Higgins R.A. Engineering Metallurgy: Applied Physical Metallurgy
Подождите немного. Документ загружается.

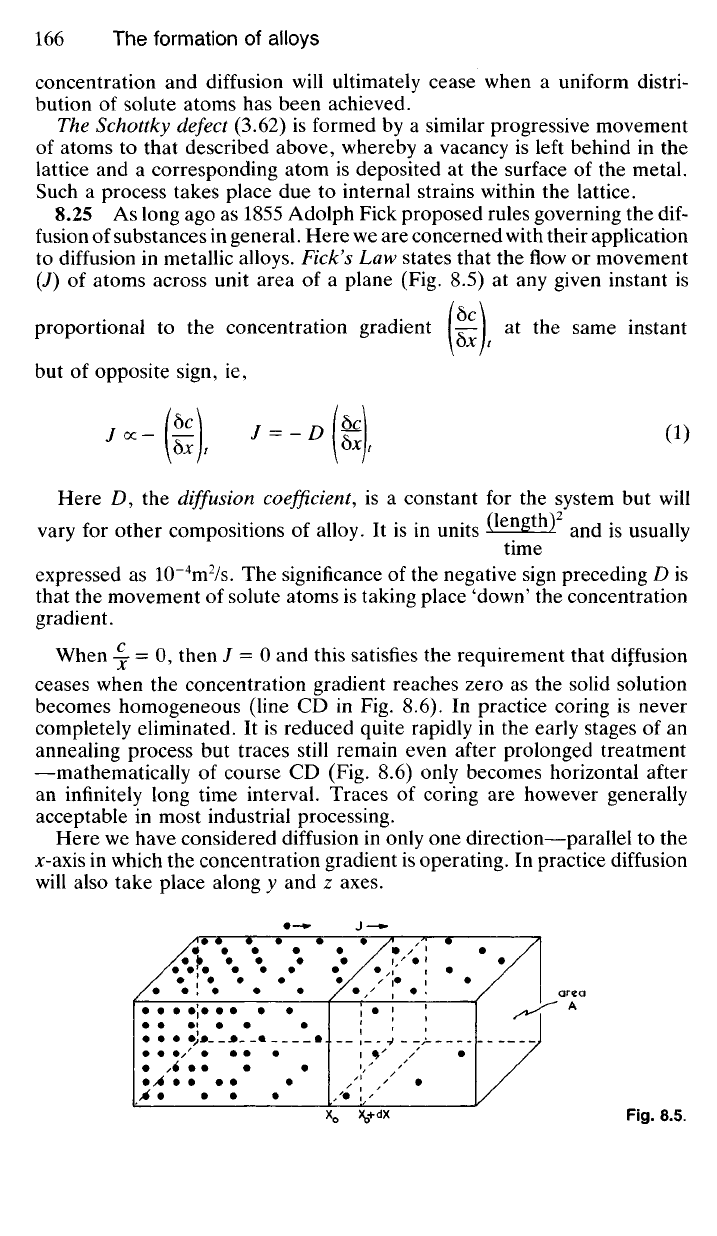
concentration and diffusion will ultimately cease when a uniform distri-
bution of solute atoms has been achieved.
The Schottky defect (3.62) is formed by a similar progressive movement
of atoms to that described above, whereby a vacancy is left behind in the
lattice and a corresponding atom is deposited at the surface of the metal.
Such a process takes place due to internal strains within the lattice.
8.25 As long ago as 1855 Adolph Fick proposed rules governing the dif-
fusion of substances in general. Here we are concerned with their application
to diffusion in metallic alloys. Fick's Law states that the flow or movement
(J) of atoms across unit area of a plane (Fig. 8.5) at any given instant is
— at the same instant
but of opposite sign, ie,
Here Z), the diffusion coefficient, is a constant for the system but will
vary for other compositions of alloy. It is in units ^
en
£—*- and is usually
time
expressed as 10~
4
m
2
/s. The significance of the negative sign preceding D is
that the movement of solute atoms is taking place 'down' the concentration
gradient.
When -j=0, then / =
O
and this satisfies the requirement that diffusion
ceases when the concentration gradient reaches zero as the solid solution
becomes homogeneous (line CD in Fig. 8.6). In practice coring is never
completely eliminated. It is reduced quite rapidly in the early stages of an
annealing process but traces still remain even after prolonged treatment
—mathematically of course CD (Fig. 8.6) only becomes horizontal after
an infinitely long time interval. Traces of coring are however generally
acceptable in most industrial processing.
Here we have considered diffusion in only one direction—parallel to the
jc-axis in which the concentration gradient is operating. In practice diffusion
will also take place along y and z axes.
Fig.
