Hermanson G. Bioconjugate Techniques, Second Edition
Подождите немного. Документ загружается.

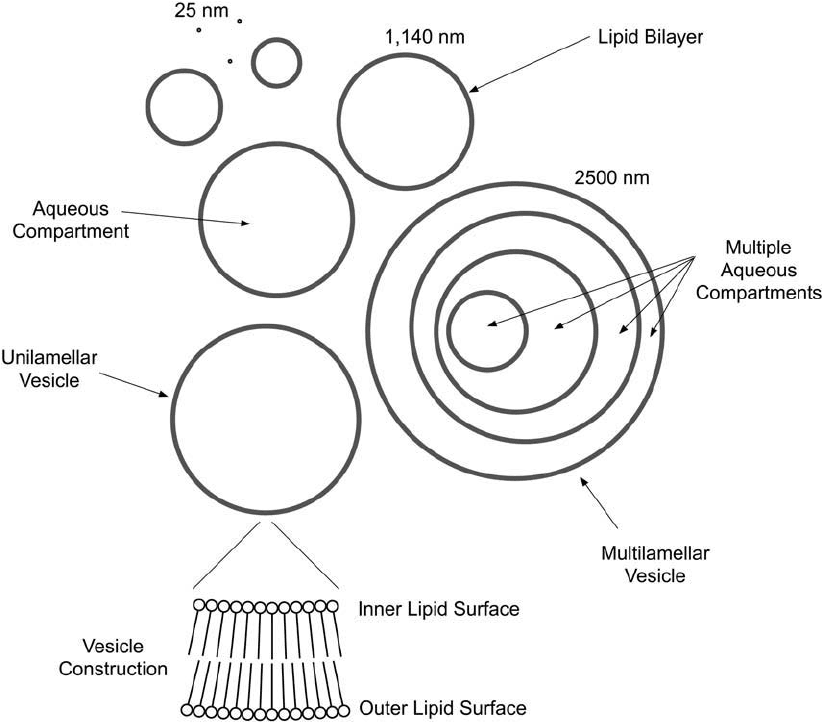
860 22. Preparation of Liposome Conjugates and Derivatives
The confi guration the bilayers can assume also can be complex. A bilayer may be in a spheri-
cal form, having one layer of hydrophilic head groups pointing outward toward the surrounding
solution and the second hydrophilic layer pointing inward toward a compartment of aque-
ous solution sequestered within the sphere. The morphology of a liposome may be classifi ed
according to the compartmentalization of aqueous regions between bilayer shells. If the aqueous
regions are segregated by only one bilayer each, the liposomes are called unilamellar vesicles
(ULV) ( Figure 22.2 ). If there is more than one bilayer surrounding each aqueous compartment,
the liposomes are termed multilamellar vesicles (MLV). ULV forms are further classifi ed as to
their relative size, although rather crudely. Thus, there can be small unilamellar vesicles (SUV;
usually thought of as less than 100 nm in diameter, with a minimum of about 25 nm) and large
unilamellar vesicles (LUV; usually greater than 100 nm in diameter, with a maximal size of about
2,500 nm). With regard to MLV, however, the bilayer structures cannot be as easily classifi ed due
Figure 22.2 The highly varied morphologies of lipid bilayer construction.
to the almost infi nite number of ways each bilayer sheet can be associated and interconnected
with the next one. MLVs typically form large complex honeycomb structures that are diffi cult
to categorize or exactly reproduce. However MLVs are the simplest to prepare, most stable, and
easiest to scale up to large production levels.
Small lipid groupings or monomers also may fuse into bigger, inverted micelles, wherein
their hydrophobic tails point outward toward other inverted micelle lipid tails. The individ-
ual structures are usually hexagonal in shape, but typically they exist as large groupings of
inverted micelles, the outer edge of which contains partial inverted micelles, exposing their
inner hydrophilic heads to the surrounding aqueous environment.
The most useful form of liposomes for bioconjugate applications consists of small, spher-
ical ULVs that possess layers of hydrophilic head groups on their inner and outer surfaces.
The inside of each vesicle can contain hydrophilic molecules that are protected from the outer
environment by the lipid shell. The outside surface can be derivatized to contain covalently
attached molecules designed to target the liposome for specifi c interactions.
1.2. Preparation of Liposomes
Mixtures of phospholipids in aqueous solution will spontaneously associate to form liposomal
structures. To prepare liposomes having morphologies useful for bioconjugate or delivery tech-
niques, it is necessary to control this assemblage to create vesicles of the proper size and shape.
