
to a
state
of
equilibrium
by
precipitating some
|3. At
room temperature such precipi-
tation will
be
unlikely
to
occur
due to the
extreme sluggishness
of
move-
ment
of B
atoms,
but if the
temperature
is
increased, diffusion will begin
and then accelerate
as the
temperature continues
to
rise.
The alloy
is
held
at
some selected temperature
so
that diffusion occurs
at
a low but
definite rate.
The
temperature chosen
is
well below
T
s
in
order
to
ensure that non-coherent precipitation does
not
occur. During this
heat-treatment clusters
of
atoms
of
both
A and B, but
with
the
overall
composition
of the
phase
|3,
slowly form groups
at
many points
in the a
lattice. These
clusters
have the important property that their
lattice structures
are continuous with that
of
the
a
matrix,
and
there
is no
discontinuous
interface
as
exists between
a and
|3, which
has
precipitated during cooling
under equilibrium conditions. This unbroken continuity
of
the two lattices
is known
as
coherency. Since
the
cluster size
is
extremely small
and the
rate
of
diffusion very slow,
a
large number
of
these coherent nuclei will
form
and the
chosen temperature will
not be
high enough
to
allow
the
formation
of a
separate (3 structure. Instead, this intermediate structure—
we will call
it
(3'—is produced,
and the
mismatching between (3'
and the a
matrix leads
to
distortion
in the a
lattice
in the
neighbourhood
of
these
nuclei. Such distortions will hinder
the
movement
of
dislocations
and so
the strength
and
hardness increase.
The
greater
the
number
of
nuclei
and
the larger they
are,
provided that coherency
is
retained,
the
greater
the
strength
and
hardness
of the
structure
as a
whole. Time
and
temperature
will influence this 'precipitation' procedure
and,
hence, also
the
ultimate
mechanical properties which result.
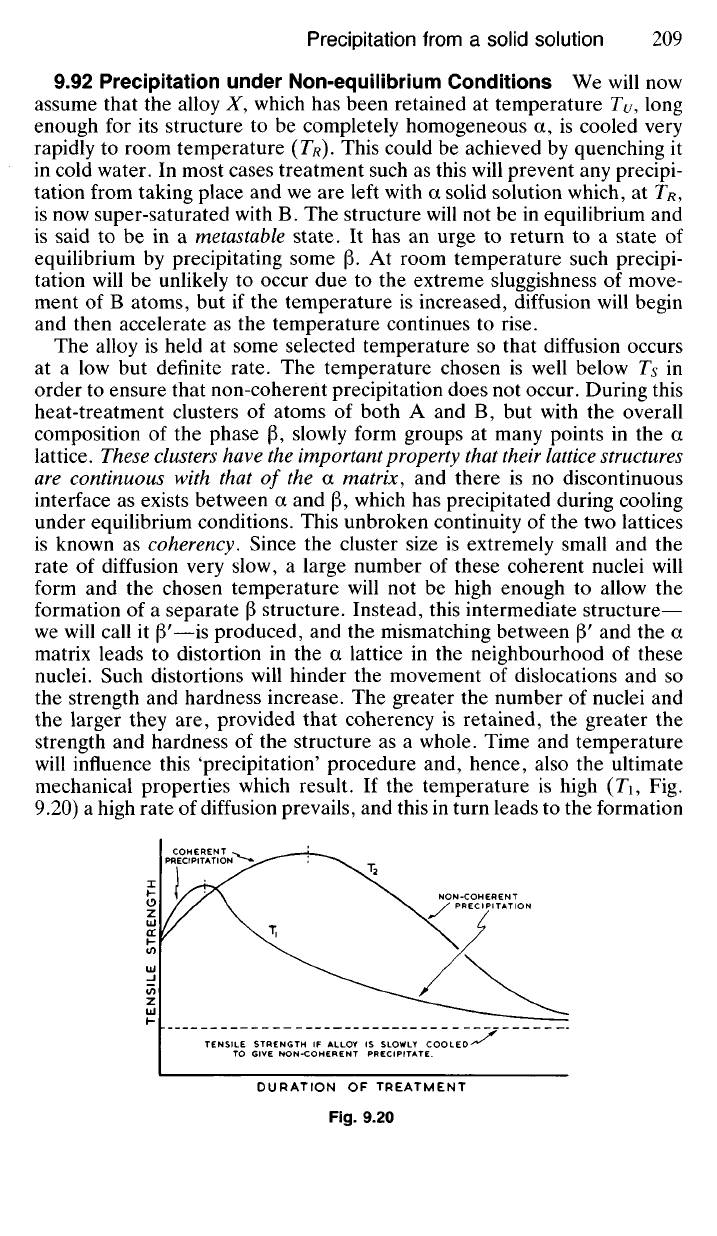
If the
temperature
is
high
(Ti, Fig.