8.5.
area
A
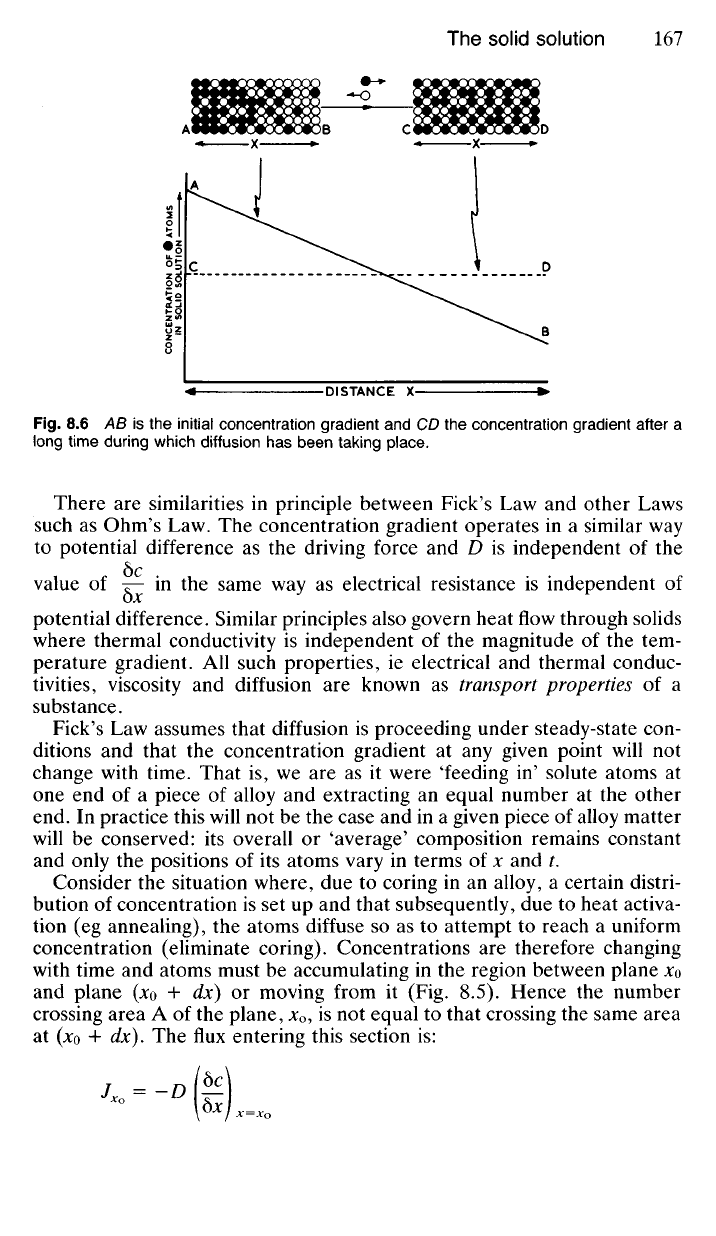
Fig.
8.6 AB is the initial concentration gradient and CD the concentration gradient after a
long time during which diffusion has been taking place.
There are similarities in principle between Fick's Law and other Laws
such as Ohm's Law. The concentration gradient operates in a similar way
to potential difference as the driving force and D is independent of the
value of j— in the same way as electrical resistance is independent of
potential difference. Similar principles also govern heat flow through solids
where thermal conductivity is independent of the magnitude of the tem-
perature gradient. All such properties, ie electrical and thermal conduc-
tivities, viscosity and diffusion are known as transport properties of a
substance.
Fick's Law assumes that diffusion is proceeding under steady-state con-
ditions and that the concentration gradient at any given point will not
change with time. That is, we are as it were 'feeding in' solute atoms at
one end of a piece of alloy and extracting an equal number at the other
end. In practice this will not be the case and in a given piece of alloy matter
will be conserved: its overall or 'average' composition remains constant
and only the positions of its atoms vary in terms of x and t.
Consider the situation where, due to coring in an alloy, a certain distri-
bution of concentration is set up and that subsequently, due to heat activa-
tion (eg annealing), the atoms diffuse so as to attempt to reach a uniform
concentration (eliminate coring). Concentrations are therefore changing
with time and atoms must be accumulating in the region between plane
Xo
and plane (JCO + dx) or moving from it (Fig. 8.5). Hence the number
crossing area A of the plane, x
o
, is not equal to that crossing the same area
at
(JCO
+ dx). The flux entering this section is:
CONCENTRATION
OF
ATOMS
IN SOLID
SOLUTION
DISTANCE X
The flux leaving
the
section
can be
written:
J*
o
+
dx
where
Jxo
+ dx = Jxo + h-
)
dx
+ • • • • + (
The
hi
g
her
terms
\
ox
J
x=x
o
can be neglected)
The rate of movement of atoms from the section Jt
0
Xo
+ dx is equal to the
difference between the two values of AJ and also equal to the volume of
the section, A.dx times the rate of decrease of c:
bJ
. , Sc
A
j
-
—. Adx = —. Adx
bx bt
or
Combining (2) with Fick's Law (1) and eliminating /:
bt bx \ bx)
or
assuming that D is a constant independent of the concentration, c. This
used to be known as Fick's Second Law of Diffusion but is now more
generally referred to as The diffusion equation. Since c depends upon x
and t it could be written as c
(Xf t)
.