Many methods are available to accomplish this goal, however all of them have at least several
steps in common: (1) dissolving the lipid mixture in organic solvent, (2) dispersion in an aque-
ous phase, and (3) fractionation to isolate the correct liposomal population.
In the fi rst stage, the desired mix of lipid components is dissolved in organic solvent (usually
chloroform:methanol (2:1 by volume)) to create a homogeneous mixture. This mixture will
include any phospholipid derivatized to contain reactive groups as well as other lipids used to
form and stabilize the bulk of the liposomal structure. During all handling procedures using
lipids or their derivatives, it is essential that the solutions be protected from oxidation and
excessive exposure to light, especially the sun. Organic solvents should be maintained under a
nitrogen or argon atmosphere to prevent introduction of oxygen. Water and buffers should be
degassed using a vacuum and bubbled with inert gas before introducing lipid components.
The correct ratio of lipid constituents is important to form stable liposomes. For instance, a
reliable liposomal composition for encapsulating aqueous substances may contain molar ratios of
lecithin:cholesterol:negatively charged phospholipid (e.g., phosphatidyl glycerol (PG)) of 0.9:1:0.1.
A composition that is typical when an activated phosphatidylethanolamine (PE) derivative is
included may contain molar ratios of phosphatidylcholine (PC):cholesterol:PG:derivatized PE of
8:10:1:1. Another typical composition using a maleimide derivative of PE without PG is PC:male-
imide-PE:cholesterol of 85:15:50 (Friede et al., 1993). In general, to maintain membrane stability,
the PE derivative should not exceed a concentration ratio of about 1–10 mol PE per 100 mol of
total lipid.
An example of a lipid mixture preparation based on mass would be to dissolve 100 mg of PC,
40 mg of cholesterol, and 10 mg of PG in 5 ml of chloroform/methanol solution. When using
activated PE components, inclusion of 10 mg of the PE derivative to this recipe will result in a
stable liposome preparation.
1. Properities and Use of Liposomes 861
862 22. Preparation of Liposome Conjugates and Derivatives
Once the desired mixture of lipid components is dissolved and homogenized in organic sol-
vent, one of several techniques may be used to disperse the liposomes in aqueous solution. These
methods may be broadly classifi ed as (1) mechanical dispersion, (2) detergent-assisted solubiliza-
tion, and (3) solvent-mediated dispersion.
Probably the most popular option is mechanical dispersion, simply because the greatest
number of methods that utilize it have been developed. When using mechanical means to form
vesicles, the lipid solution fi rst is dried to remove all traces of organic solvent prior to dis-
persion in an aqueous media. The dispersion process is the key to producing liposomal mem-
branes of the correct morphology. This method uses mechanical energy to break up large lipid
agglomerates into smaller vesicles having the optimal size and shape characteristics necessary
for encapsulation or bioconjugation.
Mechanical dispersion methods involve adding an aqueous solution (which may contain
substances to be encapsulated) to the dried, homogeneous lipid mixture and manipulating it to
effect dispersion. Major methods of mechanical dispersion include simple shaking (Bangham
et al., 1965), non-shaken aqueous contact (Reeves and Dowben, 1969), high-pressure emulsi-
fi cation (Mayhew et al., 1984), sonication (Huang, 1969), extrusion through small-pore mem-
branes (Szoka et al., 1980), and various freeze–thaw techniques (Pick, 1981). Some devices
are available commercially which automate the mechanical dispersion process, usually by high-
pressure emulsifi cation or sonication (Branson Ultrasonics Corp.).
Most of these methods result in a population of vesicles ranging from SUVs of only 25 nm
diameter to very large MLVs. Classifi cation of the desired liposomal morphology may be
done by chromatographic means using columns of Sepharose 2B or Sepharose 4B, by density-
gradient centrifugation using Ficoll or metrizamide gradients, or by dialysis.
Liposome formation by detergent-assisted solubilization utilizes the amphipathic nature of
detergent molecules to bring more effectively the lipid components into the aqueous phase for
dispersion. The detergent molecules presumably bind and mask the hydrophobic tails of lipids
from the surrounding water molecules. Detergent treatment may take place from a dried lipid
mixture or after formation of small vesicles. Usually, nonionic detergents such as the Triton X
family, alkyl glycosides, or bile salts such as sodium deoxycholate are employed for this pro-
cedure. The immediate structures which form as the detergent molecules solubilize the lipids
from a dried state are small micelles. Upon removal of the detergent from the solution, the lipid
micelles aggregate to create larger liposome structures. Liposomes of up to 1,000 Å containing a
single bilayer may be formed using detergent-assisted methods (Enoch and Strittmatter, 1979).
