Hermanson G. Bioconjugate Techniques, Second Edition
Подождите немного. Документ загружается.

880 22. Preparation of Liposome Conjugates and Derivatives
(Shek and Heath, 1983). All of the amine-reactive activation methods discussed in this section
using heterobifunctional crosslinkers may be used with PE containing liposomes to prepare
immunogen conjugates. In addition, the crosslinking methods in Chapter 19, dealing specifi -
cally with hapten–carrier conjugation, should be consulted for potential use with liposomes.
The following protocols provide suggested crosslinking strategies for producing an antigen
or hapten complex with liposomes. The fi rst procedure is a simple encapsulation of antigen
molecules. The second method involves the coupling of a sulfhydryl-containing peptide hapten
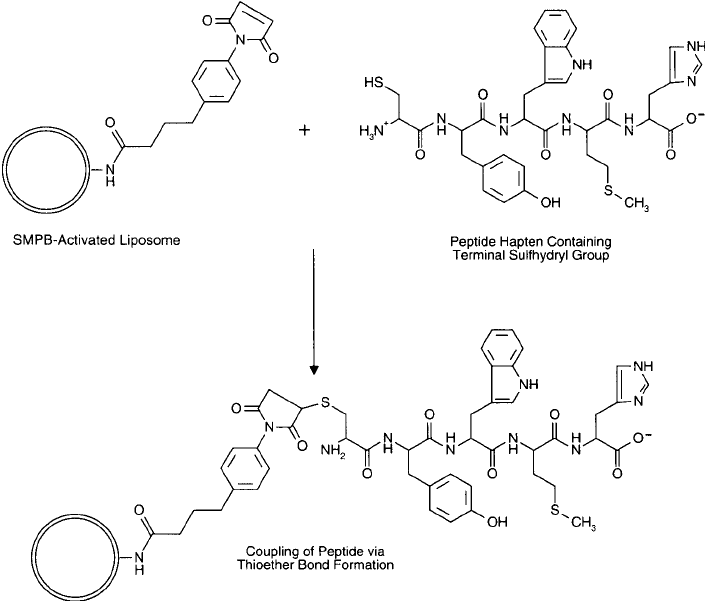
to a liposome that had been previously activated with SMPB ( Figure 22.16 ) (see Section 2, this
chapter). They are based on the methods of Van Regenmortel et al . (1988).
Protocol for the Encapsulation of Antigen into Liposomal Vesicles
1. Prepare a homogeneous lipid mixture by dissolving the desired components in chloro-
form. A suggested recipe may be to use a mixture of DPPC:DPPG:cholesterol at a molar
ratio of 7.5:2.5:5. Evaporate the chloroform using a rotary evaporator under vacuum.
Maintain lipids under nitrogen or argon to prevent air oxidation.
Figure 22.16 SMPB-activated liposomes may be modifi ed with peptide hapten molecules containing cysteine
thiol groups. The resultant immunogen may be used for immunization purposes to generate an antibody
response against the coupled peptide.
2. Dissolve the hapten or antigen to be encapsulated at a concentration of 21 mol/ml in
degassed, nitrogen-purged 10 mM HEPES, 0.15 M NaCl, pH 6.5.
3. Create liposomal vesicles using any established method (see Section 1, this chapter) by
mixing the antigen solution with the lipid mixture to obtain a fi nal concentration of
5 mg/ml lipid in the aqueous buffer. A suggested procedure may be to redissolve the lip-
ids in a minimum quantity of diethyl ether, and then mix the buffer with the ether phase
at a ratio necessary to give a 5 mg/ml concentration of lipid in the buffer. Use sonication
to emulsify the liposomal preparation. Remove diethyl ether by vacuum evaporation.
Periodically mix by vortexing to maintain a homogeneous suspension of liposomes.
4. Remove free antigen from encapsulated antigen by gel fi ltration using a column of
Sephadex G-75 or by dialysis using 10 mM HEPES, 0.15 M NaCl, pH 6.5. Store under
an inert gas at 4°C in the dark until use.
Protocol for the Coupling of Peptide Haptens Containing Sulfhydryl Groups to
Liposomal Vesicles
1. Prepare a homogeneous lipid mixture by dissolving in chloroform a mixture of DPPC:
DPPG:cholesterol:MPB-DPPE at a mole ratio of 6.3:2.12:4.25:1.5. Preparation of MPB-
activated DPPE can be found in Section 2, this chapter. Maintain all solutions under nitro-
gen or argon. The lipid derivative provides reactive maleimide groups for the coupling
of sulfhydryl-containing molecules. Evaporate the chloroform using a rotary evaporator
under vacuum.