In this system of three equations (1) is an experimental law linking the
flow of atoms at any point with the concentration gradient there, (2) is the
continuity equation expressing the fact that atoms cannot disappear whilst
(3) combines these two equations. Equation (1) applies only to steady-state
conditions where these conditions do not vary with time. Equation (3)
applies to the general case where the concentration gradient at a certain
plane changes with time. Values of bc/bt and b
2
c/bx
2
can be determined
experimentally in order to determine the value of D for a given set of con-
ditions.
Differential coefficients vary with the nature of the solute atoms, the
nature of the solvent structure and with temperature.
8.26 In Fig. 8.1, and the illustrations which follow, both solvent and
solute atoms in substitutional solid solutions have been shown of equal
size,
whilst in interstitial solid solutions solute atoms have been shown as
very small and fitting easily into the interstices of the lattice. In the real
world such is not the case; both solvent and solute atoms have been rep-
resented in this way in order not to complicate the illustration unduly.
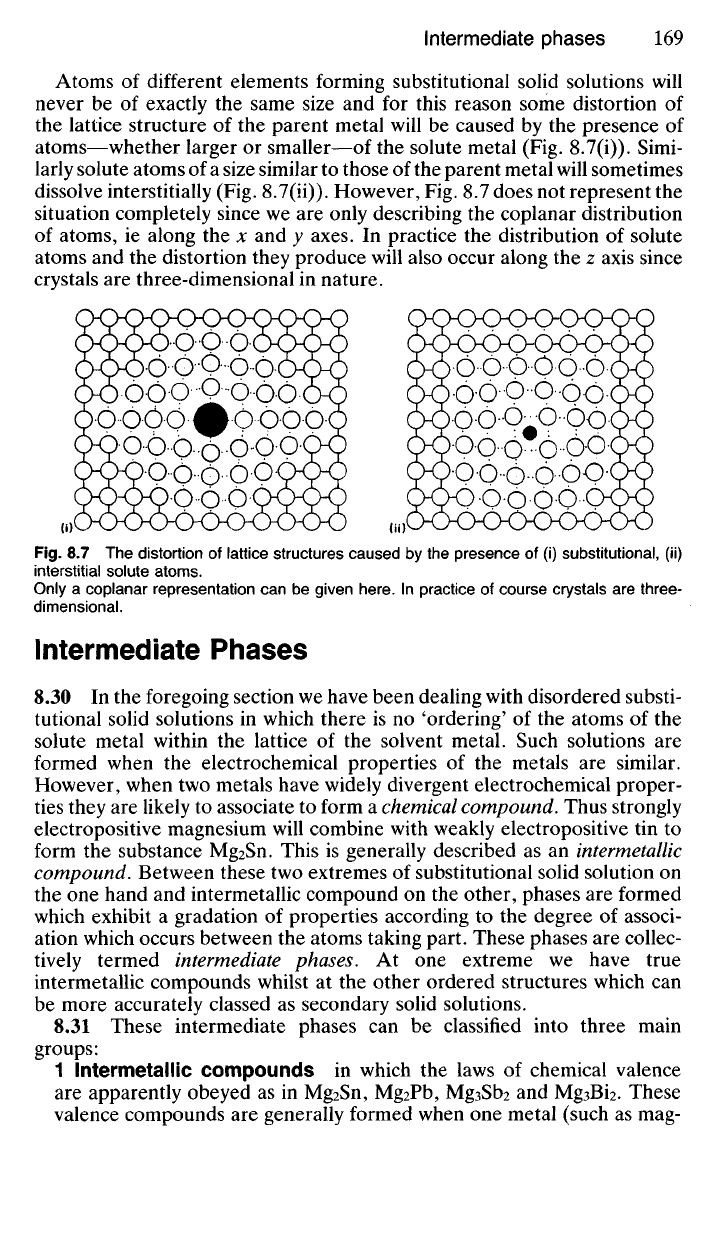
Atoms of different elements forming substitutional solid solutions will
never be of exactly the same size and for this reason some distortion of
the lattice structure of the parent metal will be caused by the presence of
atoms—whether larger or smaller—of the solute metal (Fig. 8.7(i)). Simi-
larly solute atoms of a size similar to those of the parent metal will sometimes
dissolve interstitially (Fig. 8.7(ii)). However, Fig. 8.7 does not represent the
situation completely since we are only describing the coplanar distribution
of atoms, ie along the x and y axes. In practice the distribution of solute
atoms and the distortion they produce will also occur along the z axis since
crystals are three-dimensional in nature.