Unfortunately, some detergent-removal processes also may remove other molecules that were to
be entrapped in the liposomes during formation.
Solvent-mediated dispersion techniques used to create liposomes fi rst involve dissolving the
lipid mixture in an organic solvent to create a homogeneous solution, and then introducing this
solution into an aqueous phase. The solvent may or may not be soluble in the aqueous phase to
effect this process. There also may be components dissolved in the aqueous phase to be encap-
sulated in the developing liposomes.
Perhaps the simplest solvent dispersion method is that developed by Batzri and Korn (1973).
Phospholipids and other lipids to be a part of the liposomal membrane are fi rst dissolved in
ethanol. This ethanolic solution then is rapidly injected into an aqueous solution of 0.16 M KCl
using a syringe, resulting in a maximum concentration of no more than 7.5 percent ethanol.
Using this method, single bilayer liposomes of about 25 nm diameter can be created that are
indistinguishable from those formed by mechanical sonication techniques. The main disadvan-
tages of ethanolic injection are the limited solubility of some lipids in the solvent (about 40 mM
for PC) and the dilute nature of the resultant liposome suspension. However, for the prepara-
tion of small quantities of SUVs, this method may be one of the best available.
Other solvent dispersion methods utilize solvents that are insoluble in the aqueous phase.
The key to the production of liposomes by this procedure involves the formation of a “ water-in-
oil ” emulsion. To create the proper reverse-phase emulsion, a small quantity of aqueous phase
must be introduced into a large quantity of organic phase containing the dissolved liposomes.
The result is a milky dispersion containing the “ homogenized ” liposomes. A number of tech-
niques have been developed to perform this procedure (Kim and Martin, 1981; Kim et al., 1983;
Pidgeon et al., 1986). The emulsifi cation process in each of these solvent-dispersion techniques
involves the use of mechanical means (shaking, stirring, or sonication) to effect the formation of
small droplets of aqueous solution uniformly dispersed in the lipid–organic phase.
For the preparation of large quantities of liposomes, mechanical dispersion using a commer-
cially available emulsifi er is probably the best route. For limited quantities, the use of simple
shaking or ethanolic dispersion techniques works well.
Regardless of their method of fabrication, most liposome preparations need to be further
classifi ed and purifi ed before use. To remove excess aqueous components that were not encap-
sulated during the vesicle formation process, gel fi ltration using a column of Sephadex G-50 or
dialysis can be employed. To fractionate the liposome population according to size, gel fi ltra-
tion using a column of Sepharose 2B or 4B should be done.
Small liposome vesicles often aggregate upon standing to form larger, more complex struc-
tures. Therefore, long-term storage in aqueous solution is usually not possible without major
transformations in liposome morphology. Freezing also fractures the liposomal membrane,
releasing any entrapped substances. The inclusion of cryoprotectants such as sugars or poly-
hydroxylic-containing compounds can overcome the structural degradation problems upon
freezing (Harrigan et al., 1990; Talsma et al., 1991; Park and Huang, 1992). Presumably, the
hydroxyl groups in cryoprotectants can take the place of water in hydrogen bonding activities,
thus providing structural support even under conditions in which water is removed. A procedure
by Friede et al. (1993) allows freezing and lyophilization of SUVs in the presence of 4 percent
sorbitol with complete retention of liposome integrity upon reconstitution. Thus freeze-drying
may be the best method for the long-term storage of intact liposomes.
1.3. Chemical Constituents of Liposomes
The overall composition of a liposome—its morphology, chemical constituents (including a
large variety of phospholipids and other lipids or fatty acids), charge, and any attached func-
tional groups—can affect the properties of the vesicle both in vitro and in vivo (Allison and
Gregoriadis, 1974; Alving, 1987; Therien and Shahum, 1989). Although there are literally doz-
ens of lipid components that potentially can be included in a liposomal recipe, only a handful
are commonly used.
Phospholipids are the most important of these liposomal constituents. Being the major com-
ponent of cell membranes, phospholipids are composed of a hydrophobic, fatty acid tail, and
a hydrophilic head group. The amphipathic nature of these molecules is the primary force that
drives the spontaneous formation of bilayers in aqueous solution and holds the vesicles together.