2. Create liposomal vesicles using any established method (see Section 1, this chapter) by
mixing the lipid mixture with degassed, nitrogen-purged 10 mM HEPES, 0.15 M NaCl,
pH 7.0, to obtain a fi nal concentration of 5 mg/ml lipid in the aqueous buffer. Use soni-
cation to emulsify the liposomal preparation. Remove diethyl ether by vacuum evapora-
tion. Periodically mix by vortexing to maintain a homogeneous suspension of liposomes.
3. Dissolve a sulfhydryl-containing peptide hapten at a concentration of 25 mol/ml in
degassed, nitrogen-purged 10 mM HEPES, 0.15 M NaCl, pH 7.0. Add the peptide solution
to the liposome suspension at a molar ratio necessary to obtain at least a 5:1 excess of thiol
groups to the amount of maleimide groups present (as MPB-DPPE).
4. React overnight at room temperature. Maintain an inert-gas blanket over the vessel to
prevent lipid oxidation.
5. Purify the derivatized liposomes from excess peptide by gel fi ltration using a column of
Sephadex G-75 or by dialysis. Store the immunogenic vesicles at 4°C under nitrogen or
argon and protected from light until use.
5. Preparation of Antibody–Liposome Conjugates
Covalent attachment of antibody molecules to liposomes can provide a targeting capacity to
the vesicle that can modulate its binding to specifi c antigenic determinants on cells or to mol-
ecules in solution. Antibody-bearing liposomes may possess encapsulated components that
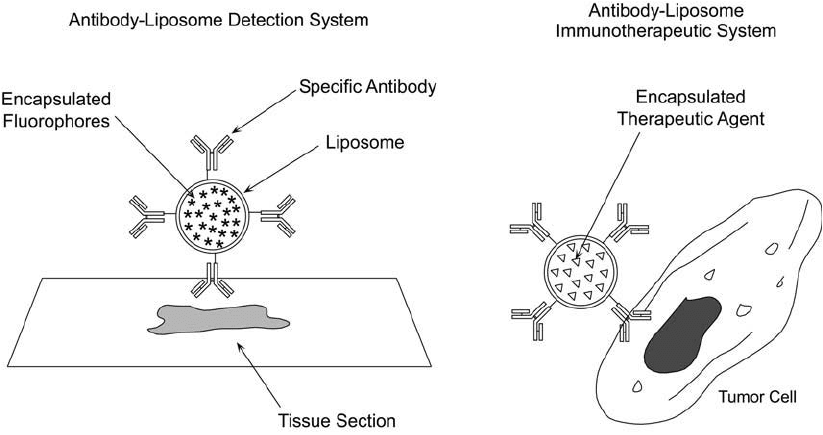
can be used for detection or therapy ( Figure 22.17 ). For instance, fl uorescent molecules encap-
sulated within antibody-targeted vesicles can be used as imaging tools or in fl ow cytometry
5. Preparation of Antibody–Liposome Conjugates 881
882 22. Preparation of Liposome Conjugates and Derivatives
(Truneh et al., 1987). Specifi c antibodies coupled to the vesicle surface can improve diagnos-
tic assays involving agglutination of latex particles (Kung et al., 1985). Liposomes possessing
antibodies directed against tumor cell antigens can deliver encapsulated toxins or drugs to the
associated cancer cells, effecting toxicity and cell death (Heath et al., 1983; Heath et al., 1984;
Matthay et al ., 1984; Straubinger et al ., 1988).
Encapsulation of chemotherapeutic agents within lipid bilayers reduces systemic toxicity
and local irritation often caused by anticancer drugs (Gabizon et al., 1986). The liposomal
membrane acts as a slow-release agent so that cytotoxic components do not come into contact
with non-tumor cells. Liposome binding to cells causes internalization and release of the encap-
sulated drugs. Antibody targeting can increase the likelihood of vesicle binding to the desired
tumor cells.