Fig.
8.7 The distortion of lattice structures caused by the presence of (i) substitutional, (ii)
interstitial solute atoms.
Only a coplanar representation can be given here. In practice of course crystals are three-
dimensional.
Intermediate Phases
8.30 In the foregoing section we have been dealing with disordered substi-
tutional solid solutions in which there is no 'ordering' of the atoms of the
solute metal within the lattice of the solvent metal. Such solutions are
formed when the electrochemical properties of the metals are similar.
However, when two metals have widely divergent electrochemical proper-
ties they are likely to associate to form a chemical compound. Thus strongly
electropositive magnesium will combine with weakly electropositive tin to
form the substance Mg
2
Sn. This is generally described as an intermetallic
compound. Between these two extremes of substitutional solid solution on
the one hand and intermetallic compound on the other, phases are formed
which exhibit a gradation of properties according to the degree of associ-
ation which occurs between the atoms taking part. These phases are collec-
tively termed intermediate phases. At one extreme we have true
intermetallic compounds whilst at the other ordered structures which can
be more accurately classed as secondary solid solutions.
8.31 These intermediate phases can be classified into three main
groups:
1 Intermetallic compounds in which the laws of chemical valence
are apparently obeyed as in Mg
2
Sn, Mg
2
Pb, Mg
3
Sb
2
and Mg
3
Bi
2
. These
valence compounds are generally formed when one metal (such as mag-
nesium) has chemical properties which are strongly metallic, and the
other metal (such as antimony, tin or bismuth) chemical properties which
are only weakly metallic and, in fact, bordering on those of non-metals.
Frequently such a compound has a melting point which is higher than
that of either of the parent metals. For example, the intermetallic com-
pound Mg
2
Sn melts at 780
0
C, whereas the parent metals magnesium and
tin melt at 650 and 232°C respectively. This is an indication of the high
strength of the chemical bond in Mg
2
Sn.
2 Electron Compounds As was shown earlier (1.70) the chemical
valence of a metal is a function of the number of electrons in the outer
'shell' of the atom, whilst the nature of the metallic bond is such that
wholesale sharing of numbers of electrons takes place in the crystal
structure of a pure metal.
In these 'electron compounds' the normal valence laws are not obeyed,
but in many instances there is a fixed ratio between the total number of
valence bonds of all the atoms involved and the total number of atoms
in the empirical formula of the compound in question.
There are three such ratios, commonly referred to as Hume-Rothery
ratios:
(i) Ratio 3/2 (21/14)—(3 structures, such as CuZn, Cu
3
Al, Cu
5
Sn,
Ag
3
Al, etc.
(ii) Ratio 21/13—y structures, such as Cu
5
Zn
8
, G19AI4, Cu
3
iSn
8
,
Ag
5
Zn
8
, Na
3
iPb
8
, etc.
(iii) Ratio 7/4 (21/12)—e structures, such as CuZn
3
, Cu
3
Sn, AgCd
3
,
Ag
5
Al
3
, etc.
Thus,
in the compound CuZn, copper has a valence of 1 and zinc a
valence of 2, giving a total of 3 valences and hence a ratio of 3 valences
to 2 atoms. In the compound Cu
3
iSn
8
copper has a valence of 1 and tin
a valence of 4. Therefore 31 valences are donated by the copper atoms
and 32 (ie, 4 X 8) by the tin atoms, making a total of 63 valences. In
all,
39 atoms are present in the empirical formula of Cu
3
iSn
8
therefore
the ratio
Total number of valences 63 21
Total number of atoms 39 13
These Hume-Rothery ratios have been valuable in relating structures
which were apparently unrelated. There are, however, many electron
compounds which do not fall into any of the three groups mentioned
above, nor are they valence compounds. Electron compounds are
formed as a result of the nature of the metallic bond and the stability of
such a substance depends upon electron concentration and the lattice
structure involved.
3 Size-factor Compounds These are intermediate phases in which
compositions and crystal structures arrange themselves in such a way as
to allow the constituent atoms to pack themselves closely together.
In the Laves phases compositions are based upon the general formula
AB
2
,
eg MgNi
2
, MgCu
2
, TiCr
2
and MnBe
2
. Their formation depends

upon the fact that the constituent atoms vary in size by about 22.5% but
that they can none the less pack closely together in crystal structures.
A very important group of size-factor compounds are the interstitial
compounds formed between some transition metals and certain small non-
metallic atoms. When the solid solubility of an interstitially dissolved
element is exceeded a compound is precipitated from the solid solution.