1. Properities and Use of Liposomes 863
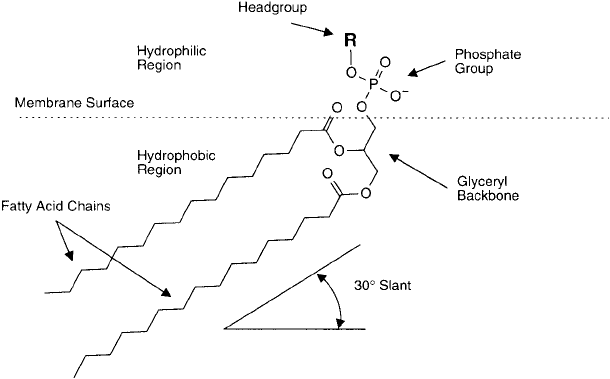
864 22. Preparation of Liposome Conjugates and Derivatives
Naturally occurring phospholipids can be isolated from a variety of sources. One of the
most common phospholipid raw materials is egg yolk. However, since the composition of egg
phospholipid is from a biological source and can vary considerably depending on age of the
eggs, the diet of the chickens, and the method of processing, newer enzymatic and synthetic
chemical methods now are being employed to manufacture the required phospholipid deriva-
tives in higher purity and yield.
Two main forms of lipid derivatives exist biologically: molecules containing a glycerol back-
bone and those containing a sphingosine backbone. The most important type for liposomal
construction is a phosphodiglyceride derivative, which consists of a glycerol backbone that links
two fatty acid molecules with a polar head group ( Figure 22.3 ). The fatty acids are acyl bonded
in ester linkages to the Nos. 1 and 2 carbon hydroxyls of the glycerol bridge. The No. 3 carbon
hydroxyl of the glycerol group is phosphorylated and possesses a negative charge at physiologi-
cal pH. This basic phosphodiglyceride construct of two fatty acids and one glycerylphosphate
group is called phosphatidic acid. This is the simplest form of phospholipid available.
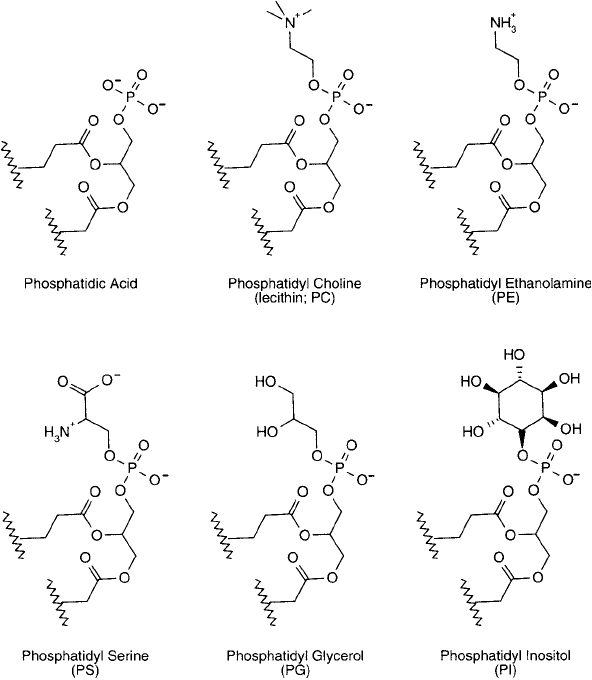
The fundamental phosphatidyl group also can be further derivatized at the phosphate to
contain an additional polar constituent. Several common derivatives of phosphodiglycerides are
naturally occurring, including PC; commonly called lecithin, phosphatidyl ethanolamine (PE),
phosphatidyl serine (PS), phosphatidyl glycerol (PG), and phosphatidyl inositol (PI) ( Figure
22.4 ). All of these phospholipids have polar groups that are linked to the phosphatidyl moiety
in a phosphate ester bond. The most abundant of these derivatives in biological cell membranes
is PC—the trimethyl derivative of PE, possessing a positive charge at physiological pH. Some or
all of these phosphodiglyceride derivatives can be mixed to create a particular liposomal recipe.