However, there are problems associated with the use of antibody–liposome conjugates for
drug delivery in vivo. Particularly, since lipid vesicles are huge compared to similar immunotoxin
conjugates (Chapter 21), their passage to particular tissue destinations may be diffi cult or
impossible. Liposomes are almost entirely limited to the reticuloendothelial system. Their abil-
ity to pass through tissue barriers to target cells in other parts of the body is limited by their
size. If liposomal conjugates can reach their intended destination, their contents are delivered
to the cells by endocytosis. Endocytic vesicles arising from the surface of cells have diameters in
the range of 1,000–1,500 Å. This limits the size of liposomes that can be used to small vesicles
that can bind to the surface of a cell and be internalized effi ciently. Large liposomes, by con-
trast, will not be internalized and therefore not be able to deliver their contents (Leserman and
Machy, 1987).
Figure 22.17 Antibody–liposome conjugates may be used as targeting reagents for detection or therapeu-
tic applications. The liposome may be constructed to contain fl uorescent molecules for detection purposes or
bioactive agents for therapy. The antibody component targets the complex for binding to specifi c antigenic
determinants.
The methods for coupling antibody molecules to liposomal surfaces are not unlike those
described for general protein coupling (Section 7, this chapter). Antibodies may be coupled
through sulfhydryl residues using liposomes-containing PE groups that are derivatized with
heterobifunctional crosslinkers such as SMCC (Chapter 5, Section 1.3), MBS (Chapter 5,
Section 1.4), SMPB (Chapter 5, Section 1.6), SIAB (Chapter 5, Section 1.5), SPDP (Chapter 5,
Section 1.1), and heterobifunctional PEG-based crosslinkers (Chapter 18, Section 2). They also
may be coupled through their amine groups using reductive amination to periodate-oxidized
glycolipids (Sections 2 and 7.6, this chapter).
6. Preparation of Biotinylated or Avidin-Conjugated Liposomes
Liposome conjugates may be used in various immunoassay procedures. The lipid vesicle can
provide a multivalent surface to accommodate numerous antigen–antibody interactions and
thus increase the sensitivity of an assay. At the same time, it can function as a vessel to carry
encapsulated detection components needed for the assay system. This type of enzyme-linked
immunosorbent assay (ELISA) is called a liposome immunosorbent assay or LISA. One method
of using liposomes in an immunoassay is to modify the surface so that it can interact to form
biotin–avidin or biotin–streptavidin complexes. The avidin–biotin interaction can be used to
increase detectability or sensitivity in immunoassay tests (Chapter 23) (Savage et al ., 1992).
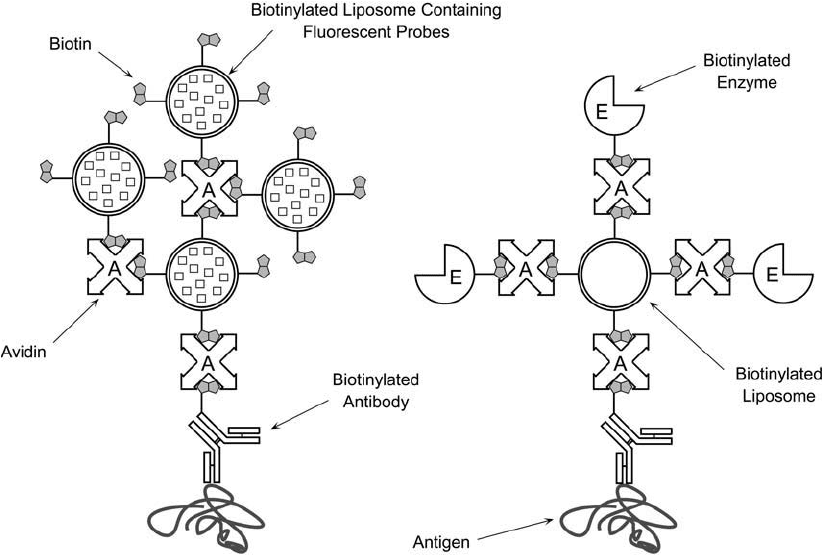
Liposomes containing biotinylated phospholipid components can be used in a bridging assay
system with avidin and a biotinylated antibody molecule, creating large multivalent complexes
able to bind antigen (Plant et al., 1989) ( Figure 22.18 ). The inside of the vesicles may con-
tain fl uorescent detection reagents that can be used to localize or quantify target analytes. One
small liposome provides up to 10
5
molecules of fl uorophore to allow excellent detectability of
a binding event. LISA systems using biotinylated liposomes to detect antigen molecules can
increase the sensitivity of an immunoassay up to 100-fold over that obtainable using tradi-
tional antibody–enzyme ELISAs.