In this type of compound the small non-metal atoms still occupy interstitial
positions but the overall crystal structure of the compound is different from
that of the original interstitial solid solution. Compounds of this type have
metallic properties and comprise hydrides, nitrides, borides and carbides
of which TiH
2
, TiN, Mn
2
N,
TiB
2
,
TaC, W
2
C, WC, Mo
2
C and Fe
3
C are
typical. All of these compounds are extremely hard and the carbides find
application in tool steels and cemented-carbide cutting materials. Fe
3
C is
of course the phase cementite of ordinary carbon steels. Many of these
carbides are extremely refractory, having melting points well in excess of
300O
0
C.
8.32 Many intermediate phases are extremely hard and brittle so that
a small ingot of such a substance may often be crushed to a powder by
gentle pressure in the jaws of a vice. This is particularly true of valence
compounds where covalent or ionic bonds tend to replace the metallic
bond, thus making the substance essentially non-metallic in its properties
as indicated by brittleness and a low electrical conductivity. Consequently
such phases are of limited use in engineering alloys and then in relatively
small proportions of the total microstructure.
Small quantities of intermediate phases—usually electron compounds—
are generally present in bearing alloys. Here the hard particles of com-
pound are embedded in a matrix of tough solid solution. The compound
resists wear and has a low coefficient of friction, whilst the solid solution
provides the necessary tough matrix capable of withstanding mechanical
shock and high compressive forces.
Intermediate phases are often easy to identify as such under the micro-
scope. Frequently they are of an unexpected colour but this is explained
by the fact that crystal structures and lattice distances are different from
those of the parent metals. (Lattice distances control the wavelength of
light reflected from a surface.) Thus Cu
3
iSn
8
is pale blue whilst Cu
2
Sb is
bright purple. Since intermediate phases are of ordered structure, coring
is not present and the microstructure appears uniform.
8.33 The good high-temperature properties of many of th£ stable inter-
metallic compounds suggests that they could be used as engineering
materials, particularly in the aerospace industries, but for their low ductility
and extremely poor impact toughness. Nevertheless research is now in
progress with a view to improving ductility and hence 'fabricability' of
some of the more promising compounds.
Thus the compound Ni
3
Al has a melting point of 1390
0
C and an ordered
FCC structure. Single crystals of the compound are very ductile, as are most
FCC structures (4.12), but in the normal polycrystalline state it suffers from
extreme grain-boundary brittleness. The addition of as little as 0.05%
boron raises the percentage elongation from zero to 50 as the fracture
mode is changed from being intercrystalline to a mixture of intercrystalline
and transcrystalline. Formability is therefore dramatically improved. This
effect is probably due to the segregation of boron at the crystal boundaries
causing some disorder in the boundary region. Stresses due to dislocation
'pile-ups' there are more evenly distributed and can be relieved by slip
across the boundary rather than by fracture along the boundary only.
Eutectics and Eutectoids
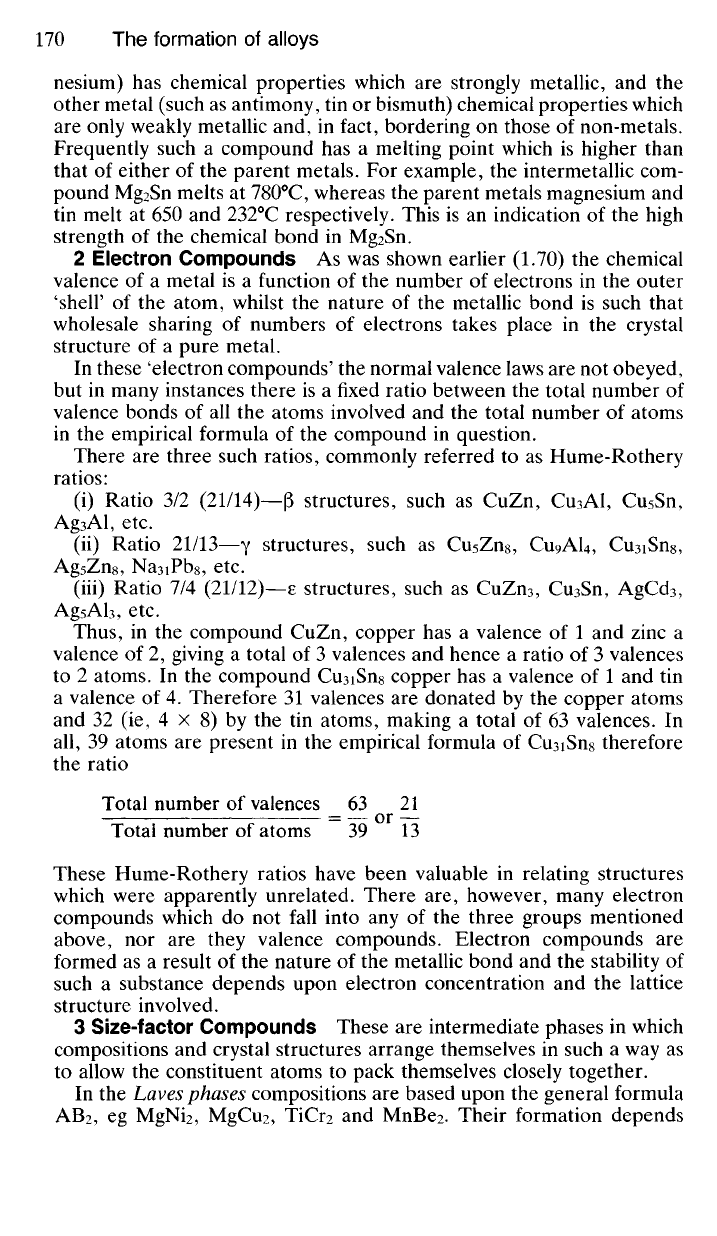
8.40 When two metals which are completely soluble in the liquid state
but completely insoluble in the solid state solidify, they do so by crystallis-
ing out as alternate layers of the two pure metals. Thus a laminated type
of structure is obtained and is termed a eutectic. This eutectic mixture is
always of a fixed composition for the two metals concerned and it melts at
a temperature below the melting points of either of the metals.