The fatty acid constituents of phosphodiglycerides can vary considerably in nature among
a number of different chain lengths and points of unsaturation. A given isolated phosphatidyl
Figure 22.3 The basic construction of phosphodiglyceride molecules within lipid bilayers. The fatty acid chains
are embedded in the hydrophobic inner region of the membrane, oriented at an angle to the plane of the mem-
brane surface. The hydrophilic head group, including the phosphate portion, points out toward the hydrophilic
aqueous environment.
derivative from a biological source usually possesses a range of fatty acid components, varying in
chain length from C16 to about C24. Some of the fatty acids also may contain points of unsatu-
ration—one or more double bonds between certain carbon atoms within the chain (Matreya
and Avanti Polar Lipids, suppliers). For instance, egg lecithin is not a single compound, but con-
tains a mixture of PC containing about 31 percent saturated fatty acid having a chain length
of 16 carbons, 16 percent saturated fatty acid with 18 carbons, about 48 percent also with 18
carbons but having at least 1–2 points of unsaturation, and the rest a variety of other fatty acid
constituents. Naturally occurring, unsaturated fatty acids typically are of the cis conformation,
not trans. The existence of unsaturation within a fatty acid is usually abbreviated as the chain
length followed by a colon and the number of double bonds. For instance, cis-9-hexadecenoic
acid (palmitoleic acid) contains one double bond at carbon 9 and it is abbreviated as C16:1.
By contrast, a given synthetic preparation of a major phospholipid possesses fatty acid constit-
uents all of identical chain length and unsaturation. A synthetic PC derivative can be purchased
that contains only, for instance, 1,2-dimyristoyl (C14) fatty acid substitutions on its glyceryl
Figure 22.4 The head-group construction of the six commonly encountered phosphatidyl derivatives.
1. Properities and Use of Liposomes 865
866 22. Preparation of Liposome Conjugates and Derivatives
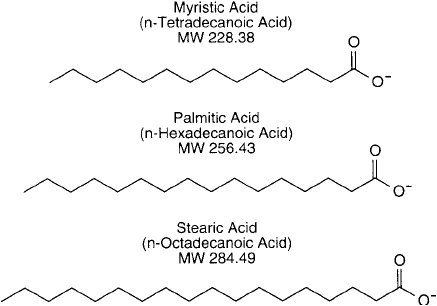
Figure 22.5 The three fatty acid components commonly used in liposome construction.
backbone (Genzyme). The use of synthetic rather than natural phospholipids for making lipo-
somes thus produces reagents of known chemical purity, which is very important for regulatory
requirements surrounding the introduction of products used topically or in vivo .
Despite the large variety of potential fatty acid components in natural-occurring phosphod-
iglycerides, only three major fatty acid derivatives of synthetic phospholipids are commonly
used in liposome preparation: (1) myristic acid ( n-tetradecanoic acid; containing 14 carbons),
(2) palmitic acid ( n-hexadecanoic acid; containing 16 carbons), and (3) stearic acid ( n -octade-
canoic acid; containing 18 carbons) ( Figure 22.5 ).
The nomenclature for associating individual fatty acid groups with particular phosphodig-
lyceride derivatives is straightforward. For instance, a phosphatidic acid (PA) derivative which
contains two myristic acid chains is commonly called dimyristoyl phosphatidic acid (DMPA).
Likewise, a PC derivative containing two palmitate chains is called dipalmitoyl phosphatidyl
choline (DPPC). Other phosphodiglyceride derivatives are similarly named.
The second form of lipid derivative that occurs naturally in membrane structures is derived
from sphingosine. Unlike the phosphodiglyceride derivatives discussed above, sphingo-
lipids contain no glycerol backbone. Instead, these lipids are constructed from a derivative of
4-sphingenine, containing an N-acyl-linked fatty acid group and possibly other constituents
off the No. 1 carbon hydroxyl group ( Figure 22.6 ). Sphingolipids are highly similar in their
construction to glyceryl lipids, in that there are two hydrophobic tails present on a 3-carbon
backbone (one of them contributed from 4-sphingenine itself and the other from the attached
fatty acid), and there also exists a hydrophilic head group. This creates the typical amphipathic
properties common to all lipid membrane components.
The simplest form of sphingolipid, ceramide, contains a fatty acid group, but no additional
components on the No. 1 hydroxyl. Major derivatives of ceramide at the 1-hydroxyl position
include a positively charged phosphocholine compound, called sphingomyelin, a glucose deriv-
ative, called glucosylcerebroside, and other complex carbohydrate derivatives, termed gan-
gliosides ( Figure 22.7 ). Gangliosides are involved in various cellular recognition phenomena,
including being part of the blood group determinants, A, B, and O, in humans.