9.20)
a
high rate
of
diffusion prevails, and this
in
turn leads
to
the formation
TENSILE
STRENGTH
COHERENT
PRECIPITATION
NON-COHERENT
PRECIPITATION
TENSILE STRENGTH IF ALLOY IS SLOWLY COOLED
TO GIVE NON-COHERENT PRECIPITATE.
DURATION
OF
TREATMENT
Fig.
9.20
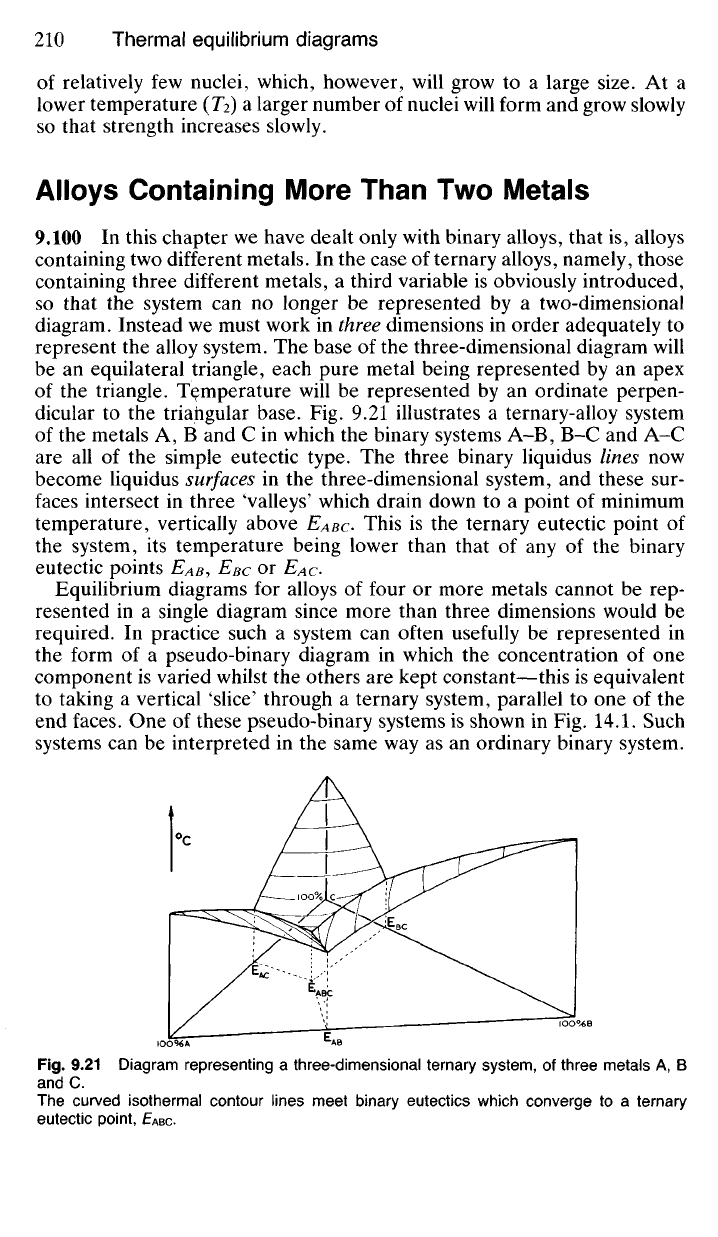
of relatively few nuclei, which, however, will grow to a large size. At a
lower temperature (TY) a larger number of nuclei will form and grow slowly
so that strength increases slowly.