Consider the two metals cadmium and bismuth which are completely
soluble in each other as liquids but completely insoluble as solids. If
increasing amounts of cadmium are added to bismuth then the freezing
point of bismuth (TIl
0
C) is progressively depressed (along AE in Fig. 8.8).
Similarly if increasing amounts of bismuth are added to cadmium then the
freezing point of cadmium (321°C) decreases progressively (along BE).
These depression-of-freezing point lines meet at a minimum in E which is
called the eutectic point. This indicates the composition of the eutectic
mixture (60% wt. Bi/ 40% wt. Cd) and also the temperature (140
0
C) at
which it freezes. The relative thicknesses of the layers of bismuth and
cadmium will be so adjusted as to give an overall eutectic composition as
indicated by E. Suppose the overall composition of a bismuth-cadmium
alloy were given by X. This indicates that bismuth is present in the molten
alloy in excess of the eutectic composition. Therefore some bismuth will
crystallise out first until by the time that the temperature has fallen to
TEMPERATURE
0
C
COMPOSITION (°/owt.)
Fig.
8.8 The
eutectic point
(E) for
cadmium/bismuth alloys.
32I°C
IOOCtf
OBi

140
0
C the remaining liquid will be of composition E. This liquid will then
crystallise out as eutectic. Similarly if the overall composition of the initial
liquid mixture were given by Y then some cadmium would solidify first until
the remaining liquid were of composition E. Eutectic would then form as
before, ie as alternate layers of pure bismuth and pure cadmium. The
mechanism of this type of crystallisation will be discussed more fully in the
next chapter (9.40).
8.41 In a eutectic of two pure metals there is no question whatsoever
of solution, since the layers of the pure metals forming the eutectic can be
seen quite clearly under the microscope, at magnifications usually between
100 and 500, as definite separate entities. The formation of the eutectic is
therefore a result of insolubility being introduced when the alloy solidifies.
At this point it must be admitted that it is very doubtful whether com-
plete insolubility exists in the solid state in any alloy system. There is nearly
always some solid solubility however slight it may be. However, if the two
metals in question are partially soluble in each other in the solid state, we
may still obtain a eutectic on solidification, but it will be a eutectic
composed of alternate layers of two saturated solid solutions. One layer
will consist of metal A saturated with metal B, and the other a layer of
metal B saturated with metal A. This statement may at first cause some
confusion in the mind of the reader, but it must be understood that the
two layers are not one and the same as far as composition is concerned,
since one layer is rich in metal A and the other is rich in metal B. Consider
as a parallel case in liquid solutions the substances water and ether. If a
few spots of ether are added to a test-tube nearly filled with water and the
tube shaken, a single solution of ether in water will result. If, on the other
hand, a few spots of water are added to a test-tube nearly filled with ether,
and the tube shaken, a single solution of water in ether will result. When,
however, we put into the test-tube equal volumes of ether and water and
again shake, we find that we are left with two layers. The upper layer will be
ether saturated with water, and the lower layer will be water saturated
with ether. The upper layer is a solution containing most of the ether, and
the lower layer a different solution containing most of the water. We have
a similar situation in the case of metallic solid solutions, in that one phase
of a eutectic may be a solid solution rich in metal A and the other phase
a solid solution rich in metal B.
8.42 Not all eutectics, whether of pure metals or solid solutions, appear
under the microscope in the well-defined laminated form mentioned above.