The use of sphingolipids in liposome formation is possible due to the natural amphipathic
properties of the molecules. Some sphingolipids can lend structural advantages to the integrity
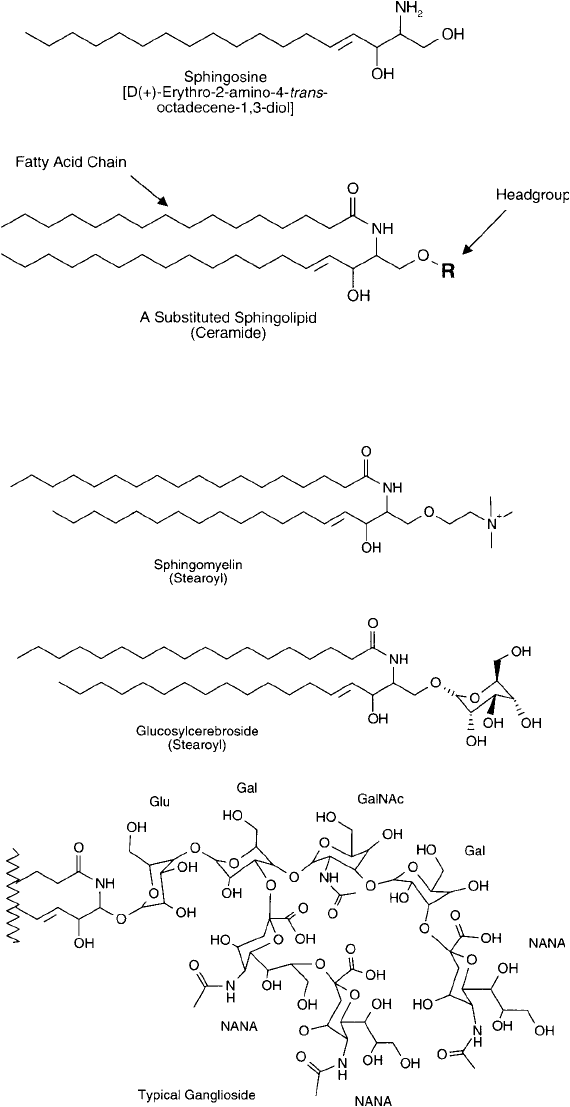
Figure 22.6 Sphingolipids are constructed of sphingosine derivatives containing an acylated fatty acid and a
head group attached to the hydroxyl.
Figure 22.7 Common sphingolipid derivatives include small and highly complex head groups.
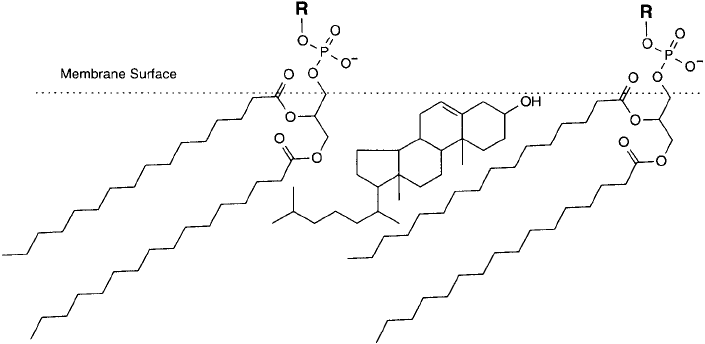
868 22. Preparation of Liposome Conjugates and Derivatives
Figure 22.8 The orientation of cholesterol in phospholipid bilayers.
of liposomal membranes. Sphingomyelin, for example, is capable of hydrogen bonding with
adjacent glyceryl lipids, thus increasing the order and stability of the vesicle construction. This
stability may translate into a lower potential for passage of molecules through the membrane
bilayer, forming vesicles that are better able to retain their contents than more fl uid liposome
constructions. The temperature of phase transition in sphingolipid-containing membranes is
often greater than membranes constructed of only phosphodiglyceride derivatives. Liposomes
containing sphingomyelin or gangliosides also have prolonged lifetimes in vivo (Gregoriadis
and Senior, 1980; Allen and Chonn, 1987) and may be advantageous for creating liposome
immunogen complexes.
The main disadvantage of incorporating sphingolipids in liposomes is their high cost.