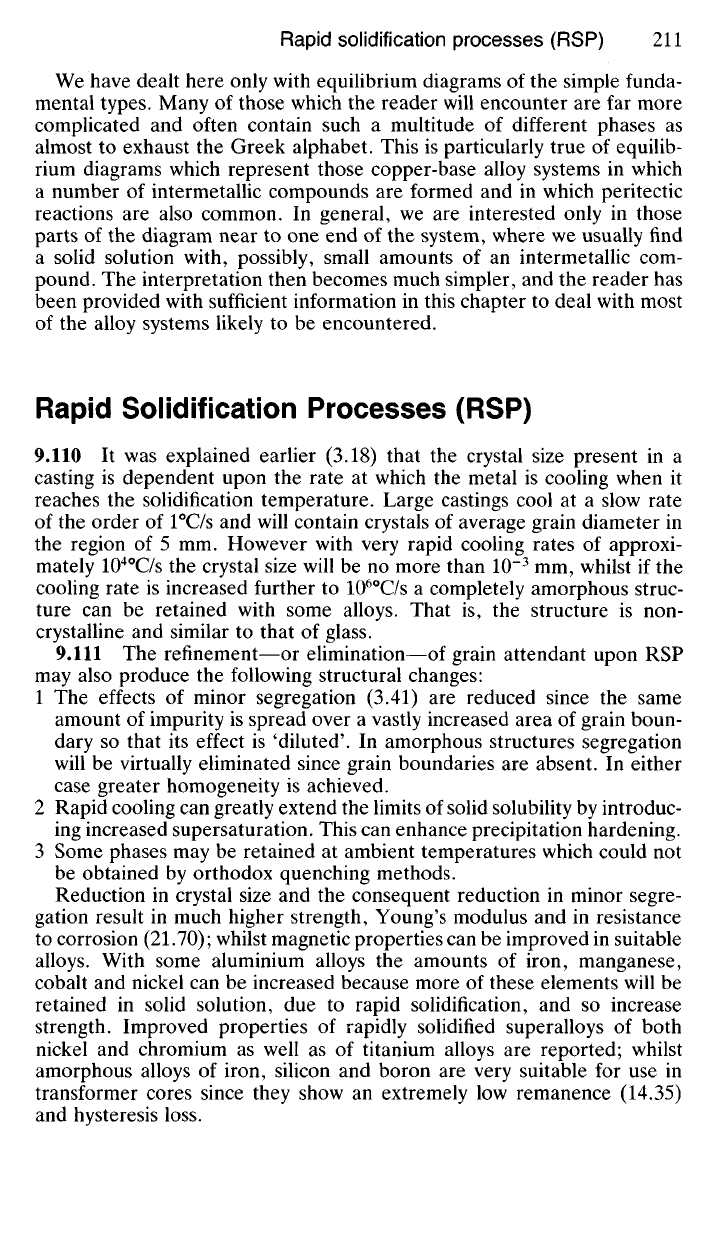
Alloys Containing More Than Two Metals
9.100 In this chapter we have dealt only with binary alloys, that is, alloys
containing two different metals. In the case of ternary alloys, namely, those
containing three different metals, a third variable is obviously introduced,
so that the system can no longer be represented by a two-dimensional
diagram. Instead we must work in three dimensions in order adequately to
represent the alloy system. The base of the three-dimensional diagram will
be an equilateral triangle, each pure metal being represented by an apex
of the triangle. Temperature will be represented by an ordinate perpen-
dicular to the triangular base. Fig. 9.21 illustrates a ternary-alloy system
of the metals A, B and C in which the binary systems A-B, B-C and A-C
are all of the simple eutectic type. The three binary liquidus lines now
become liquidus surfaces in the three-dimensional system, and these sur-
faces intersect in three 'valleys' which drain down to a point of minimum
temperature, vertically above E
A
BC- This is the ternary eutectic point of
the system, its temperature being lower than that of any of the binary
eutectic points E
A
B, E
B
C or E
A
c
Equilibrium diagrams for alloys of four or more metals cannot be rep-
resented in a single diagram since more than three dimensions would be
required. In practice such a system can often usefully be represented in
the form of a pseudo-binary diagram in which the concentration of one
component is varied whilst the others are kept constant—this is equivalent
to taking a vertical 'slice' through a ternary system, parallel to one of the
end faces. One of these pseudo-binary systems is shown in Fig. 14.1. Such
systems can be interpreted in the same way as an ordinary binary system.