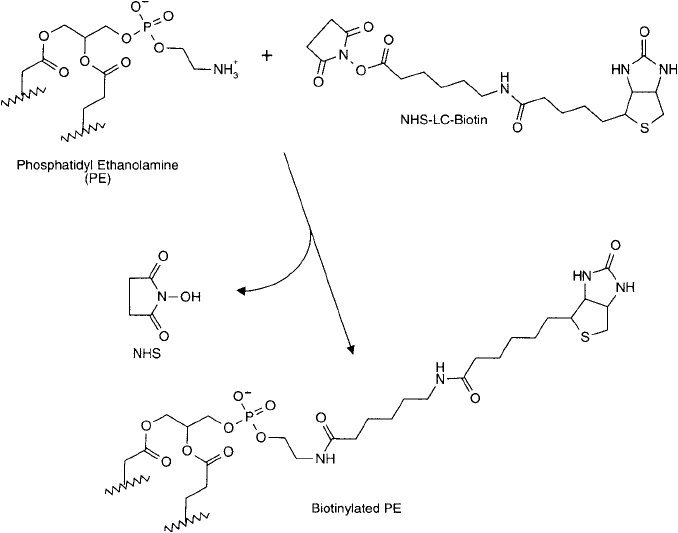
Biotinylated liposomes usually are created by modifi cation of PE components with an amine-
reactive biotin derivative, for example NHS-LC-Biotin (Chapter 11, Section 1). The NHS ester
reacts with the primary amine of PE residues, forming an amide bond linkage ( Figure 22.19 ). A
better choice of biotinylation agent may be to use the NHS-PEG
n
-biotin compounds (Chapter
18), because the hydrophilic PEG spacer provides better accessibility in the aqueous environ-
ment than a hydrophobic biotin spacer. Since the modifi cation occurs at the hydrophilic end of
the phospholipid molecule, after vesicle formation the biotin component protrudes out from
the liposomal surface. In this confi guration, the surface-immobilized biotins are able to bind
(strept)avidin molecules present in the outer aqueous medium.
However, since many of the traditional biotinylation reagents, such as NHS-LC-biotin con-
tain hydrophobic spacers, their use with amphipathic liposomal constructions may not be
entirely appropriate. A better choice may be to use a hydrophilic PEG-based biotin compound
that creates a water-soluble biotin modifi cation on the outer aqueous surface of the liposome
bilayer. Biotinylation reagents of this type are discussed in Chapter 18, Section 3.
Biotinylation may be done before or after liposome formation, but having a stock supply
of biotin-modifi ed PE is an advantage, since it can then be used to test a number of liposomal
recipes. In addition, only a very small percent of the total lipid should be biotinylated to pre-
vent avidin-induced aggregation in the absence of antigen. It is diffi cult to control precisely
6. Preparation of Biotinylated or Avidin-Conjugated Liposomes 883
884 22. Preparation of Liposome Conjugates and Derivatives
the biotin content if direct biotinylation of intact liposomes is done. Using pure biotinylated
phospholipid allows incorporation of discrete amounts of biotin binding sites into the fi nal
liposomal membrane. The preparation of biotinylated PE (B-PE) can be done similar to the
methods described for activation of PE with SMPB (Section 2, this chapter) or it may be
obtained commercially.
The following method for the formation of a biotinylated liposome is adapted from Plant
et al ., 1989). It assumes prior production of B-PE.
Protocol
1. Prepare a biotinylated liposome construct by fi rst dissolving in chloroform, the lipids
DMPC:cholesterol:dicetylphosphate (Sigma) at mole ratios of 5:4:1, and adding to this
solution 0.1 mol percent B-PE. Larger mole ratios of B-PE to total lipid may result in
nonspecifi c aggregation of liposomes in the presence of avidin. Maintain all lipids under
an inert atmosphere to prevent oxidation.
2. Evaporate 2 mol of total homogenized lipid in chloroform using a stream of nitrogen or
a rotary vacuum evaporator.
Figure 22.18 Biotinylated liposomes may be used in immunoassay systems to enhance the signal for detection
or measurement of specifi c analytes. The liposome components may be constructed to include fl uorescent mol-
ecules to facilitate detection of antigens within tissue sections.