Purifi ed phosphodiglyceride derivatives may be obtained in bulk quantities and in highly
defi ned synthetic preparations, whereas sphingolipid derivatives are not so readily available in
similar purity.
Another signifi cant component of many liposome preparations is cholesterol. In natural cell
membranes, cholesterol makes up about 10–50 percent of the total lipid. For liposome prepa-
ration, it is typical to include a mole ratio of about 50 percent cholesterol in the total lipid rec-
ipe. The addition of cholesterol to phospholipid bilayers alters the properties of the resultant
membrane in important ways. As it dissolves in the membrane, cholesterol orients itself with
its polar hydroxyl group pointed toward the aqueous outer environment, approximately even,
in a three-dimensional sense, with the glyceryl backbone of the bilayer ’s phosphodiglyceride
components ( Figure 22.8 ). Structurally, cholesterol is a rigid component in membrane construc-
tion, not having the same freedom of movement that the fatty acid tails of phosphodiglycerides
possess. Adjacent phospholipid molecules are restricted in their freedom of movement through-
out the length of their fatty acid chains that are abutting the cholesterol molecules. However,
since the cholesterol components have the effect of creating spaces in the uniform hydrophobic
morphology of the bilayer, the portion of the fatty acid chains below the abutted regions are
increased in their freedom of movement.
Cholesterol ’s presence in liposome membranes has the effect of decreasing or even abolish-
ing (at high cholesterol concentrations) the phase transition from the gel state to the fl uid or
liquid crystal state that occurs with increasing temperature. It also can modulate the permeabil-
ity and fl uidity of the associated membrane—increasing both parameters at temperatures below
the phase transition point and decreasing both above the phase transition temperature. Most
liposomal recipes include cholesterol as an integral component in membrane construction.
1.4. Functional Groups of Phospholipids
For the production of liposomal conjugates, lipid derivatives must be incorporated into the
bilayer construction that contain available functional groups able to be chemically crosslinked
or modifi ed. A number of phosphodiglyceride compounds can be employed for conjugation
purposes. Each of these components contains a head group that can be directly derivatized or
chemically modifi ed to contain a reactive group. For instance, several lipid derivatives contain
amine groups that can be utilized in nucleophilic reactions with crosslinkers or other modifi ca-
tion reagents. These include PE, PS, and stearylamine. Carboxyl-containing molecules include
all the individual fatty acids as well as PS. These can be coupled to amine-containing mole-
cules by the use of the carbodiimide reaction (Chapter 3, Section 1). Hydroxyl-containing lipids
include PG, fatty acid alcohols, PI, and various gangliosides and cerebrosides of sphingolipid
derivation. Lipids possessing hydroxyl groups on adjacent carbon atoms, such as those contain-
ing sugar constituents, may be oxidized with sodium periodate to produce reactive aldehyde
residues (Chapter 1, Section 4.4). Coupling aldehydes to amine-containing molecules can be
accomplished by reductive amination (Chapter 3, Section 4). Finally, the phosphate groups of
PA residues may be used to conjugate with amine-containing molecules in a method similar
to modifi cation of the 5 -phosphates of DNA probes. This is done through the use of the car-
bodiimide reaction with 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) (Chapter 27,
Section 2.1). Figure 22.9 shows the structures and reactive sites of these lipid functional groups.
When liposomes are used as part of a conjugate system, the targeting molecules usually are
attached covalently to these head group functional groups using standard crosslinking chemis-
tries (Derksen and Scherphof, 1985). Although all of the above-mentioned functional groups
on lipid molecules can be used for the conjugation process, most often the derivatization reac-
tion is done off the PE constituents within the liposomal mixture. The primary amine modifi ca-
tion off the glycerylphosphate head of PE provides an ideal functional group for activation and
subsequent coupling of targeting or detection molecules (Shek and Heath, 1983). Liposomes
may be constructed with reactive groups already prepared on their PE constituents, all set to
be conjugated with selected molecules having the correct functional group. Stock preparations
of activated liposomes may even be prepared and lyophilized to be used as needed in coupling
macromolecules (Friede et al., 1993). All of the amine-reactive conjugation methods discussed
in this section may be used with PE-containing liposomes.
2. Derivatization and Activation of Lipid Components
Two approaches for the activation of lipid components may be used to create reactive groups in
liposomes. A purifi ed lipid may be activated prior to incorporation into the bilayer construction
2. Derivatization and Activation of Lipid Components 869