Fig.
9.21 Diagram representing a three-dimensional ternary
system,
of three metals A, B
and
C.
The
curved isothermal contour lines meet binary eutectics which converge to a ternary
eutectic
point,
EABC-
We have dealt here only with equilibrium diagrams of the simple funda-
mental types. Many of those which the reader will encounter are far more
complicated and often contain such a multitude of different phases as
almost to exhaust the Greek alphabet. This is particularly true of equilib-
rium diagrams which represent those copper-base alloy systems in which
a number of intermetallic compounds are formed and in which peritectic
reactions are also common. In general, we are interested only in those
parts of the diagram near to one end of the system, where we usually find
a solid solution with, possibly, small amounts of an intermetallic com-
pound. The interpretation then becomes much simpler, and the reader has
been provided with sufficient information in this chapter to deal with most
of the alloy systems likely to be encountered.
Rapid Solidification Processes (RSP)
9.110 It was explained earlier (3.18) that the crystal size present in a
casting is dependent upon the rate at which the metal is cooling when it
reaches the solidification temperature. Large castings cool at a slow rate
of the order of l°C/s and will contain crystals of average grain diameter in
the region of 5 mm. However with very rapid cooling rates of approxi-
mately 10
4o
C/s the crystal size will be no more than 10~
3
mm, whilst if the
cooling rate is increased further to 10
6o
C/s a completely amorphous struc-
ture can be retained with some alloys. That is, the structure is non-
crystalline and similar to that of glass.
9.111 The refinement—or elimination—of grain attendant upon RSP
may also produce the following structural changes:
1 The effects of minor segregation (3.41) are reduced since the same
amount of impurity is spread over a vastly increased area of grain boun-
dary so that its effect is 'diluted'. In amorphous structures segregation
will be virtually eliminated since grain boundaries are absent. In either
case greater homogeneity is achieved.
2 Rapid cooling can greatly extend the limits of solid solubility by introduc-
ing increased supersaturation. This can enhance precipitation hardening.
3 Some phases may be retained at ambient temperatures which could not
be obtained by orthodox quenching methods.
Reduction in crystal size and the consequent reduction in minor segre-
gation result in much higher strength, Young's modulus and in resistance
to corrosion (21.70); whilst magnetic properties can be improved in suitable
alloys. With some aluminium alloys the amounts of iron, manganese,
cobalt and nickel can be increased because more of these elements will be
retained in solid solution, due to rapid solidification, and so increase
strength. Improved properties of rapidly solidified superalloys of both
nickel and chromium as well as of titanium alloys are reported; whilst
amorphous alloys of iron, silicon and boron are very suitable for use in
transformer cores since they show an extremely low remanence (14.35)
and hysteresis loss.
9.112 The practical difficulties involved in attaining very high rates of
cooling limit rapid solidification products to either very fine powder or thin
foil. Nevertheless methods of achieving the high rates necessary to produce
extremely small crystals or, ultimately, an amorphous structure, are ingeni-
ous and varied.
Smooth spherical powders in a wide range of alloys can be made by
pouring the molten alloy through a small-diameter refractory nozzle.
Below the nozzle jets of a suitable inert gas impinge on the metal stream
'atomising' it and scattering it as small droplets. These are rapidly cooled
by the gas and collected as a fine powder at the base of the 'atomisation
tower'.