3. Redissolve the dried lipid in 50 l of dry isopropanol.
4. Take the lipid solution up into a syringe and inject it into 1 ml of degassed, nitrogen-
purged 20 mM Tris, 0.15 M NaCl, pH 7.4, which is being vigorously stirred using a
vortex mixer. To encapsulate a fl uorescent dye using this procedure, include 100 mM
5,6-carboxyfl uorescein (or another suitable fl uorophore) in the buffer solution before
adding the lipids. The fl uorescent probe used in the encapsulation procedure should be
chemically nonreactive so that no lipid components are covalently modifi ed during the
process.
The biotinylated liposomes prepared by this procedure may be stored under an inert-gas
atmosphere at 4°C for long periods without degradation.
7. Conjugation of Proteins to Liposomes
Covalent attachment of proteins to the surface of liposomal bilayers is done through reactive
sites created on the head groups of phospholipids with the intermediary use of a crosslinker
or other activating agent. The lipid functional groups described in Section 1 of this chapter are
modifi ed according to the methods discussed in Section 2 to be reactive toward specifi c target
Figure 22.19 Biotinylated liposomes may be formed using biotinylated PE. Reaction of NHS-LC-biotin with PE
results in amide bond linkages and a long spacer arm terminating in a biotin group.
7. Conjugation of Proteins to Liposomes 885
886 22. Preparation of Liposome Conjugates and Derivatives
groups in proteins. Conjugation of liposomes with proteins may be done with homobifunctional
or heterobifunctional crosslinking reagents, carbodiimides, reductive amination, by NHS ester
activation of carboxylates, or through the noncovalent use of the avidin–biotin interaction.
Characterizing the resultant complex for the amount of protein per liposome is somewhat
more diffi cult than in other protein conjugation applications. The protein–liposome composi-
tion is highly dependent on the size of each liposomal particle, the amount of protein charged
to the reaction, and the mole quantity of reactive lipid present in the bilayer construction. An
approach to solving this problem is presented by Hutchinson et al. (1989). In analyzing at least
17 different protein–liposome preparations, the ratio of protein:lipid content ( g protein/ g
lipid) in most of the complexes ranged from a low of about 4 to as much as 675. In some
instances, however, up to 6,000 molecules of a particular protein could be incorporated into
each liposome.
Coupling of protein molecules to liposomes occasionally may induce vesicle aggregation.
This may be due to the unique properties or concentration of the protein used, or it may be
a result of liposome-to-liposome crosslinking during the conjugation process. Adjusting the
amount of protein charged to the reaction as well as the relative amounts of crosslinking rea-
gents employed may have to be done to solve an aggregation problem.
The following sections present suggested protocols for creating protein-bearing liposomes.
Each method utilizes specifi c lipid modifi cations to form reactive groups capable of targeting
amines, sulfhydryls, aldehydes, or carboxylates on the protein molecules.
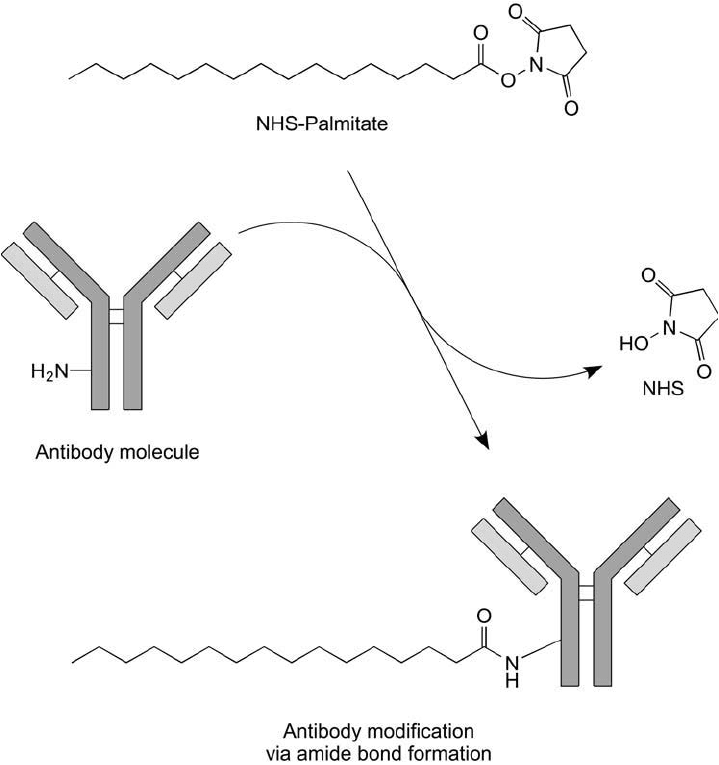
7.1. Coupling via the NHS Ester of Palmitic Acid
Huang et al. (1980) coupled monoclonal antibodies to liposomes using an NHS ester modifi ca-
tion of palmitic acid incorporated into the bilayer construction (Lapidot et al., 1967). The NHS
ester reacts with amine groups on the protein molecule, producing stable amide bond linkages
(Figure 22.20 ). The specifi city of the antibody-bearing liposomes for mouse L-929 cells was
documented, illustrating the preservation of antibody binding activity.