Usually layers of one of the phases are embedded in a matrix of the other
phase, and quite often these layers are broken or disjointed. Fig. 17.2 illus-
trates the aluminium-silicon eutectic structure in which the thinner silicon
layers (the eutectic mixture contains only 11.7% silicon) are broken into
plate form and are embedded in an aluminium matrix. This is indeed fortu-
nate since silicon is brittle and if present as continuous layers would seriously
impair the properties of this very useful alloy.
Again, surface tension may influence crystallisation and the final eutectic
may consist of globules of one phase embedded in a matrix of the other.
The effect of surface tension will be accentuated by slow rates of cooling
which allow layers of one phase to break up; first, into smaller, thicker
plates,
and ultimately, into rounded globules (Fig. 11.8).
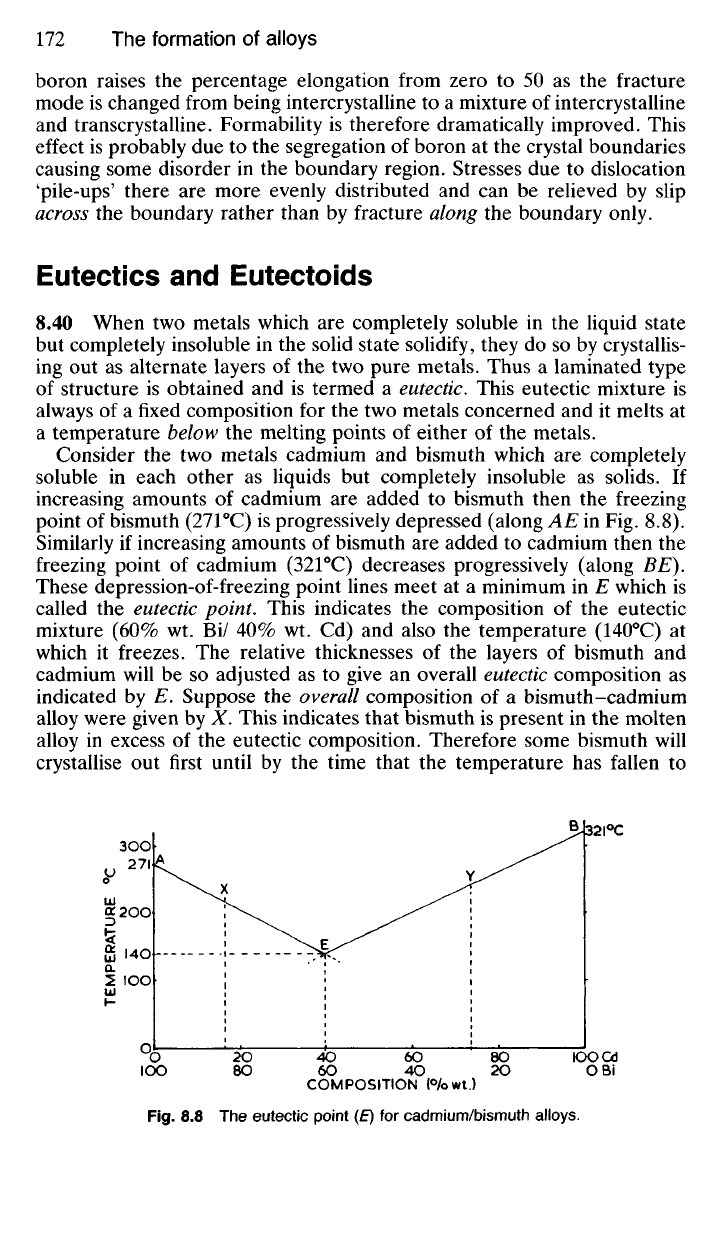
8.43 Sometimes a solid solution which has already formed in an alloy
transforms at a lower temperature to a eutectic type of structure. When
this happens, the eutectic type of structure produced is called a eutectoid,
since it was not formed from a liquid solution like a eutectic, but from a
solid solution. The transformation of the solid solution, austenite, to the
eutectoid, pearlite (7.55), is an example of this type of change. Cementite
nuclei form at random at the crystal boundaries of the austenite, and these
nuclei initiate the growth of cementite plates in the direction in which the
concentration of carbon in the austenite is highest. As a result of the
extraction of the carbon from the surrounding austenite to form cementite,
ferrite will nucleate alongside the cementite. In this way cementite and
ferrite plates will develop alongside each other (Fig. 8.9)
Fig.
8.9 The mechanism of the austenite -» pearlite transformation.
If an alloy is cooled very rapidly from a temperature above that at which
the eutectoid begins to form, the solid solution (or possibly a modified
form of it such as martensite in steels) is often retained. This demonstrates
that the eutectoid has not formed from the liquid but had been produced
at a later stage.