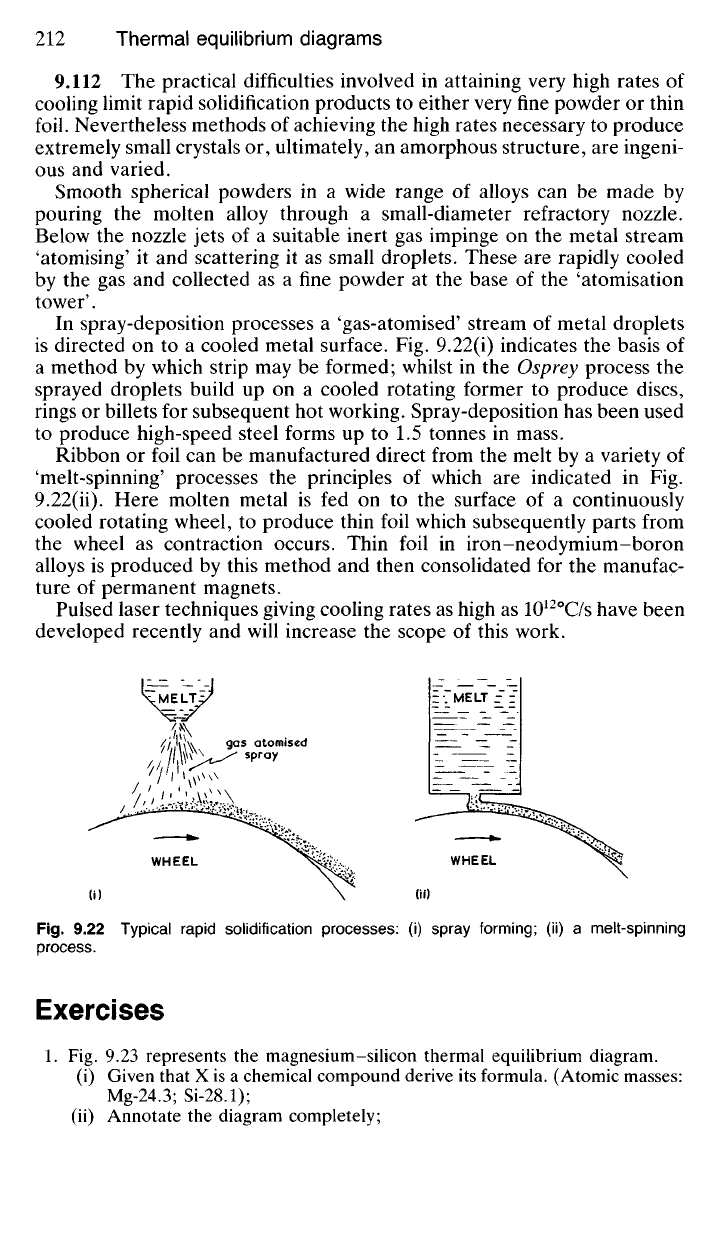
In spray-deposition processes a 'gas-atomised' stream of metal droplets
is directed on to a cooled metal surface. Fig. 9.22(i) indicates the basis of
a method by which strip may be formed; whilst in the Osprey process the
sprayed droplets build up on a cooled rotating former to produce discs,
rings or billets for subsequent hot working. Spray-deposition has been used
to produce high-speed steel forms up to 1.5 tonnes in mass.
Ribbon or foil can be manufactured direct from the melt by a variety of
'melt-spinning' processes the principles of which are indicated in Fig.
9.22(ii). Here molten metal is fed on to the surface of a continuously
cooled rotating wheel, to produce thin foil which subsequently parts from
the wheel as contraction occurs. Thin foil in iron-neodymium-boron
alloys is produced by this method and then consolidated for the manufac-
ture of permanent magnets.
Pulsed laser techniques giving cooling rates as high as 10
12o
C/s have been
developed recently and will increase the scope of this work.
Fig.
9.22 Typical rapid solidification processes: (i) spray forming; (ii) a melt-spinning
process.
Exercises
1.
Fig. 9.23 represents the magnesium-silicon thermal equilibrium diagram,
(i) Given that X is a chemical compound derive its formula. (Atomic masses:
Mg-24.3;
Si-28.1);
(ii) Annotate the diagram completely;
WHEEL
WHEEL
gas atomised
spray
MELT
MELT

Fig.
9.23.
i
(iii) What phases
can
co-exist
at E?
(iv) What will happen
if the
temperature
of the
alloy represented
by E is
raised
by a
small amount?
(v) What
are the
compositions
of the
phases present
in an
alloy containing
80%
Si-20%
Mg
overall,
at a
temperature
of
1100
0
C?
(vi)
In
what proportions
by
mass will
the
phases
in (v) be
present?
(vii) Describe, step
by
step, what happens
as an
alloy containing 50%
of
each
element cools slowly from 1200
0
C
to
700
0
C;
(viii) What will
be the
compositions
of the
phases present
at
700
0
C?