Protocol
Preparation of NHS–Palmitate
1. Dissolve 3.45 mg of N-hydroxysuccinimide (NHS) in 30 ml dry ethyl acetate.
2. Add 30 mmol of palmitic acid to the NHS solution. Maintain a nitrogen blanket over the
solution.
3. Dissolve 6.18 g of dicyclohexyl carbodiimide (DCC; Chapter 3, Section 1.4) in 10 ml of
ethyl acetate and add it to the NHS/palmitic acid solution.
4. React overnight at room temperature under a nitrogen blanket.
5. Remove the insoluble dicyclohexyl urea (DCU) by-product by fi ltration using a glass fi ber
fi lter pad and vacuum.
6. Remove solvent from the fi ltered solution by using a rotary evaporator under vacuum.
7. The NHS–palmitate may be purifi ed by recrystallization using ethanol. Dissolve the acti-
vated fatty acid in a minimum quantity of hot ethanol. Immediately upon dissolving,
fi lter it through a fi lter funnel containing a fl uted glass fi ber fi lter pad, both of which have
been warmed to the same temperature as the ethanol solution. Allow the NHS–palmitate
to recrystallize overnight at room temperature. Remove solvent from the recrystallized
solid by fi ltration. Dry under vacuum in a desiccator.
8. Analyze the NHS–palmitate for purity using TLC on silica plates. Develop using a sol-
vent mixture of chloroform:petroleum diethyl ether (bp 40–60°C) of 8:2. Excess NHS
and NHS–palmitate may be detected by staining with 10 percent hydroxylamine in 0.1 N
NaOH, followed after 2 minutes by a 5 percent solution of FeCl
3
in 1.2 N HCl (creates
red colored spots).
Figure 22.20 The NHS ester derivative of palmitic acid may be used to couple antibody molecules through
amide bonds. These complexes then may be incorporated into liposomes.
7. Conjugation of Proteins to Liposomes 887
888 22. Preparation of Liposome Conjugates and Derivatives
Coupling of Protein to NHS–Palmitate
1. Add 2 mg of protein to 44 g NHS–palmitate in 20 mM sodium phosphate, 0.15 M NaCl,
pH 7.4, containing 2 percent deoxycholate.
2. Incubate at 37°C for 10 hours.
3. Remove excess palmitic acid by chromatography on a column of Sephadex G-75. Use
PBS, pH 7.4, containing 0.15 percent deoxycholate to perform the gel fi ltration. Collect
the fractions containing derivatized protein, as monitored by absorbance at 280 nm.
Addition of Protein–Palmitate Conjugate to Liposomal Membranes
Since the protein–palmitate derivative can ’t be dissolved in organic solvent during homogeniza-
tion of lipid to form liposomal membranes, it must be inserted into intact liposomes by deter-
gent dialysis.
1. Construct a liposome by dissolving the desired lipids in chloroform to homogenize fully
the mixture, drying them to remove solvent, and using any established method of form-
ing bilayer vesicles in aqueous solution (i.e., sonication; see Section 1, this chapter).
2. Add protein–palmitate conjugate to the formed liposomes in a ratio of 20:1 (w/w). Add
concentrated deoxycholate to give a fi nal concentration of 0.7 percent. Mix thoroughly
using a vortex mixer.
3. Dialyze the liposome preparation against PBS, pH 7.4.
4. The liposome vesicles may be characterized for size by chromatography on a column of
Sepharose 4B.