8.50 Many useful alloys consist structurally of a single uniform solid
solution in which increased strength is developed as suggested in the follow-
ing section (8.60). Such alloys are generally also ductile and corrosion
austenite
cementite
ferrite
resistant (21.71). Yet other useful alloys are of duplex structure, that is,
they contain two different phases in the microstructure. These two different
phases may be solid solutions in a eutectic type of structure or they may
be a solid solution along with some form of intermediate phase. Since
many intermediate phases are weak and brittle it is usually only those
which are hard and fairly strong which find use in metallurgical alloys, and
then only in relatively small quantities. In addition to their use in bearing
alloys as already mentioned, such phases may be employed to increase
strength by some form of precipitation hardening process (9.92) or disper-
sion hardening mechanism (8.62).
It is the function of the metallurgist, by control of his alloy compositions
along with suitable mechanical and thermal treatments, to produce alloys
which will provide a set of physical and mechanical properties which fulfil
the requirements of the engineer.
Strengthening Mechanisms in Alloys
8.60 As mentioned earlier in this chapter, the main reason for alloying
is to increase the yield strength of a metal. This involves impeding the
movement of dislocations by making alterations to the structure on
approximately the atomic scale.
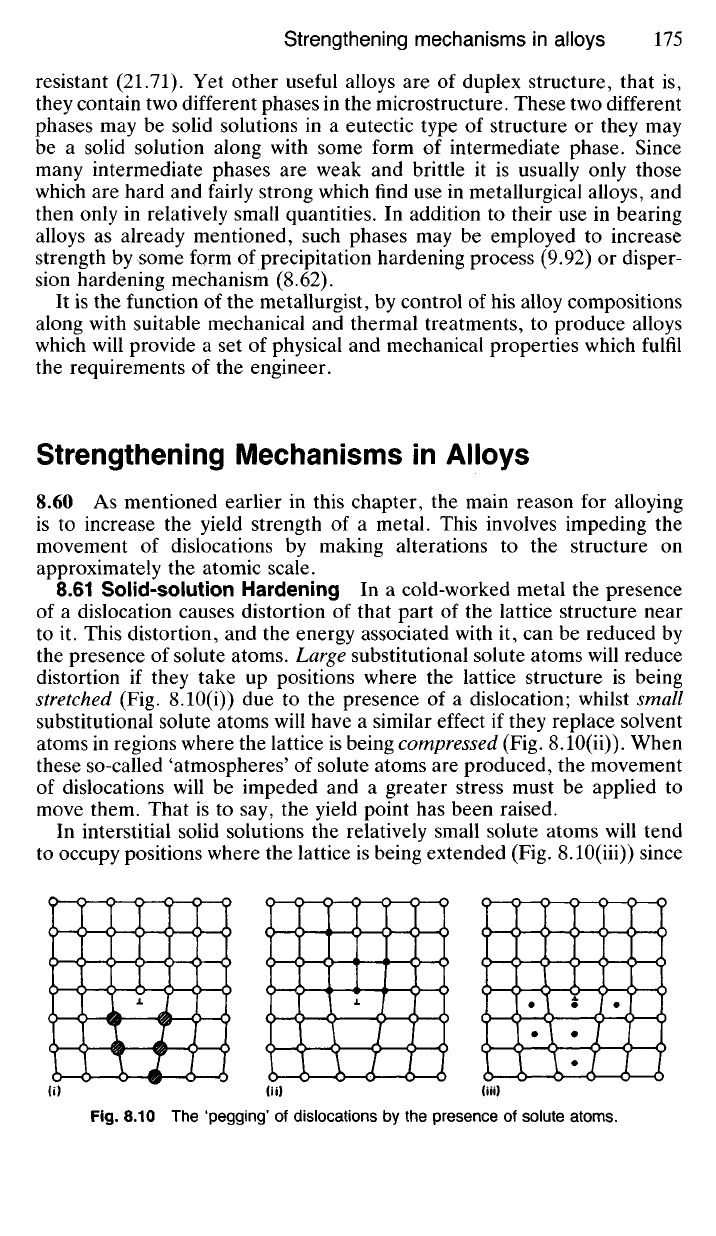
8.61 Solid-solution Hardening In a cold-worked metal the presence
of a dislocation causes distortion of that part of the lattice structure near
to it. This distortion, and the energy associated with it, can be reduced by
the presence of solute atoms. Large substitutional solute atoms will reduce
distortion if they take up positions where the lattice structure is being
stretched (Fig. 8.10(i)) due to the presence of a dislocation; whilst small
substitutional solute atoms will have a similar effect if they replace solvent
atoms in regions where the lattice is being compressed (Fig. 8.10(ii)). When
these so-called 'atmospheres' of solute atoms are produced, the movement
of dislocations will be impeded and a greater stress must be applied to
move them. That is to say, the yield point has been raised.
In interstitial solid solutions the relatively small solute atoms will tend
to occupy positions where the lattice is being extended (Fig. 8.10(Hi)) since
Fig.
8.10 The 'pegging' of dislocations by the presence of solute atoms.