(ix)
In
what proportions will
the
phases
in
(viii)
be
present?
2.
Beryllium (m.pt. 1282°C)
and
silicon (m.pt. 1414°C)
are
completely soluble
as
liquids
but
completely insoluble
as
solids. They form
a
eutectic
at
1090
0
C
containing 61% silicon.
Draw
the
thermal equilibrium diagram
and
explain, with
the aid of
sketches,
what happens when alloys containing
(i)
10% silicon,
(ii)
70% silicon, solidify
completely. (9.40)
3.
Fig. 9.24
represents
the
bismuth-antimony thermal equilibrium diagram.
(a) Would
you
expect
the
diagram
to be of
this type? Give reasons.
(b) Consider
an
alloy containing 70% Sb-30%
Bi
which
is
cooling slowly:
(i)
At
what temperature will solidification begin?
(ii) What will
be the
composition
of the
first solid
to
form?
(iii) What will
be the
compositions
of the
phases present
at
500
0
C?
(iv)
In
what proportions
by
mass will
the
phases
in (iii) be
present?
(v) What will
be the
composition
of
the last trace
of
liquid which solidifies?
(vi)
At
what temperature will solidification
be
complete?
(c) Explain what would happen
if
the alloy containing 70% Sb-30%
Bi
cooled
liquid
liquid
+ solid
solid
Fig.
9.24.
antimony (%>wt.)
0
C
0
C
liquid
silicon
(% wt.)
rapidly from 600
0
C to 300
0
C. Sketch the type of microstructure you would
expect in the solid alloy. (9.50)
4.
Again referring to Fig. 9.24, at what temperature will a 60% Sb-40% Bi alloy
contain 25% by mass of liquid and 75% by mass of solid? What will be the
compositions of liquid and solid at this temperature?
5.
Two metals A and B have melting points 600
0
C and 450
0
C respectively. The
following results indicate the temperatures associated with discontinuities in
the cooling curves of the alloys indicated:
%B 10 20 35 50 55 60 75 90 95
0
C 545 495 410 330 300 315 365 415 430
0
C 450 300 300 300 — 300 300 300 370
If the maximum and minimum percentage solubilities of the two metals are
20%
B in A, 10% B in A, and 10% A in B, 5% A in B, sketch and label the
equilibrium diagram. (Assume solubility lines are straight.)
(i) At what temperature will an alloy containing 30% B begin to solidify?
(ii) Describe the cooling of an alloy containing 30% B and sketch typical
microstructures.
(iii) What proportions of a and (3 would you expect in the eutectic alloy at the
eutectic temperature and at
0
0
C?
(9.60)
6. Di*aw a thermal equilibrium diagram representing the system between two
metals, X and Y, given the following data:
(i) X melts at 1000
0
C and Y at 800
0
C;
(ii) X is soluble in Y in the solid state to the extent of 10.0% at 700
0
C and
2.0% at 0
0
C;
(iii) Y is soluble in X in the solid state to the extent of 20.0% at 700
0
C and
8.0% at 0
0
C;
(iv) a eutectic is formed at 700
0
C containing 40.0% X and 60.0% Y.
Describe what happens when an alloy containing 70.0% X solidifies and
cools slowly to 0
0
C.
Sketch the microstructures of an alloy containing 15.0% Y (a) after it
has cooled slowly to 0
0
C; (b) after it has been heated for some time at
700
0
C and then water quenched. (9.60 and 9.92)
7. Fig. 9.25 shows part of the aluminium-magnesium thermal equilibrium
diagram.
(i) What is the maximum solid solubility of magnesium in aluminium?
(ii) Over what temperature range will an alloy containing 7% Mg exist as a
single solid phase?
(iii) At what temperature does an alloy containing 5% Mg begin to melt on
heating?
(iv) An alloy containing 16% Mg is at 520
0
C. What are the compositions of
the phases present?
(v) In what proportions will the phases in (iv) be present?
(vi) What is the percentage increase in solubility of magnesium in aluminium
in an alloy containing 12% Mg as the temperature rises slowly from 60
0
C
to 350
0
C?