7.2. Coupling via Biotinylated PE Lipid Derivatives
Biotinylated PE (B-PE) incorporated into liposomal membranes can be used to interact non-
covalently with (strept)avidin–protein conjugates or with other biotinylated proteins using
(strept)avidin as a bridging molecule (Plant et al., 1989). It is important that a long-chain spacer
be used in constructing the B-PE derivative to allow enough spatial separation from the bilayer
surface to accommodate avidin docking (Hashimoto et al., 1986). Thus, any biotinylated pro-
tein can be coupled to the liposome surface through the strength of the (strept)avidin–biotin
interaction. Section 6 (this chapter) describes the preparation of B-PE derivatives and their addi-
tion into vesicle construction. Incubation of (strept)avidin conjugates or (strept)avidin plus a
biotinylated protein with the biotinylated liposome in PBS, pH 7.4, will form essentially nonre-
versible complexes, immobilizing the proteins to the outer surface of the bilayers. This method
can be used to couple biotin-modifi ed antibody molecules to liposomes ( Figure 22.21 ). Removal
of noncomplexed protein may be done using gel fi ltration chromatography on a column of
Sephadex G-50 or G-75.
7.3. Conjugation via Carbodiimide Coupling to PE Lipid Derivatives
Underivatized PE in liposomal membranes contains an amine group that can participate in the
carbodiimide reaction with carboxylate groups on proteins or other molecules (Dunnick et al .,
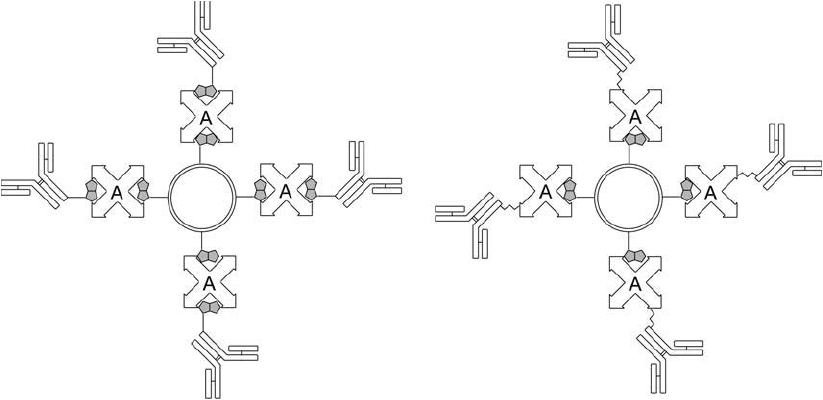
Figure 22.21 Antibodies may be conjugated to liposomes using an indirect approach incorporating a
(strept)avidin–biotin system. Biotinylated liposomes may be complexed with biotinylated antibodies using
(strept)avidin as a bridging molecule or may be complexed with an antibody–(strept)avidin conjugate.
1975). The water-soluble carbodiimide EDC (Chapter 3, Section 1.1) activates carboxylate
groups to form active-ester intermediates that can react with PE to form an amide linkage.
Unfortunately, EDC coupling of proteins to surfaces often results in considerable protein-to-
protein crosslinking, since proteins contain an abundance of both amines and carboxylates.
There also is potential for vesicle aggregation by proteins coupling to more than one liposome.
Martin et al. (1990) suggest avoiding this polymerization problem by fi rst blocking the amine
groups of the protein with citraconic acid, which has been used successfully with antibodies
(Jansons and Mallett, 1980).
However, even with the potential for protein–protein conjugation, carbodiimide coupling of
peptides and proteins to liposomes can be done with EDC without blocking polypeptide amines.
The approach is similar to that described for the EDC conjugation of hapten molecules to car-
rier proteins to form immunogen complexes (Chapter 19, Section 3). Thus, this method may be
used to prepare peptide hapten–liposome conjugates for immunization purposes ( Figure 22.22 ).
The procedure also works particularly well for coupling molecules containing only carboxylates
to the amines on the liposomes.
Protocol
1. Prepare liposomes containing PE by any desired method. For instance, the common recipe
mentioned in Section 1 (this chapter) that involves mixing PC:cholesterol:PG:PE in a molar
ratio of 8:10:1:1 may be used. Thoroughly emulsify the liposome construction to obtain
a good population of SUVs. The fi nal liposome suspension should be in 20 mM sodium
phosphate, 0.15 M NaCl, pH 7.2. Adjust the concentration to about 5 mg lipid/ml buffer.
7. Conjugation of Proteins to Liposomes 889