(vii) Sketch the structure of an alloy containing 10% Mg (a) slowly cooled
from 450
0
C; (b) water quenched from 450
0
C
(viii) Which will be the stronger in (vii)—(a) or (b)l (9.90)
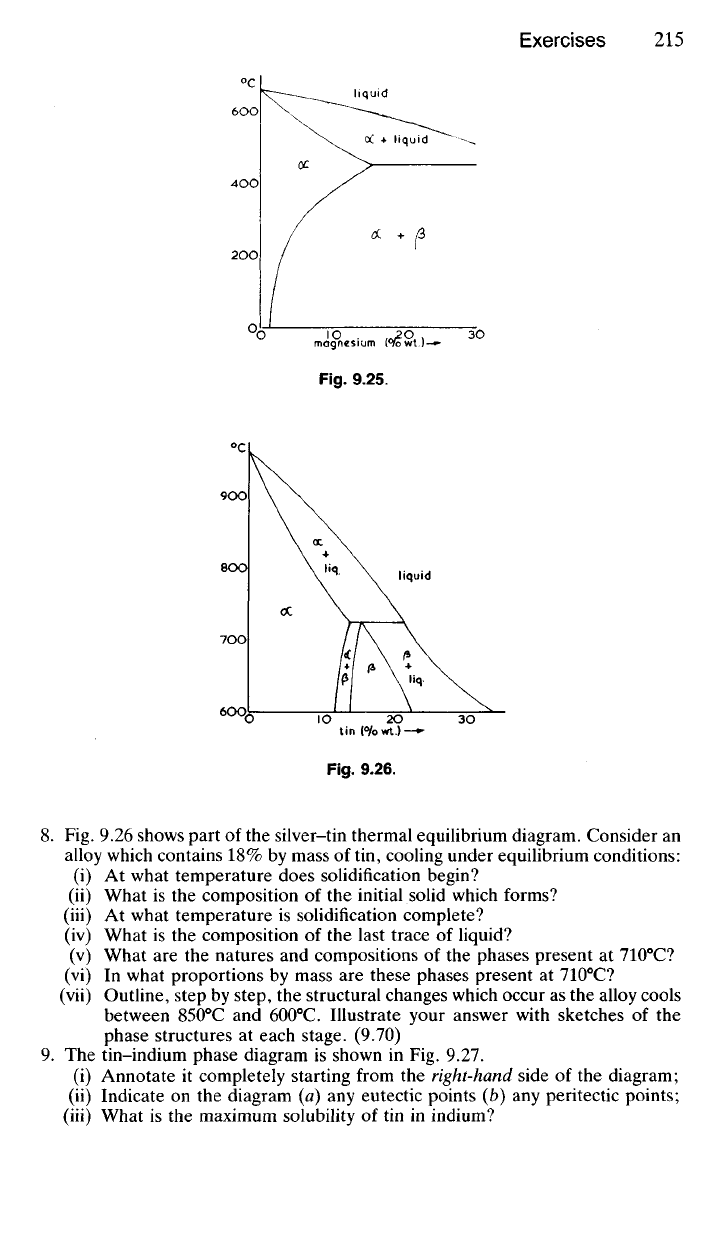
Fig.
9.26.
8. Fig. 9.26 shows part of the silver-tin thermal equilibrium diagram. Consider an
alloy which contains 18% by mass of tin, cooling under equilibrium conditions:
(i) At what temperature does solidification begin?
(ii) What is the composition of the initial solid which forms?
(iii) At what temperature is solidification complete?
(iv) What is the composition of the last trace of liquid?
(v) What are the natures and compositions of the phases present at 710
0
C?
(vi) In what proportions by mass are these phases present at 710
0
C?
(vii) Outline, step by step, the structural changes which occur as the alloy cools
between 850
0
C and 600
0
C. Illustrate your answer with sketches of the
phase structures at each stage. (9.70)
9. The tin-indium phase diagram is shown in Fig. 9.27.
(i) Annotate it completely starting from the right-hand side of the diagram;
(ii) Indicate on the diagram (a) any eutectic points (b) any peritectic points;
(iii) What is the maximum solubility of tin in indium?
°c
liquid
oC + liquid
OC
CU + /3
magnesium (
0
ToWt.)
Fig.
9.25.
°c
liquid
tin
(°/owt.)
